Abstract
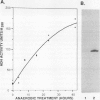
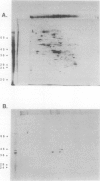
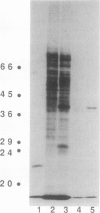
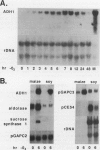
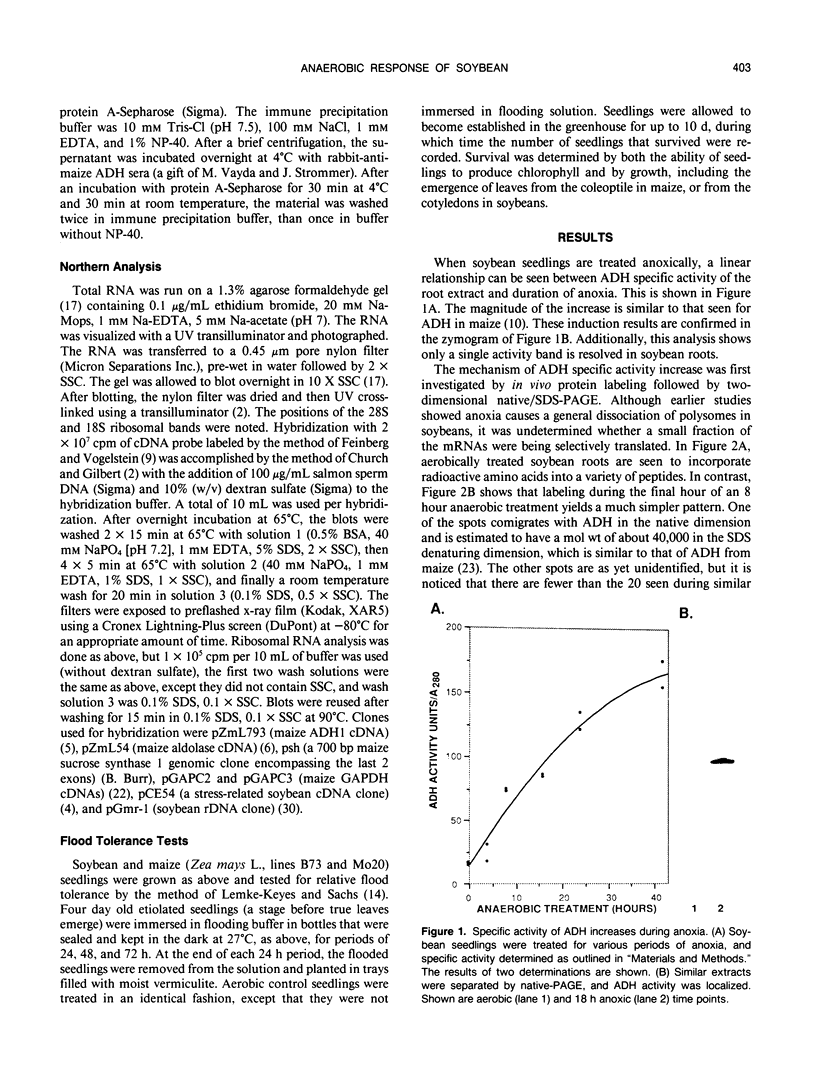
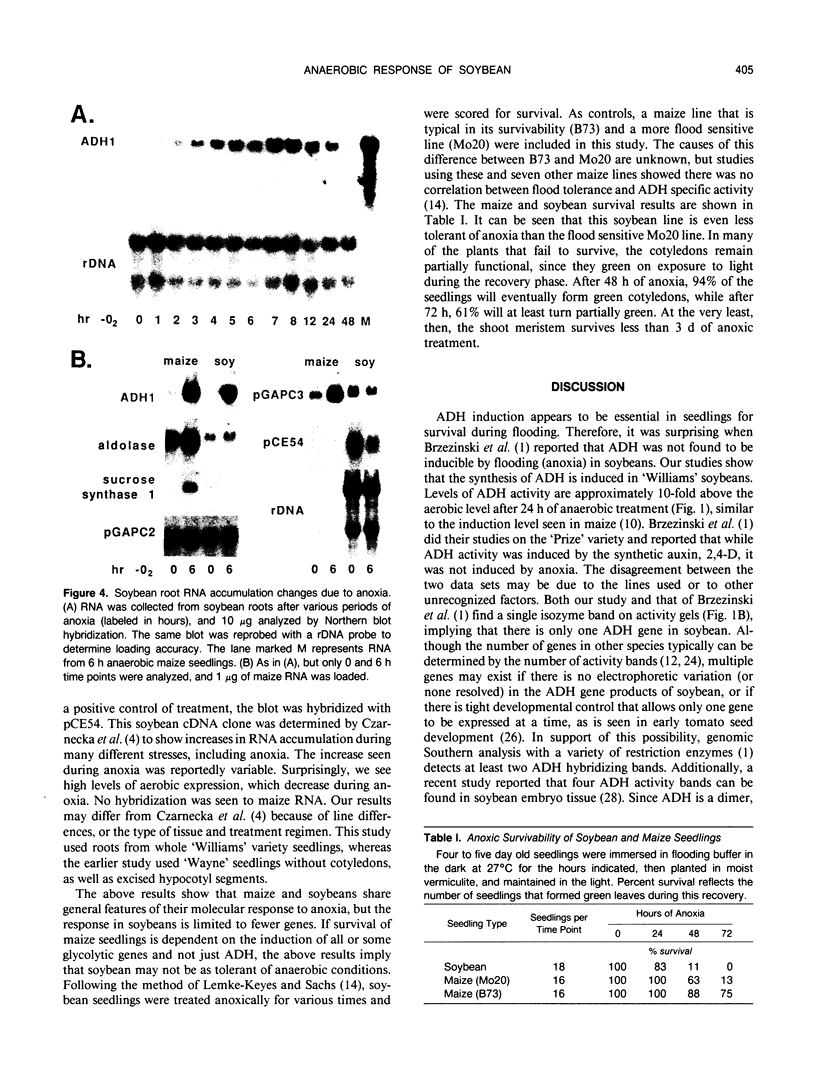
The effect of anoxia on roots of soybean (Glycine max [L.] Merr., variety `Williams') was studied at various levels and the results compared to those from previously studied species. While alcohol dehydrogenase (ADH) activity is induced in a manner similar to other plant species, other aspects of the anaerobic response are unique to soybean. A variety of molecular clones was used to analyze changes in soybean and maize RNA levels. Increased RNA accumulation was observed in both species with a maize ADH clone, while a maize aldolase and one of the two different maize glyceraldehyde-3-phosphate dehydrogenase cDNA clones showed induction only in maize. A maize sucrose synthase 1 clone showed induction in maize but no hybridization to soybean RNA samples. The reduction in the number of anaerobically inducible soybean genes relative to maize is consistent with in vivo and in vitro protein synthesis results. Only four major proteins are labeled during anoxia in soybean, one corresponding to ADH, while maize has been reported to have about 20. In either species, in vitro translation yields similar products with RNA from anaerobic and pre-stress plants, indicative of translational control during anoxia. These results are discussed in relation to the differential tolerance of maize and soybean to anaerobic stress.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brzezinski R., Talbot B. G., Brown D., Klimuszko D., Blakeley S. D., Thirion J. P. Characterization of alcohol dehydrogenase in young soybean seedlings. Biochem Genet. 1986 Oct;24(9-10):643–656. doi: 10.1007/BF00498999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E. S., Gerlach W. L., Pryor A. J., Bennetzen J. L., Inglis A., Llewellyn D., Sachs M. M., Ferl R. J., Peacock W. J. Molecular analysis of the alcohol dehydrogenase (Adh1) gene of maize. Nucleic Acids Res. 1984 May 11;12(9):3983–4000. doi: 10.1093/nar/12.9.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E. S., Gerlach W. L., Walker J. C., Lavin M., Peacock W. J. Anaerobically regulated aldolase gene of maize. A chimaeric origin? J Mol Biol. 1988 Aug 20;202(4):759–767. doi: 10.1016/0022-2836(88)90556-6. [DOI] [PubMed] [Google Scholar]
- Ellis J. G., Llewellyn D. J., Dennis E. S., Peacock W. J. Maize Adh-1 promoter sequences control anaerobic regulation: addition of upstream promoter elements from constitutive genes is necessary for expression in tobacco. EMBO J. 1987 Jan;6(1):11–16. doi: 10.1002/j.1460-2075.1987.tb04711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- HAGEMAN R. H., FLESHER D. The effect of an anaerobic environment on the activity of alcohol dehydrogenase and other enzymes of corn seedings. Arch Biochem Biophys. 1960 Apr;87:203–209. doi: 10.1016/0003-9861(60)90161-2. [DOI] [PubMed] [Google Scholar]
- Irish E. E., Schwartz D. Activation of low and null activity isozymes of maize alcohol dehydrogenase by antibodies. Mol Gen Genet. 1987 Jun;208(1-2):271–278. doi: 10.1007/BF00330453. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Dolferus R., Van den Bossche D. Isolation and biochemical analysis of ethyl methanesulfonate-induced alcohol dehydrogenase null mutants of arabidopsis thaliana (L.) Heynh. Biochem Genet. 1988 Feb;26(1-2):105–122. doi: 10.1007/BF00555492. [DOI] [PubMed] [Google Scholar]
- Jen G., Thach R. E. Inhibition of host translation in encephalomyocarditis virus-infected L cells: a novel mechanism. J Virol. 1982 Jul;43(1):250–261. doi: 10.1128/jvi.43.1.250-261.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. Y., Key J. L. Dissocation and reassembly of polyribosomes in relation to protein synthesis in the soybean root. J Mol Biol. 1967 Jun 14;26(2):237–247. doi: 10.1016/0022-2836(67)90294-x. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Przybyla A. E., Chirgwin J. M. Isolation of RNA using guanidinium salts. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- Morell M., Copeland L. Sucrose synthase of soybean nodules. Plant Physiol. 1985 May;78(1):149–154. doi: 10.1104/pp.78.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Russell D. A., Sachs M. M. Differential expression and sequence analysis of the maize glyceraldehyde-3-phosphate dehydrogenase gene family. Plant Cell. 1989 Aug;1(8):793–803. doi: 10.1105/tpc.1.8.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Schwartz D., Endo T. Alcohol Dehydrogenase Polymorphism in Maize-simple and Compound Loci. Genetics. 1966 Apr;53(4):709–715. doi: 10.1093/genetics/53.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer B., Werr W., Starlinger P., Bennett D. C., Zokolica M., Freeling M. The Shrunken gene on chromosome 9 of Zea mays L is expressed in various plant tissues and encodes an anaerobic protein. Mol Gen Genet. 1986 Dec;205(3):461–468. doi: 10.1007/BF00338083. [DOI] [PubMed] [Google Scholar]
- Tanksley S. D., Jones R. A. Effects of O2 stress on tomato alcohol dehydrogenase activity: description of a second ADH coding genes. Biochem Genet. 1981 Apr;19(3-4):397–409. doi: 10.1007/BF00504283. [DOI] [PubMed] [Google Scholar]
- Thummler F., Verma D. P. Nodulin-100 of soybean is the subunit of sucrose synthase regulated by the availability of free heme in nodules. J Biol Chem. 1987 Oct 25;262(30):14730–14736. [PubMed] [Google Scholar]
- Walker J. C., Howard E. A., Dennis E. S., Peacock W. J. DNA sequences required for anaerobic expression of the maize alcohol dehydrogenase 1 gene. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6624–6628. doi: 10.1073/pnas.84.19.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer E. A., Jupe E. R., Walbot V. Ribosomal gene structure, variation and inheritance in maize and its ancestors. Genetics. 1988 Dec;120(4):1125–1136. doi: 10.1093/genetics/120.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]