Abstract
Although the immunogenicity of formalin-fixed paraffin-embedded tissue sections can decrease during storage and transport, the exact mechanism of antigenic loss and how to prevent it are not clear. Herein, we investigated changes in the expression of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER-2), E-cadherin, and Ki-67 in human breast tissue microarray (TMA) tissue sections stored for up to 3 months in dry and wet conditions. The positive rates of ER and PR expression were minimally changed after 3 months of storage, but the Allred scores of ER and PR stored in humid conditions decreased remarkably in comparison to fresh-cut tissue. The HER-2 antigenicity and RNA integrity of breast TMA sections stored in dry conditions diminished gradually with storage time, whereas the immunoreactivity and RNA quality of HER-2 in humid conditions decreased sharply as storage length increased. The area and intensity of E-cadherin staining in tissue sections stored in dry conditions did not change significantly and were minimally changed after 3 months, respectively. In contrast, the area and intensity of E-cadherin staining in tissue sections stored in humid conditions decreased significantly as storage length increased. Finally, the Ki-67 labeling index of tissue sections stored for 3 months in dry (9% decrease) and wet (31.9% decrease) conditions was decreased in comparison to fresh sections. In conclusion, these results indicate that water is a crucial factor for protein and RNA degradation in stored tissue sections, and detailed guidelines are required in the clinic.
Keywords: antigenicity, formalin fixed, immunohistochemistry, in situ hybridization, storage condition, tissue microarray
Introduction
In an era of personalized therapy, various ancillary molecular tests are conducted with patient tissue specimens after histopathological examination to determine prognoses and design treatment strategies. In particular, transcriptional profiling analysis relies on surgical tissue specimen quality.1,2 However, long-term preservation of high-quality specimens, such as frozen tissue, is not practical in a routine clinical care environment. The tissues obtained from patients are usually processed into formalin-fixed, paraffin-embedded (FFPE) blocks for diagnostic histopathology and archival storage.
Immunohistochemistry (IHC) is well established and commonly used in histopathology for diagnosis, prognosis, and biomarker identification, but its use is often plagued by poor reproducibility and inconsistent interpretation. Although there have been recent efforts toward improving IHC reagents, standardization, and automation,3,4 there are several factors that can affect IHC staining quality. These include preanalytical conditions (e.g., cold ischemia, fixation, and processing time), section thickness, and paraffin block and slide storage conditions.5–9 For the best staining quality, a fresh paraffin section should be used, but use of stored slides is inevitable. Prior studies have demonstrated that humidity and a long-term delay between cutting sections and IHC staining (section aging) can cause antigen decay, whereas cold temperature storage of paraffin blocks or sections prevents antigen degradation.10–12
In contrast, there are reports that antigenicity in stored paraffin blocks is preserved for several decades with very slow and minimal antigen decay.13,14 Although scientists believe that oxidation processes and archival conditions are involved in the loss of antigenicity in stored paraffin blocks and slides, the exact mechanism is unclear. To overcome these problems, several storage methods have been developed. Some methods recommend storage in a refrigerator or paraffin coating,5,9,10 but these are controversial and cumbersome to use, which make them difficult to apply in the clinic.
The guidelines of the American Society of Clinical Oncology and the College of American Pathologists (ASCO/CAP) require both IHC and in situ hybridization (ISH) tests for the assessment of diagnostic and prognostic markers in cancer tissue specimens. ISH allows the detection of gene expression or genetic alterations at the cellular level within histological sections and has become an important diagnostic tool. It serves as an alternative to conventional IHC for many markers, including human epidermal growth factor receptor 2 (HER-2),15 phosphatase and tensin homolog,16 and programmed cell death ligand 1.17 In situ transcriptional profiling of single cells provides important insights into normal biological function and disease processes. In this context, RNA assays using FFPE tissues have become important for prognoses. Studies have shown that the quality of RNA from FFPE tissues relies on proper fixation, processing, tissue block storage, and age.1,2,18–20
Breast cancer is a key example of how molecular and histological characterization play a fundamental role in treatment decisions. In most clinical pathology laboratories, IHC for estrogen receptor (ER), progesterone receptor (PR), and HER-2 is used for subtyping of breast cancer, and IHC examination for E-cadherin is also used when lobular carcinoma is suspected.21,22 Ki-67 expression (cutoff value >25%) provides strong prognostic information in breast cancer patients.23
In examining the expression of these major markers, slide quality management is important.8,10,24 According to recently updated ASCO/CAP guidelines for breast biomarker reporting, variables that can affect test results include cold ischemia time, fixation time, type of fixative, any tissue treatment that could potentially alter immunoreactivity (decalcification), the status of internal and external controls, adequacy of sample for evaluation, primary antibody clone, and regulatory status (Food and Drug Administration cleared vs. laboratory-developed test). For HER-2 expression, ASCO/CAP guidelines recommend not to use unstained slides that have been cut more than 6 weeks before analysis.25 However, there is no definite storage regulation for other subtype-determining or prognosis-related markers.
To expand our previous finding that water negatively impacts protein and RNA stability in FFPE tissue sections, we analyzed changes in the IHC staining of breast cancer subtype markers based on two storage methods at room temperature: vacuum packed with a desiccant or in a humidity chamber. In addition, we also investigated HER-2 messenger RNA (mRNA) level by assessing c-erbB2 expression in stored breast cancer tissue microarray (TMA) slides using RNA-ISH technology. IHC and RNA-ISH signal intensities were assessed by digital image analysis. Furthermore, we evaluated whether loss of bioanalytes from experimentally stored human breast TMA sections affected breast biomarkers accordant with the current ASCO/CAP clinical practice guidelines.
Materials and Methods
Specimens and TMA construction
This study included 100 consecutive breast cancer cases, which were resected surgically at Kangbuk Samsung Hospital between January and December 2010. Cases with neo-adjuvant chemotherapy were not included. Based on the histology, the total cohort consisted of 97 cases of invasive carcinoma and three cases of invasive lobular carcinoma. The patients' clinical data, including age at operation, sex, type of operation, and adjuvant chemotherapy, were obtained from electronic medical records. All slides of 100 breast cases were reviewed and pathologic findings, including histologic grade, tumor size, and lymph node metastasis, were recorded.
The molecular subtype was determined on the basis of IHC results (ER, PR, HER-2, and Ki-67), which were routinely performed with breast cancer tissue post-operation. TMA blocks were produced with 2-mm-diameter cores from representative paraffin blocks of each tumor, as described previously.26 This study protocol was approved by the Institutional Review Board at Kangbuk Samsung Hospital (IRB No. 2020-08-029-001, Seoul, Republic of Korea).
Storage conditions of unstained slides
The unstained breast TMA slides were produced at 3 months, 1 month, 1 week, and 1 day before the scheduled date of the IHC and RNA-ISH tests. The 1-day-old slides were used as the controls for both tests. Sections were 5-μm thick, and slides were baked in an oven for 60 minutes. These slides were stored using either a vacuum packaging unit (Foodsaver; Jarden Corporation, Providence, RI) with a desiccant (Drierite, anhydrous calcium sulfate; W.A. Hammond Company, Xenia, OH) or a humidity chamber at room temperature. The humidity chamber was built using a plastic container (11 × 8 × 6 cm) containing 100 mL distilled water. The slides were placed on a rack above the water and sealed in the plastic container (humidity, 94%–96%). Then, they were collected and used for IHC staining or RNA-ISH at the same time to minimize intraexperimental variability.
IHC staining
Breast TMA sections from each condition were deparaffinized and rehydrated through xylenes and serial alcohol solutions, respectively. Slides were incubated for 10 minutes at 3% H2O2 to quench endogenous peroxidase activity after antigen retrieval. Detailed IHC conditions are described in Table 1. We used the IHC conditions that were established previously in our laboratory. The antigen-antibody reaction was detected with Dako Envision + Dual Link system-horseradish peroxidase (Dako, Carpinteria, CA) and visualized with 3,3′-diaminobenzidine (Dako). Mouse or rabbit immunoglobulin G and the omission of the primary antibody were used as a negative control. Slides were counterstained with hematoxylin and cover-slipped.
Table 1.
Antibodies Used for Immunohistochemistry
| Antibody | Vendor | Clonality | Cat. no. | Incubation | Dilution | Antigen retrieval |
|---|---|---|---|---|---|---|
| ER | Dako | mAb Ms (1D5) | M7047 | 30 minutes at RT | 1/500 | 20 minutes, pressure cooker, pH 9 |
| PR | Dako | mAb Ms (PgR636) | M3569 | 30 minutes at RT | 1/1000 | 20 minutes, pressure cooker, pH 9 |
| HER-2 | Dako | pAb Rb (c-erbB-2) | A0485 | 30 minutes at RT | 1/2000 | 20 minutes, pressure cooker, pH 6 |
| E-cadherin | Dako | mAb Ms (NCH-38) | M3612 | 30 minutes at RT | 1/500 | 20 minutes, pressure cooker, pH 9 |
| Ki-67 | Cell marque | mAb Rb (SP6) | 275R-15 | 30 minutes at RT | 1/250 | 20 minutes, pressure cooker, pH 9 |
ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2; PR, progesterone receptor; RT, room temperature.
The stained slides were scanned by an Aperio AT2 digital scanner with a 40 × objective (Leica Biosystems Inc., Buffalo Grove, IL). Representative images of each core were analyzed using Visiopharm software version 4.5.1.324 (Visiopharm, Hørsholm, Denmark). For ER and PR, nuclear staining was recorded separately as staining intensity (0 = negative, 1 = weak, 2 = moderate, and 3 = strong) and proportion; the scores were calculated using the Allred score system.27 Membrane HER-2 and E-cadherin expression were quantified by staining area and intensity. HER-2 positivity was determined using the HER-2 scoring system from the ASCO/CAP clinical practice guideline-focused update, with scores of 0 and 1 considered negative, 2 equivocal, and 3 positive.28 The Ki-67 labeling index was calculated using the fraction of tumor cell nuclei stained at moderate or higher intensity.
In situ hybridization
Chromogenic in situ detection of HER-2 mRNA was performed manually using the RNAScope in situ Hybridization Kit (Advanced Cell Diagnostics, Hayward, CA) according to the manufacturer's instructions. Briefly, the slides were deparaffinized, boiled with pretreatment reagent for 15 minutes, and then digested with protease at 40°C for 30 minutes, followed by hybridization for 2 hours at 40°C with probe-Hs-Erb-B2 receptor tyrosine kinase 2 (ERBB2) (Cat. No. 310081; Advanced Cell Diagnostics). In addition, peptidylprolyl isomerase B (Probe-Hs-PPIB; Cat. No. 313901; Advanced Cell Diagnostics) and Bacillus subtilis dihydrodipicolinate reductase (Probe-DapB; Cat. No. 310043; Advanced Cell Diagnostics) were used as positive and negative controls, respectively. Specific probe binding sites were visualized with the RNAScope 2.5 HD Reagent Kit (Brown) (Cat. No. 322370; Advanced Cell Diagnostics) and lightly counterstained with hematoxylin. Positive staining was detected as brown dots in the nucleus and/or cytoplasm.
Digital images were acquired using an Aperio AT2 digital scanner with a 40 × objective (Leica Biosystems Inc.). Captured digital images were imported into computer-assisted image analyzing software (Visiopharm v6.9.1) for quantification of ERBB2 mRNA expression. After training the system by digitally “painting” examples of the nucleus in the image, tumor nuclei were defined, and the cytoplasm was further defined by outlining the nucleus. Finally, the detection system was digitally trained to identify brown 3,3′-diaminobenzidine dots of HER-2. The mean value of brown dots per tumor cell in the representative areas of each TMA was calculated.
Statistical analyses
Variables were expressed as mean ± standard deviation (SD) or percentage and frequency. The differences between fresh and 1-week, 1-month, and 3-month samples from each group were analyzed using a paired t-test for continuous variables and using the McNemar's test for categorical variables, as appropriate. An interaction test to estimate the significant differences between groups over time was calculated using the linear mixed model for continuous variables and the generalized estimating equations method for categorical variables, as appropriate. A two-tailed p-value <0.05 was considered significant. All statistical analysis was performed using SPSS 24 (IBM SPSS Statistics 24; IBM Corporation, Armonk, NY).
Results
Patient characteristics
The patients' clinicopathological characteristics are summarized in Supplementary Table S1. All patients were female and the median age at operation was 50 years (range, 32–76 years). The mean tumor size was 2.2 cm (range, 0.7–5.7 cm). Twenty-five patients (25.0%) were the American Joint Committee on Cancer stage I, 43 (43.0%) were stage II, 28 (28.0%) were stage III, and 4 (4.0%) were stage IV. LN metastasis was detected in 48 (48.0%) patients. The biological subtypes of breast cancer included luminal A (n = 64, 64.0%), luminal B (n = 23, 23.0%), HER-2-enriched (n = 6, 6.0%), and triple-negative breast cancer (n = 7, 7.0%).
ER and PR expression
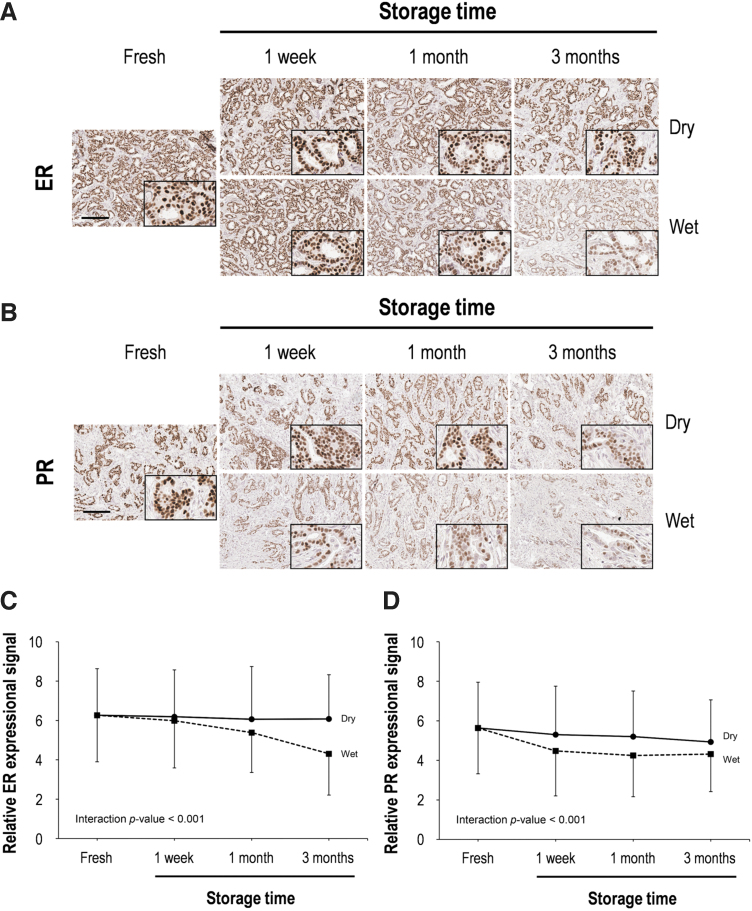
Of 100 patients, 89–94 interpretable samples were obtained for IHC and molecular assays. To assess the effects of storage period and humidity on the antigenicity of human breast FFPE TMA sections, ER and PR expression were evaluated by IHC staining (Fig. 1). Typical nuclear staining for ER and PR was detected in all tissue sections stored in dry conditions. In contrast, the ER and PR staining of tissue sections stored in the humidity chamber for 3 months were significantly weakened in intensity and number of stained cells (Fig. 1A, B). Using the Allred scoring system, we confirmed that the total score (TS) and intensity score (IS) for ER were significantly decreased after 3 months of storage in dry conditions, whereas there was no notable difference in ER proportion score (PS) among the dry conditions (Table 2).
FIG. 1.
Immunohistochemical assessment of ER and PR according to storage time in both dry and wet conditions. Representative images of ER (A) and PR (B) immunohistochemical staining in human breast tissue. High magnification images are shown in the inset. Scale bar, 100 μm. Quantitative analysis of ER (C) and PR (D) immunohistochemical staining. Data are presented as mean ± SD. ER, estrogen receptor; PR, progesterone receptor; SD, standard deviation.
Table 2.
Allred Scores for Estrogen Receptor and Progesterone Receptor Evaluation in Breast Cancer Tissues Stored Experimentally
| Dry |
Wet |
||||||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD | p | n | Mean ± SD | p | ||
| ER | |||||||
| PS | Fresh | 94 | 4.07 ± 1.56 | 94 | 4.07 ± 1.56 | ||
| 1 Week | 94 | 4.09 ± 1.54 | 0.829 | 94 | 4.01 ± 1.59 | 0.241 | |
| 1 Month | 92 | 4.01 ± 1.73 | 0.277 | 94 | 3.72 ± 1.41 | <0.001 | |
| 3 Months | 89 | 4.12 ± 1.51 | 0.798 | 92 | 3.09 ± 1.64 | <0.001 | |
| IS | Fresh | 94 | 2.19 ± 0.88 | 94 | 2.19 ± 0.88 | ||
| 1 Week | 94 | 2.11 ± 0.93 | 0.103 | 94 | 1.98 ± 0.90 | <0.001 | |
| 1 Month | 92 | 2.09 ± 1.02 | 0.068 | 94 | 1.66 ± 0.74 | <0.001 | |
| 3 Months | 89 | 1.99 ± 0.85 | <0.001 | 92 | 1.24 ± 0.60 | <0.001 | |
| TS | Fresh | 94 | 6.27 ± 2.37 | 94 | 6.27 ± 2.37 | ||
| 1 Week | 94 | 6.19 ± 2.38 | 0.373 | 94 | 5.99 ± 2.40 | 0.004 | |
| 1 Month | 92 | 6.10 ± 2.67 | 0.104 | 94 | 5.38 ± 2.03 | <0.001 | |
| 3 Months | 89 | 6.11 ± 2.23 | 0.003 | 92 | 4.33 ± 2.11 | <0.001 | |
| PR | |||||||
| PS | Fresh | 93 | 3.76 ± 1.56 | 94 | 3.78 ± 1.55 | ||
| 1 Week | 93 | 3.55 ± 1.64 | 0.004 | 94 | 3.07 ± 1.56 | <0.001 | |
| 1 Month | 93 | 3.55 ± 1.58 | 0.005 | 93 | 3.04 ± 1.50 | <0.001 | |
| 3 Months | 91 | 3.44 ± 1.48 | 0.001 | 94 | 3.10 ± 1.31 | <0.001 | |
| IS | Fresh | 93 | 1.86 ± 0.87 | 94 | 1.86 ± 0.86 | ||
| 1 Week | 93 | 1.75 ± 0.92 | 0.032 | 94 | 1.40 ± 0.83 | <0.001 | |
| 1 Month | 93 | 1.66 ± 0.84 | <0.001 | 93 | 1.20 ± 0.72 | <0.001 | |
| 3 Months | 91 | 1.49 ± 0.78 | <0.001 | 94 | 1.22 ± 0.72 | <0.001 | |
| TS | Fresh | 93 | 5.62 ± 2.33 | 94 | 5.64 ± 2.32 | ||
| 1 Week | 93 | 5.30 ± 2.45 | 0.002 | 94 | 4.48 ± 2.28 | <0.001 | |
| 1 Month | 93 | 5.20 ± 2.31 | <0.001 | 93 | 4.25 ± 2.09 | <0.001 | |
| 3 Months | 91 | 4.93 ± 2.12 | <0.001 | 94 | 4.32 ± 1.90 | <0.001 | |
IS, intensity score; PS, proportion score; SD, standard deviation; TS, total score.
On the other hand, there were significant decreases for PS, IS, and TS for ER in sections stored in the humidity chamber. This remarkable decrease was detected at 1 week for IS and TS and 1 month for PS. The PS, IS, and TS scores for ER after 3 months in the humidity chamber were reduced by 24.1%, 43.4%, and 30.9%, respectively, in comparison to fresh-cut tissue sections.
Loss of PR antigenicity started earlier than ER (Fig. 1C, D), and there was a significant reduction in all three scores (PS, IS, and TS) for PR in all experimentally stored breast cancer tissues (Table 2). The PS, IS, and TS scores for PR were 91.5%, 80.1%, and 87.7% of the fresh-cut scores, respectively, after 3 months in the dry chamber. Meanwhile, these scores for PR after 3 months in the humidity chamber were 18%, 34.4%, and 23.4% respective reductions of the fresh-cut scores. Overall, the antigenicity of ER and PR was better preserved in dry chamber conditions in comparison to the humidity chamber (Fig. 1C, D).
Using the current ASCO/CAP clinical practice guidelines, the positive rates for ER and PR expression in fresh-cut breast cancer tissues were 86.2% and 83.9%, respectively. Fortunately, these positive rates were minimally changed during 3 months in either dry or humid conditions (Supplementary Table S2).
HER-2 expression
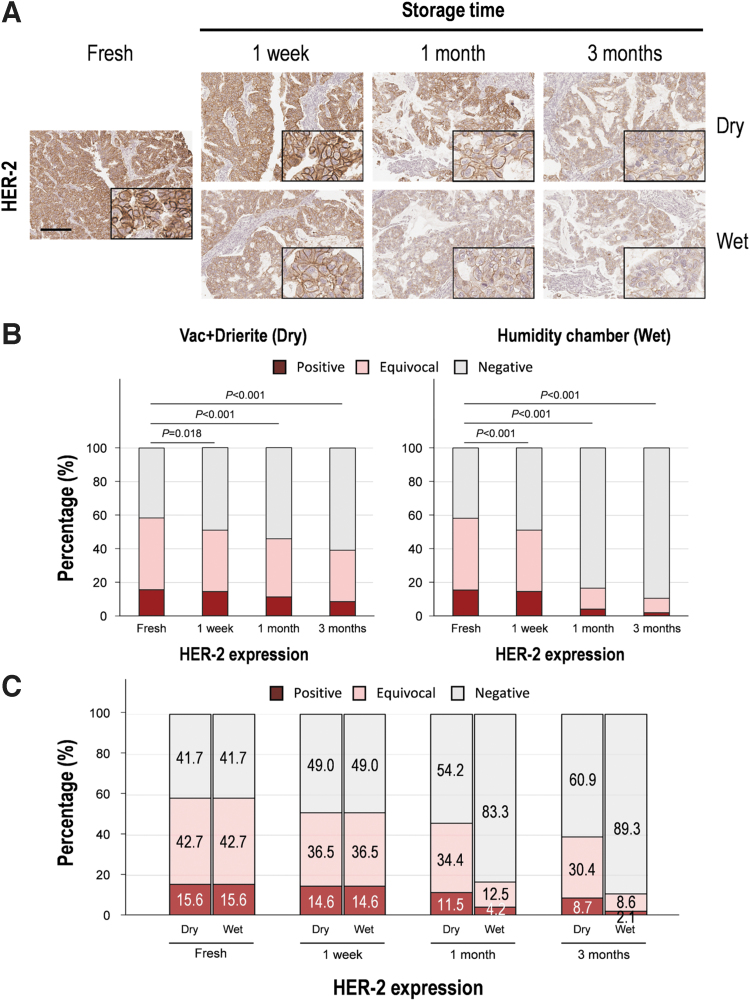
The membranous staining of HER-2 was well maintained for up to 1 week in both dry and wet conditions (Fig. 2). To objectively evaluate the change in antigenicity, we measured the area and intensity of HER-2 staining using digital image analysis. The stained area and intensity were maintained up to 1 week in dry condition, but the wet condition showed a significant decrease in stained area and intensity after 1 week (Table 3). The HER-2-stained area of tissue sections stored in dry conditions was relatively stable for up to 1 month (12.6% reduction compared to the fresh section), whereas the HER-2-stained area in humid conditions declined significantly after 1 week of storage (28.9% reduction, p < 0.001) compared to fresh tissue sections. Similarly, we confirmed that the intensity of HER-2 staining in stored tissue was more stable in dry conditions than in humid conditions (Table 3).
FIG. 2.
Evaluation of HER-2 immunochemical staining according to storage time in both dry and wet conditions. (A) Representative images of HER-2 immunohistochemical staining in human breast tissue. High magnification images are shown in the inset. Scale bar, 100 μm. (B) Quantitative analysis of HER-2 immunohistochemical staining. The data are presented as mean ± SD. (C) Change of HER-2-positive rate according to storage time in both dry and wet conditions. HER-2, human epidermal growth factor receptor 2.
Table 3.
Area and Intensity of Human Epidermal Growth Factor Receptor 2 Expression in Breast Cancer Tissues Stored Experimentally
| Dry |
Wet |
|||||
|---|---|---|---|---|---|---|
| n | Mean ± SD | p | n | Mean ± SD | p | |
| Area | ||||||
| Fresh | 96 | 26.85 ± 41.06 | 95 | 27.05 ± 41.24 | ||
| 1 Week | 96 | 26.66 ± 38.97 | 0.902 | 95 | 19.24 ± 30.56 | <0.001 |
| 1 Month | 95 | 23.46 ± 35.60 | 0.047 | 96 | 17.30 ± 28.54 | <0.001 |
| 3 Months | 92 | 18.94 ± 33.70 | 0.004 | 93 | 8.71 ± 19.61 | <0.001 |
| Intensity | ||||||
| Fresh | 96 | 1.11 ± 0.22 | 95 | 1.11 ± 0.22 | ||
| 1 Week | 96 | 1.09 ± 0.23 | 0.064 | 95 | 1.01 ± 0.20 | <0.001 |
| 1 Month | 95 | 1.08 ± 0.22 | 0.022 | 96 | 0.97 ± 0.16 | <0.001 |
| 3 Months | 92 | 1.03 ± 0.21 | <0.001 | 93 | 0.90 ± 0.18 | <0.001 |
Based on the current ASCO/CAP clinical practice guidelines, positive, equivocal, and negative HER-2 expression values were 15.6%, 42.7%, and 41.7% in fresh sections, respectively. In dry conditions, these values changed at 1 month (positive, equivocal, and negative rates of 11.5%, 34.4%, and 54.2%) and significantly lost antigenicity at 3 months (positive, equivocal, and negative rates of 8.7%, 30.4%, and 60.9%) (Fig. 2C). In wet conditions, HER-2-positive, HER-2-equivocal, and HER-2-negative rates were 4.2%, 12.5%, and 83.3%, respectively, at 1 month, whereas they were 2.1%, 8.6%, and 89.3% at 3 months, and the results showed a remarkable decrease in HER-2 positivity (p < 0.001; Fig. 2C).
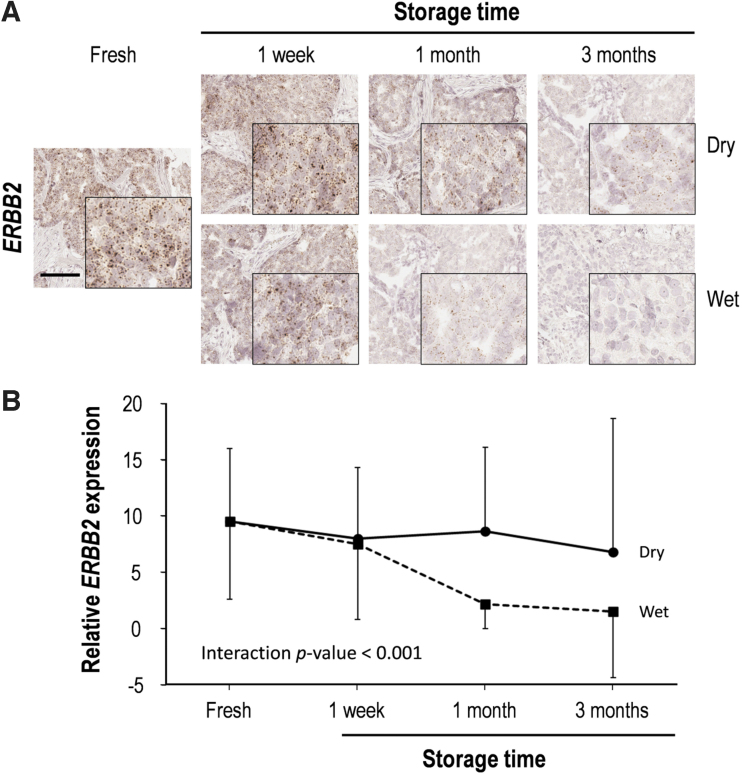
To appreciate the impacts of storage period and humidity on RNA integrity, we evaluated RNA quantity in breast cancer tissue sections, stored in dry or humid chambers for 3 months, using manual RNAScope ISH with ERBB2 probes (Fig. 3). HER-2 RNA expression showed a modest decrease after 1 week storage in both conditions (Fig. 3; Supplementary Table S3). In comparison to fresh-cut slides, HER-2 RNA expression gradually decreased in dry conditions as the storage length increased, with 15.3%, 16.2%, and 26.3% decrements at 1 week, 1 month, and 3 months. In contrast, there was a sharp decrease in HER-2 RNA expression after 1 month in humid conditions (1 week, 1 month, and 3 months; respective 21.5%, 76.2%, and 83.8% decrease in comparison to fresh-cut scores) (Supplementary Table S3).
FIG. 3.
RNA quality according to storage time in both dry and wet conditions. RNA quality was assessed by RNAScope in situ hybridization. (A) Representative RNAScope in situ hybridization images of ERBB2 in human breast tissue. High magnification images are shown in the inset. Scale bar, 100 μm. (B) Quantitative analysis of ERBB2 expression. Data are presented as mean ± SD. ERBB2, Erb-B2 receptor tyrosine kinase 2.
E-cadherin and Ki-67 expression
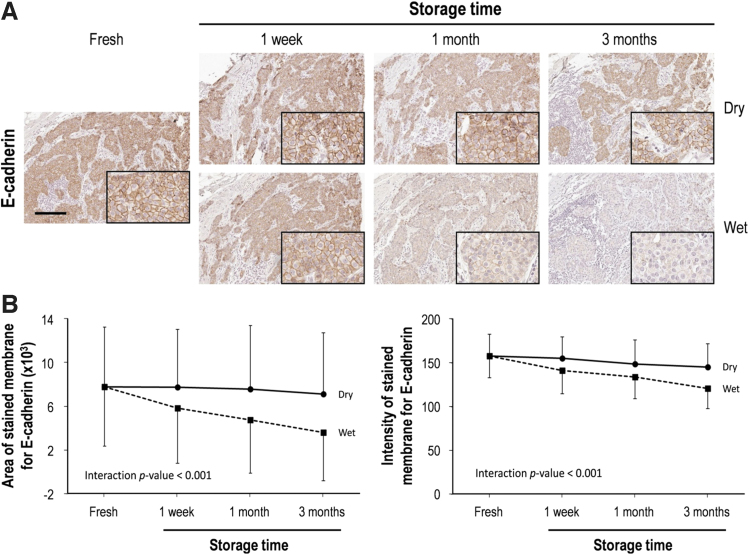
E-cadherin was stained in the cellular membrane of all cases of ductal carcinoma histology (93 of 96 cases, 96.9%) (Fig. 4A). In a detailed analysis of the stained area and intensity, the stained area of E-cadherin expression for the tissue sections stored in dry conditions was not changed significantly, while the intensity of staining changed slightly after 3 months (7.6% decrease in comparison to fresh cut) in the dry chamber (Fig. 4B).
FIG. 4.
E-cadherin immunohistochemical staining of human breast cancer tissue for slides stored over time and in dry and wet storage conditions. (A) Representative immunohistochemical images of E-cadherin before and after storage in both dry and wet conditions. High magnification images are shown in the inset. Scale bar, 100 μm. (B) Quantitative analysis of the immunohistochemical stained area and intensity of E-cadherin according to storage conditions. Data are presented as mean ± SD.
In contrast, the area and intensity of E-cadherin staining for the tissue sections stored in the humidity chamber decreased significantly as storage length increased. In comparison to fresh-cut sections, the stained area of E-cadherin was decreased by 25.3%, 38.9%, and 53.5% in the tissue sections stored in the humid conditions for 1 week, 1 month, and 3 months, respectively. The staining intensity for E-cadherin was also significantly reduced after 3 months in humid conditions (23.4% decrease in comparison to fresh cut) (Fig. 4B).
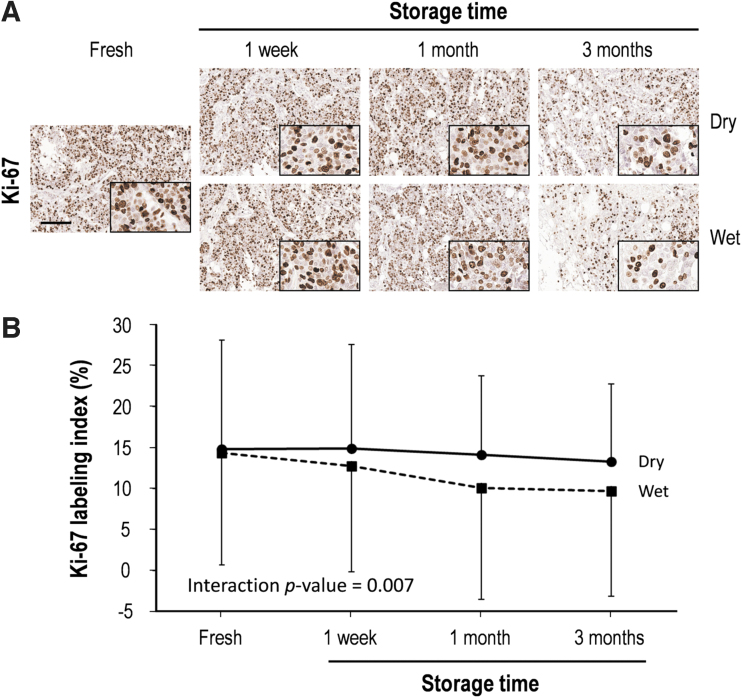
The Ki-67 immunostaining index showed a mean of 14.7% in fresh-cut breast cancer tissue sections, but this decreased gradually under both dry and wet conditions, with an especially prominent reduction under wet conditions (Fig. 5). In detail, the Ki-67 labeling index of tissue sections stored for 3 months (9% decrease in comparison to fresh cut) in the dry chamber was well maintained, while that after 1 week of storage in humid conditions started to decrease, with particular decrements after 3 months (31.9% decrease in comparison to fresh cut) (Supplementary Table S4).
FIG. 5.
Ki-67 labeling index of human breast cancer tissue for slides stored over time and in dry and wet storage conditions. (A) Representative immunohistochemical images of Ki-67 before and after storage in dry or wet conditions. High magnification images are shown in the inset. Scale bar, 100 μm. (B) Quantitative analysis of the Ki-67 labeling index according to storage conditions. Ki-67 labeling index was defined as the percentage of Ki-67 antigen-positive cells. Data are presented as mean ± SD.
Discussion
In this study, we used breast cancer TMA sections to compare changes in protein and RNA quality under dry and humid storage conditions. As expected, the intensity of IHC staining significantly decreased for all tested markers (ER, PR, HER-2, E-cadherin, and Ki-67) in TMA sections stored long term in humid conditions versus fresh tissue. Immunoreactivity was successfully detected with minimal reduction in staining intensity for all five markers in breast cancer TMA sections stored in dry conditions for 3 months. On the other hand, the RNA integrity of HER-2 was significantly reduced in both dry and humid conditions after 1 week of storage.
We have published previous data suggesting that endogenous and exogenous water play an important role in the loss of antigenicity in mouse FFPE tissues through hydrolysis. These results confirmed that RNA is substantially more sensitive than protein to humid storage conditions. Furthermore, we identified individual analyte specificity by IHC. The staining intensity decreased gradually in all markers as storage time increased, and the dry condition was more effective in preserving antigenicity than the humid condition.
Many factors, including storage time, temperature, humidity, photo-oxidation, exposure to sunlight, and drying, can affect IHC staining quality.12,13,29–33 Among these, we focused on storage duration and humidity, because clinical pathology laboratories routinely store unstained slides at room temperature and sometimes expose them to wet conditions. Several previous studies have reported refrigerator storage as a preventer of antigen degradation,6,29,34 but storing slides at 4°C might be suboptimal due to hydrolysis from exogenous water.12 Omilian et al reported recently that storage in a desiccator at room temperature was the most effective method to maintain antigenicity.9 Our study corroborated this finding, with the immunoreactivity of five markers (ER, PR, HER-2, E-cadherin, and Ki-67) being significantly protected in tissue sections stored in dry conditions in comparison to those in humid conditions. These results support our hypothesis that antigen degradation in FFPE tissue sections is due to hydrolysis during storage.
Because loss of ER and PR expression in tissue sections stored in various storage conditions has been reported, we analyzed the Allred scores and positivity for these two markers. In this study, a significant change of total Allred scores for ER was observed after 3 months of storage (mean ± SD; 6.11 ± 2.23) in dry conditions and after 1 week of storage (mean ± SD; 5.99 ± 2.40) in humid conditions. Total Allred scores for PR significantly changed after 1 week of storage in both conditions (Table 2). However, the positive percentage remained stable for up to 3 months in both storage conditions (Supplementary Table 1 ). This discrepancy between Allred score and positivity for ER and PR might be the consequence of a low threshold for positivity in the semiquantitative Allred score system and high positivity rates for ER (86.2%) and PR (84.0%) on fresh-cut slides.
A prior study reported that the ER-positive rate in breast TMA sections stored for 6 months at room temperature was similar to that of fresh sections, whereas the PR-positive rate was moderately disparate between the two conditions (stored vs. fresh tissue sections; 56.3 vs. 64.1).24 In addition, Tabbara et al reported that ER and PR immunoreactivity were well preserved in air-dried ThinPreps stored for 56 days at room temperature.35 These results suggest that ER and PR status can be evaluated in tissue sections stored at room temperature by IHC staining. Qualitative ER and PR analysis can be performed with specimens stored under dry conditions for a relatively long time. This will provide flexibility in performing ER and PR analysis for retrospective studies.
HER-2 is an invaluable indicator in the management of patients with breast cancer because it is the sole predictive marker for HER-2-targeted therapy. Thus, precise assessment of HER-2 status is an essential step for treatment of patients with breast cancer. However, long-term storage at high temperatures negatively impacts HER-2 antigen stability.6,29,33 Furthermore, cold storage of tissue sections also results in significant antigenicity loss, despite some benefits in stability.32,36 Our study shows that HER-2 staining is more stable in dry conditions than in humid conditions, which was confirmed using the ASCO/CAP guideline for HER-2 status evaluation. However, positive (15.6%), equivocal (42.7%), and negative (41.7%) HER-2 expression of fresh tissue sections remarkably changed in dry (11.5%, 34.4%, and 54.2%, respectively) as well as in humid (4.2%, 12.5%, and 83.3%) conditions even at 1 month (Fig. 2).
This significant decrease of positive or equivocal cases is clinically important because the candidates for HER-2-targeted therapy cannot be selected properly. Thus, we suggest that, for HER-2 IHC, it is necessary to establish more stringent storage guidelines, including duration and humidity. Equivocal cases of HER-2 (IHC 2+) require further assessment by fluorescence in situ hybridization. This study confirmed our previous findings that RNA is more susceptible to degradation during tissue processing and storage than is protein, suggesting that RNA-based assays can be more negatively affected in terms of data stability and reproducibility than protein-based methods in basic and clinical research.1,37
The negative impact of water on human breast tissue section storage has not yet been acknowledged. We revealed previously that protein hydrolysis caused by inadequate dehydration during tissue processing is responsible for reduced immunoreactivity over time.12 These findings confirm that exogenous water in stored sections plays a critical role in antigen instability. Loss of IHC staining intensity was accelerated in humid conditions compared to dry conditions for all five markers. DiVito et al reported that the antigenicity of cytokeratin, ER, and Ki-67 in stored tissue sections might be protected by combined nitrogen gas storage and paraffin coating storage because oxidation is blocked.13
However, paraffin dipping is impractical because it is too difficult to completely remove the paraffin from the slide, and it reduces staining intensity and positivity. Nitrogen gas storage may require specialized equipment as well. Interestingly, van den Broek and van den Vijver reported greater antigen degradation in membrane proteins than in cytoplasmic and nuclear proteins.32 Although loss of HER-2 membrane staining was detected in relatively short storage times (1 week) in dry conditions, E-cadherin membrane staining was stable up to 3 months in dry storage. These results suggest that the quality of IHC staining mainly depends on the characteristics of the target molecule and the IHC protocol, including antibody, detection system, and scoring method.
There are several limitations in this study. First, we did not consider storage temperature or special storage methods, such as paraffin dipping. This is because our initial focus was on storage of unstained slides in the clinical pathology laboratory, excluding complex methods of slide production. Second, because this study was performed using TMA blocks, the results of whole tumor sections were not included. In particular, the intratumoral heterogeneity of HER-2 IHC staining was reported in gastric cancer, so further research on whole tumor sections is needed.38,39 Third, we stained stored and fresh sections simultaneously using an in-house standard IHC protocol, and digital image analysis with established algorithms was used to analyze the results. Thus, IHC staining results might differ in other laboratories depending on IHC protocol and scoring system.
Fourth, this study did not include detailed clinical or survival data on the patients, so we could not verify the clinical impact of antigenicity loss, such as choice of treatment. Finally, validation of HER-2 gene amplification was not performed in this study. In conclusion, we showed that TMA slides that were vacuum sealed and stored under dry conditions slowly lost antigenicity, but the degree of degradation differed depending on the antigen. Furthermore, RNA degradation is more susceptible compared with proteins even in short-term storage. Notably, the elimination of humidity in the storage method is beneficial in the protection of protein and RNA quantity during section storage. This storage methodology offers the advantage of delaying the loss of immunogenicity over time and can be easily incorporated into clinical practices.
Supplementary Material
Authors' Contributions
K.K. and J.Y.C. conceived the study and devised the experimental design. K.K., K.Y., C.P., and J.Y.C. performed the experiments. K.K., M.Y.L., J.W.K., J.Y.C., and S.M.H. contributed to interpretation of the results. K.K. and J.Y.C. wrote the article in consultation with S.M.H. All authors provided critical feedback, contributed to the research and writing, and reviewed the final article.
Author Disclosure Statement
The authors declare that there is no conflict of interest. The authors alone are responsible for the content and writing of the article.
Funding Information
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Supplementary Material
References
- 1. Chung JY, Braunschweig T, Williams R, et al. Factors in tissue handling and processing that impact RNA obtained from formalin-fixed, paraffin-embedded tissue. J Histochem Cytochem 2008;56(11):1033–1042; doi: 10.1369/jhc.2008.951863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hewitt SM, Lewis FA, Cao Y, et al. Tissue handling and specimen preparation in surgical pathology: Issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med 2008;132(12):1929–1935; doi: 10.1043/1543-2165-132.12.1929 [DOI] [PubMed] [Google Scholar]
- 3. Goldstein NS, Hewitt SM, Taylor CR, et al. Recommendations for improved standardization of immunohistochemistry. Appl Immunohistochem Mol Morphol 2007;15(2):124–133; doi: 10.1097/PAI.0b013e31804c7283 [DOI] [PubMed] [Google Scholar]
- 4. Mayr D, Heim S, Werhan C, et al. Comprehensive immunohistochemical analysis of Her-2/neu oncoprotein overexpression in breast cancer: HercepTest (Dako) for manual testing and Her-2/neuTest 4B5 (Ventana) for Ventana BenchMark automatic staining system with correlation to results of fluorescence in situ hybridization (FISH). Virchows Arch 2009;454(3):241–248; doi: 10.1007/s00428-009-0728-8 [DOI] [PubMed] [Google Scholar]
- 5. Economou M, Schoni L, Hammer C, et al. Proper paraffin slide storage is crucial for translational research projects involving immunohistochemistry stains. Clin Transl Med 2014;3(1):4; doi: 10.1186/2001-1326-3-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacobs TW, Prioleau JE, Stillman IE, et al. Loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. J Natl Cancer Inst 1996;88(15):1054–1059; doi: 10.1093/jnci/88.15.1054 [DOI] [PubMed] [Google Scholar]
- 7. Khoury T. Delay to formalin fixation (cold ischemia time) effect on breast cancer molecules. Am J Clin Pathol 2018;149(4):275–292; doi: 10.1093/ajcp/aqx164 [DOI] [PubMed] [Google Scholar]
- 8. Mirlacher M, Kasper M, Storz M, et al. Influence of slide aging on results of translational research studies using immunohistochemistry. Mod Pathol 2004;17(11):1414–1420; doi: 10.1038/modpathol.3800208 [DOI] [PubMed] [Google Scholar]
- 9. Omilian AR, Zirpoli GR, Cheng TD, et al. Storage conditions and immunoreactivity of breast cancer subtyping markers in tissue microarray sections. Appl Immunohistochem Mol Morphol 2020;28(4):267–273; doi: 10.1097/PAI.0000000000000756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grillo F, Pigozzi S, Ceriolo P, et al. Factors affecting immunoreactivity in long-term storage of formalin-fixed paraffin-embedded tissue sections. Histochem Cell Biol 2015;144(1):93–99; doi: 10.1007/s00418-015-1316-4 [DOI] [PubMed] [Google Scholar]
- 11. Olapade-Olaopa EO, Ogunbiyi JO, MacKay EH, et al. Further characterization of storage-related alterations in immunoreactivity of archival tissue sections and its implications for collaborative multicenter immunohistochemical studies. Appl Immunohistochem Mol Morphol 2001;9(3):261–266; doi: 10.1097/00129039-200109000-00011 [DOI] [PubMed] [Google Scholar]
- 12. Xie R, Chung JY, Ylaya K, et al. Factors influencing the degradation of archival formalin-fixed paraffin-embedded tissue sections. J Histochem Cytochem 2011;59(4):356–365; doi: 10.1369/0022155411398488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DiVito KA, Charette LA, Rimm DL, et al. Long-term preservation of antigenicity on tissue microarrays. Lab Invest 2004;84(8):1071–1078; doi: 10.1038/labinvest.3700131 [DOI] [PubMed] [Google Scholar]
- 14. Litlekalsoy J, Vatne V, Hostmark JG, et al. Immunohistochemical markers in urinary bladder carcinomas from paraffin-embedded archival tissue after storage for 5–70 years. BJU Int 2007;99(5):1013–1019; doi: 10.1111/j.1464-410X.2006.06699.x [DOI] [PubMed] [Google Scholar]
- 15. Vassilakopoulou M, Togun T, Dafni U, et al. In situ quantitative measurement of HER2mRNA predicts benefit from trastuzumab-containing chemotherapy in a cohort of metastatic breast cancer patients. PLoS One 2014;9(6):e99131; doi: 10.1371/journal.pone.0099131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bingham V, Ong CW, James J, et al. PTEN mRNA detection by chromogenic, RNA in situ technologies: A reliable alternative to PTEN immunohistochemistry. Hum Pathol 2016;47(1):95–103; doi: 10.1016/j.humpath.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 17. Yu H, Batenchuk C, Badzio A, et al. PD-L1 expression by two complementary diagnostic assays and mRNA in situ hybridization in small cell lung cancer. J Thorac Oncol 2017;12(1):110–120; doi: 10.1016/j.jtho.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cronin M, Pho M, Dutta D, et al. Measurement of gene expression in archival paraffin-embedded tissues: Development and performance of a 92-gene reverse transcriptase-polymerase chain reaction assay. Am J Pathol 2004;164(1):35–42; doi: 10.1016/S0002-9440(10)63093-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muller BM, Kronenwett R, Hennig G, et al. Quantitative determination of estrogen receptor, progesterone receptor, and HER2 mRNA in formalin-fixed paraffin-embedded tissue—A new option for predictive biomarker assessment in breast cancer. Diagn Mol Pathol 2011;20(1):1–10; doi: 10.1097/PDM.0b013e3181e3630c [DOI] [PubMed] [Google Scholar]
- 20. von Ahlfen S, Missel A, Bendrat K, et al. Determinants of RNA quality from FFPE samples. PLoS One 2007;2(12):e1261; doi: 10.1371/journal.pone.0001261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gandara-Cortes M, Vazquez-Boquete A, Fernandez-Rodriguez B, et al. Breast cancer subtype discrimination using standardized 4-IHC and digital image analysis. Virchows Arch 2018;472(2):195–203; doi: 10.1007/s00428-017-2194-z [DOI] [PubMed] [Google Scholar]
- 22. Rakha EA, Patel A, Powe DG, et al. Clinical and biological significance of E-cadherin protein expression in invasive lobular carcinoma of the breast. Am J Surg Pathol 2010;34(10):1472–1479; doi: 10.1097/PAS.0b013e3181f01916 [DOI] [PubMed] [Google Scholar]
- 23. Petrelli F, Viale G, Cabiddu M, et al. Prognostic value of different cut-off levels of Ki-67 in breast cancer: A systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat 2015;153(3):477–491; doi: 10.1007/s10549-015-3559-0 [DOI] [PubMed] [Google Scholar]
- 24. Fergenbaum JH, Garcia-Closas M, Hewitt SM, et al. Loss of antigenicity in stored sections of breast cancer tissue microarrays. Cancer Epidemiol Biomarkers Prev 2004;13(4):667–672; doi: 10.1158/1055-9965.667.13.4 [DOI] [PubMed] [Google Scholar]
- 25. Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol 2020;38(12):1346–1366; doi: 10.1200/JCO.19.02309 [DOI] [PubMed] [Google Scholar]
- 26. Shin S, Kim K, Kim HR, et al. Deubiquitylation and stabilization of Notch1 intracellular domain by ubiquitin-specific protease 8 enhance tumorigenesis in breast cancer. Cell Death Differ 2020;27(4):1341–1354; doi: 10.1038/s41418-019-0419-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Allred DC, Harvey JM, Berardo M, et al. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 1998;11(2):155–168. [PubMed] [Google Scholar]
- 28. Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 2018;36(20):2105–2122; doi: 10.1200/JCO.2018.77.8738 [DOI] [PubMed] [Google Scholar]
- 29. Bertheau P, Cazals-Hatem D, Meignin V, et al. Variability of immunohistochemical reactivity on stored paraffin slides. J Clin Pathol 1998;51(5):370–374; doi: 10.1136/jcp.51.5.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blind C, Koepenik A, Pacyna-Gengelbach M, et al. Antigenicity testing by immunohistochemistry after tissue oxidation. J Clin Pathol 2008;61(1):79–83; doi: 10.1136/jcp.2007.047340 [DOI] [PubMed] [Google Scholar]
- 31. Ramos-Vara JA, Webster JD, DuSold D, et al. Immunohistochemical evaluation of the effects of paraffin section storage on biomarker stability. Vet Pathol 2014;51(1):102–109; doi: 10.1177/0300985813476067 [DOI] [PubMed] [Google Scholar]
- 32. van den Broek LJ, van de Vijver MJ. Assessment of problems in diagnostic and research immunohistochemistry associated with epitope instability in stored paraffin sections. Appl Immunohistochem Mol Morphol 2000;8(4):316–321. [PubMed] [Google Scholar]
- 33. Wester K, Wahlund E, Sundstrom C, et al. Paraffin section storage and immunohistochemistry. Effects of time, temperature, fixation, and retrieval protocol with emphasis on p53 protein and MIB1 antigen. Appl Immunohistochem Mol Morphol 2000;8(1):61–70. [PubMed] [Google Scholar]
- 34. Gelb AB, Freeman VA, Astrow SH. Evaluation of methods for preserving PTEN antigenicity in stored paraffin sections. Appl Immunohistochem Mol Morphol 2011;19(6):569–573; doi: 10.1097/PAI.0b013e318217a3d3 [DOI] [PubMed] [Google Scholar]
- 35. Tabbara SO, Sidawy MK, Frost AR, et al. The stability of estrogen and progesterone receptor expression on breast carcinoma cells stored as PreservCyt suspensions and as ThinPrep slides. Cancer 1998;84(6):355–360; doi: [DOI] [PubMed] [Google Scholar]
- 36. Grabau D A NO, Hansen S, Nielsen MM, et al. Influence of storage temperature and high-temperature antigen retrieval buffers on results of immunohistochemical staining in sections stored for long periods. Appl Immunohistochem 1998;6:209–213; doi: 10.1097/00022744-199812000-00006 [DOI] [Google Scholar]
- 37. Chung JY, Song JS, Ylaya K, et al. Histomorphological and molecular assessments of the fixation times comparing formalin and ethanol-based fixatives. J Histochem Cytochem 2018;66(2):121–135; doi: 10.1369/0022155417741467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaito A, Kuwata T, Tokunaga M, et al. HER2 heterogeneity is a poor prognosticator for HER2-positive gastric cancer. World J Clin Cases 2019;7(15):1964–1977; doi: 10.12998/wjcc.v7.i15.1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wakatsuki T, Yamamoto N, Sano T, et al. Clinical impact of intratumoral HER2 heterogeneity on trastuzumab efficacy in patients with HER2-positive gastric cancer. J Gastroenterol 2018;53(11):1186–1195; doi: 10.1007/s00535-018-1464-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.