Abstract
The upper Housatonic River and Woods Pond (Lenox, Mass.), a shallow impoundment on the river, are contaminated with polychlorinated biphenyls (PCBs), the residue of partially dechlorinated Aroclor 1260. Certain PCB congeners have the ability to activate or “prime” anaerobic microorganisms in Woods Pond sediment to reductively dehalogenate the Aroclor 1260 residue. We proposed that brominated biphenyls might have the same effect and tested the priming activities of 14 mono-, di-, and tribrominated biphenyls (350 μM) in anaerobic microcosms of sediment from Woods Pond. All of the brominated biphenyls were completely dehalogenated to biphenyl, and 13 of them primed PCB dechlorination. Measured in terms of chlorine removal and decrease in the proportion of hexa- through nonachlorobiphenyls, the microbial PCB dechlorination primed by several brominated biphenyls was nearly twice as effective as that primed by chlorinated biphenyls. Congeners containing a meta bromine primed Dechlorination Process N (flanked meta dechlorination), and congeners containing an unflanked para bromine primed Dechlorination Process P (flanked para dechlorination). Two ortho-substituted congeners, 2-bromobiphenyl and 2,6-dibromobiphenyl (2-BB and 26-BB), also primed Process N dechlorination. The most effective primers were 26-BB, 245-BB, 25-3-BB, and 25-4-BB. The microbial dechlorination primed by 26-BB converted ∼75% of the hexa- through nonachlorobiphenyls to tri- and tetrachlorobiphenyls in 100 days and removed ∼75% of the PCBs that are most persistent in humans. These results represent a major step toward identifying an effective method for accelerating PCB dechlorination in situ. The challenge now is to identify naturally occurring compounds that are safe and effective primers.
Polychlorinated biphenyls (PCBs) are pollutants that persist in river, lake, and harbor sediments wherever they were manufactured or used. Concerns about their bioaccumulation and potential toxicity to humans and wildlife have spurred interest in novel approaches for remediation. Industrial PCB mixtures, such as Aroclors, provide a daunting challenge for microbial dechlorination and degradation, because they each contain 60 to 90 different molecular species known as congeners. Each congener consists of a biphenyl skeleton substituted with 1 to 10 chlorines (Fig. 1). Fortunately, microorganisms in many PCB-contaminated sediments already have the ability to dechlorinate many of the PCB congeners (10, 12–14, 22, 24, 29, 36; see reference 3 for a review). Microbial dechlorination can play an important role in natural restoration because it decreases the toxicity of PCBs and increases their degradability (5, 25, 26). Accordingly, one goal of our research is to discover innovative approaches for enhancing or accelerating microbial PCB dehalogenation in situ.
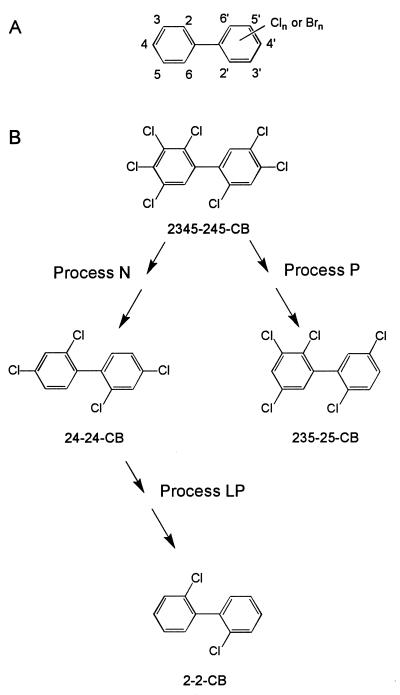
FIG. 1.
Structure of halogenated biphenyls, showing the numbering scheme (A) and the dechlorination of a major heptachlorobiphenyl by Process N, Processes N plus LP, and Process P (B). Each chlorinated or brominated biphenyl congener can have from 1 to 10 halogens (chlorines or bromines) at the positions shown in panel A. The halogens at positions 2 and 6 on either ring are designated ortho, those at carbons 3 and 5 are designated meta, and those at carbon 4 are designated para. Panel B shows one of the major components of Aroclor 1260 and the terminal dechlorination products produced from this congener by Process N, Processes N plus LP, and Process P. The most extensive chlorine removal results from sequential dechlorination by Processes N and LP (27).
The upper Housatonic River, including Woods Pond, is contaminated with partially dechlorinated Aroclor 1260 (2, 4), and previous studies have indicated that its sediments harbor several discrete populations of PCB-dechlorinating microorganisms (1, 8, 32, 37). Despite rigorous attempts, none of these microorganisms has been isolated or obtained in a sediment-free culture (38). Hence, we are limited to describing these populations in terms of their PCB dechlorination activity. Three major PCB dechlorination activities, known as Dechlorination Processes N, P, and LP, have been observed in Woods Pond sediment (1, 8, 32, 37). (A microbial dechlorination process is a set or series of dechlorination reactions that determines the substrate range, the specific chlorines targeted, and the sequence of dechlorination [3].) Several lines of evidence indicate that three discrete microbial populations are responsible for Dechlorination Processes N, P, and LP, as discussed below.
(i) The dehalogenation specificities of these three dechlorination processes are distinctly different. Process N primarily removes flanked meta chlorines from chlorophenyl groups with the following substitution patterns: 2,3,4- (234-), 236-, 245-, 2345-, 2346-, and 23456- (3) (chlorines that are removed are underlined). Process P removes flanked para chlorines from 234-, 245-, and 2345-chlorophenyl groups (1), and Process LP primarily removes the unflanked para chlorines from 24- and 246-chlorophenyl groups (8). Figure 1B shows a heptachlorobiphenyl that is a major component of Aroclor 1260 and the terminal dechlorination products produced from it by Processes N, P, and the combination of N and LP.
(ii) Dechlorination Processes N, P, and LP have different temperature ranges. Process N occurs at 8 to 30°C, Process P occurs at 12 to 34°C, and Process LP occurs at 18 to 30°C (37).
(iii) These three dechlorination processes can be activated separately by specific primers. Process N is primed by 236-CB, 2346-CB, and 23456-CB (see below for an explanation of chemical abbreviations) (32, 37); Process P is primed by 25-34-CB, 24-34-CB, and 245-CB (1, 32); and Process LP is primed by 246-CB (8, 37).
We hypothesized that PCB primers stimulate and support the growth of PCB dechlorinators, perhaps by serving as electron acceptors. This hypothesis is strongly supported by the recent finding that adding a high concentration of PCBs to microcosms of anaerobic sediment resulted in a nearly 200-fold transient increase in the number of PCB dechlorinators concurrent with the dechlorination of the PCBs (21).
Morris and colleagues demonstrated that microorganisms from two PCB-contaminated sediments with no history of brominated biphenyl contamination could partially dehalogenate hexabrominated biphenyls, whereas microorganisms from a sediment that was not contaminated with halogenated biphenyls could not (23). Based on their findings, these authors proposed that PCB-dechlorinating microorganisms might be able to dehalogenate brominated biphenyls. Likewise, we recently found that the anaerobic microorganisms in Woods Pond sediment can dehalogenate many mono-, di-, and tribrominated biphenyls even though the upper Housatonic River has no history of contamination with brominated biphenyls (6). Our data suggested that PCB-dehalogenating microorganisms in Woods Pond might exhibit relaxed specificity for brominated biphenyl dehalogenation. Since many of the brominated biphenyls are completely dehalogenated to biphenyl in a relatively short period of time (6), we reasoned that they might be good candidates for priming PCB dechlorination.
In this paper we report the results obtained when various mono-, di-, and tribrominated biphenyls were tested for the ability to prime microbial dechlorination of the partially dechlorinated Aroclor 1260 in Woods Pond sediment. Most of the brominated biphenyls primed Process N or Process P dechlorination. Our results demonstrate that compounds other than PCBs can activate extensive PCB dechlorination in sediment.
(A preliminary report of these findings was presented at a conference on microbial dehalogenation held in 1992 [7].)
MATERIALS AND METHODS
Chemicals.
In this paper each PCB and brominated biphenyl congener is identified by listing the substituted position(s) on each ring, separated by a hyphen, and followed by the designation -CB (chlorinated biphenyl) or -BB (brominated biphenyl) (Fig. 1). For example, 2,6-dibromobiphenyl is abbreviated 26-BB, and 2,3′,4′,5-tetrachlorobiphenyl is abbreviated 25-34-CB. All commercially available mono-, di-, and tribrominated biphenyls (97 to 99% pure as determined by gas chromatography [GC] with a flame ionization detector [FID]) were purchased from Accu-Standard (New Haven, Conn.) or from Ultra Scientific (North Kingstown, R.I.). Chemists at GE Corporate Research and Development synthesized highly purified 26-BB (99.93% pure as determined by GC-FID) for use in our quantitative experiments. l-Malic acid (cell culture reagent quality; catalog no. M-7387) was purchased from Sigma Chemical Co. (St. Louis, Mo.), and the pH was adjusted to 7 with sodium hydroxide.
Slurry preparation and incubation.
Multiple core samples (depth, 45 cm) of sediment were collected from the west side of Woods Pond (Lenox, Mass.) (2), a shallow impoundment on the Housatonic River. The core samples were pooled in glass jars, filled to the top with site water, and stored at 4°C until they were used. On a dry weight basis, each gram of sediment contained 30 μg of partially dechlorinated Aroclor 1260 and approximately 7,000 μg of weathered hydrocarbon oil. Slurries were prepared under an atmosphere of 95 to 97% nitrogen and 3 to 5% hydrogen in an anaerobic chamber by mixing wet sediment (2 volumes) with glass-distilled water (3 volumes). Bromobiphenyl congeners were added from concentrated stock solutions prepared in acetone. The final concentration of acetone was 0.5% (vol/vol). Unless indicated otherwise, the final concentration of each brominated biphenyl was 350 μmol per liter. This concentration is well above the aqueous solubility of brominated biphenyls; hence, these compounds likely partition into the organic material in the sediment, including the excess oil. Therefore, when referring to halogenated biphenyls, we use the term micromolar (μM) to mean micromoles per liter of slurry. In most cases, disodium malate was also added to a final concentration of 10 mM because previous experiments showed that malate accelerated dehalogenation (6). No other nutrients were added. The serum bottles were crimp sealed with Teflon-lined butyl rubber septa. Sterile controls were prepared by pasteurization (75°C, 20 min), followed by incubation (23 to 25°C, 24 h) and autoclaving (121°C, 3 h). Live controls were incubated with malate and acetone at final concentrations of 10 mM and 0.5%, respectively. Experimental samples and controls were incubated in the dark at 23 to 25°C. All live incubations became methanogenic within 1 to 2 weeks. The quantitative data shown for 26-BB (see Fig. 2 through 4) were derived from triplicate samples. All other congeners were tested in duplicate.
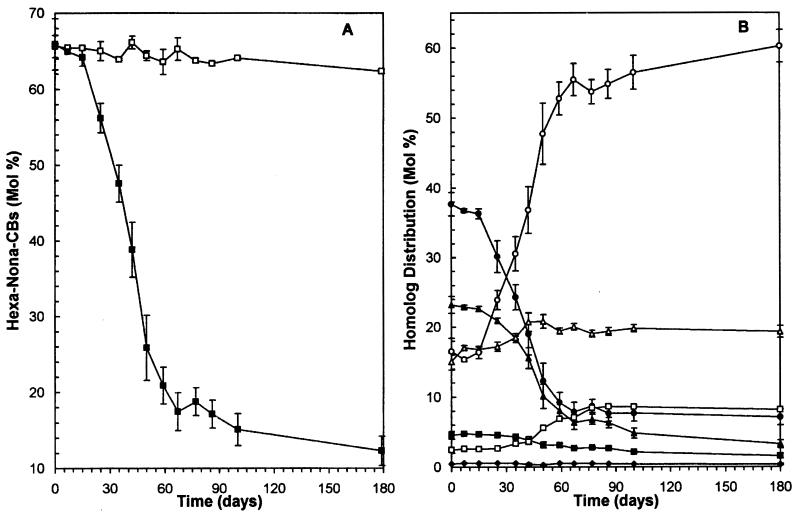
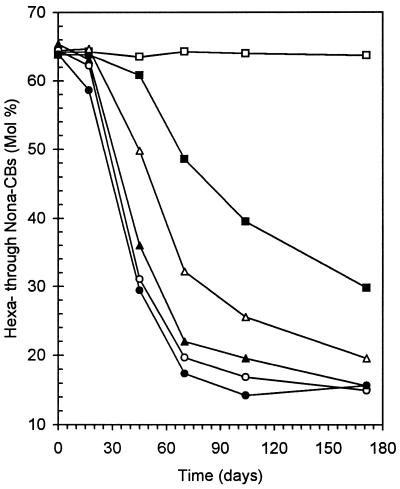
FIG. 2.
Time course of dechlorination of Aroclor 1260 primed by 26-BB (450 μM) in Woods Pond sediment. (A) Change in the total hexa- through nonachlorobiphenyls (Hexa-Nona-CBs) as a function of time. Symbols: □, live controls; ▪, 26-BB-primed samples. (B) Change in PCB homolog distribution in 26-BB-primed samples as a function of time. Symbols: ⧫, nonachlorobiphenyls; ▪, octachlorobiphenyls; ▴, heptachlorobiphenyls; •, hexachlorobiphenyls; ▵, pentachlorobiphenyls; ○, tetrachlorobiphenyls; □, trichlorobiphenyls. The data shown are averages for triplicate samples ± standard deviations (shown as error bars); if no bar is evident, the deviation was smaller than the size of the symbol.
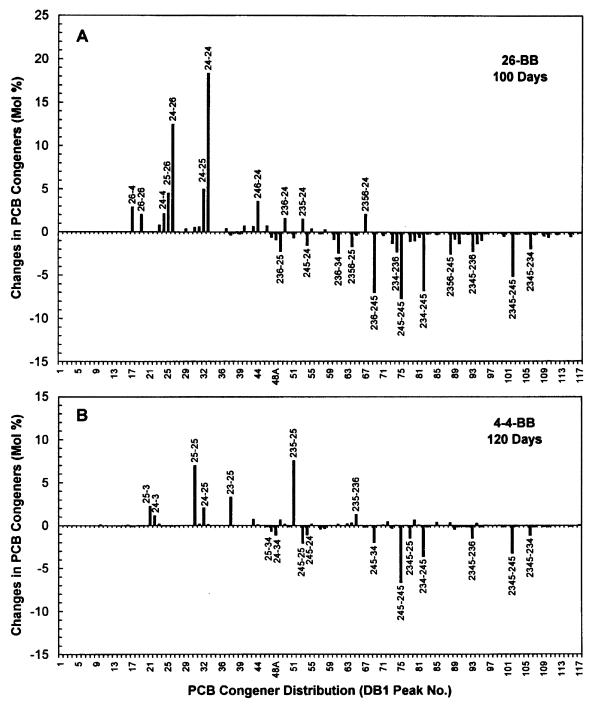
FIG. 4.
Absolute differences in the PCB congener distributions of the Aroclor 1260 residues (controls minus experimental samples) as a result of priming with 26-BB (450 μM) (A) and priming with 4-4-BB (350 μM) (B). The PCB congener designations indicate the positions of the chlorine atoms on each phenyl ring, and the hyphen represents separation of the rings. The data shown are averages for duplicate samples primed with 4-4-BB (obtained at 120 days) and for triplicate samples primed with 26-BB (obtained at 100 days). The scale is the same for both panels.
Extraction and analysis of PCBs and brominated biphenyls. (i) Extraction.
Aliquots (1 ml) of the 30-ml slurries were sampled periodically and extracted in vials with Teflon-lined foam-backed screw caps by vigorous shaking (24 h) with anhydrous diethyl ether (5 volumes). We used elemental mercury (0.25 volume) or acid-reduced copper filings (∼0.3 g) to remove sulfur (17). Quantitative comparisons of the mole percent (mol%) congener distributions for samples extracted by this simple procedure and by a rigorous Soxhlet procedure (16) demonstrated no significant differences (28); hence, we routinely used the simple one-step extraction procedure.
(ii) GC analysis.
Dehalogenation of the brominated biphenyls was monitored by GC with a mass spectrometer operated in the selected ion mode as previously described (6). Debromination products were identified as described previously (6). PCB dechlorination was monitored by high-resolution capillary GC analysis with a Ni63 electron capture detector (ECD). We used a Hewlett-Packard model 5890 GC-ECD operated in splitless mode and equipped with a DB-1 (polydimethylsiloxane) capillary column (length, 30 m; inside diameter, 0.25 mm; phase thickness, 0.25 μm; J & W Scientific, Inc., Folsom, Calif.). The injector and detector temperatures were 250 and 300°C, respectively. We used the following multistage temperature program. The initial temperature was 40°C; the temperature was increased at a rate of 20°C/min to 160°C, kept at 160°C for 3 min, increased at a rate of 2°C/min to 200°C and then at a rate of 8°C/min to 260°C, and kept at 260°C for 15 min. We used helium as the carrier gas at a column flow rate of 1.5 ml/min and nitrogen as the makeup gas at a flow rate of 6 ml/min. The septum purge flow rate was 3 ml/min, and the splitless vent flow rate was 57 ml/min. We are able to resolve 118 PCB peaks using these conditions (19).
We carried out congener-specific quantitation of the PCBs (see below) on samples primed with 26-BB and 4-4-BB. These brominated biphenyls primed Dechlorination Processes N and P, respectively. A few minor PCB components in the samples could not be quantified because they coeluted with the primers. These were GC peaks 14 (252-CB, 4-4-CB) and 15 (24-2-CB) for samples primed with 26-BB, and GC peaks 38 (23-24-CB, 236-3-CB) and 39 (26-34-CB, 236-4-CB, 234-2-CB, 25-35-CB) for samples primed with 4-4-BB. In each case, the components of the obscured peaks constitute less than 1.25 mol% of the total PCBs in the sediment (1, 32). No significant Process P or Process N dechlorination products eluted in the obscured peaks.
We monitored changes in the chromatographic profile of the PCBs for incubations with each of the other brominated biphenyls by visually comparing relative peak heights throughout the chromatograms. Then we compared the dechlorination substrates and products to those reported for the eight PCB dechlorination processes that have been described (1, 3, 32, 37). Two clear patterns of PCB dechlorination were observed, patterns P and N.
We were not able to obtain quantitative congener-specific analyses of the PCBs in samples primed by brominated biphenyls other than 26-BB and 4-4-BB because important portions of the chromatogram region where PCBs elute were obscured by most of the brominated biphenyls. The ECD responses for brominated compounds are much greater than those for PCBs, and in most samples one or more significant PCB peaks in Aroclor 1260 were obscured by coelution with the brominated biphenyls and unidentified halogenated contaminants in the brominated biphenyls. For example, the tribrominated biphenyls and their contaminants eluted in the same region of the chromatogram as tetrachlorinated biphenyls and concealed several substantial peaks in this region. Therefore, for samples primed with all brominated biphenyls other than 26-BB and 4-4-BB, we scored the extent of Process N or Process P dechlorination relative to the dechlorination primed by 26-BB or 4-4-BB by using a peak ratio procedure. For samples primed by 26-BB and 4-4-BB, we selected several reference peaks that are known to be unaffected by dechlorination (1, 32) and determined the ratios of the heights of the peaks of key PCB dechlorination substrates and products to the heights of these reference peaks. We then determined the ratios of the heights of the same peaks in the samples primed by other brominated biphenyls. These values were used to score the extent of dechlorination in each sample relative to the extent of dechlorination primed by 26-BB or 4-4-BB, as appropriate.
(iii) Quantitative congener-specific PCB analysis.
Previous GC-mass spectrometry analyses of the partially dechlorinated PCBs in Woods Pond sediment (2) and of the extensively dechlorinated PCBs resulting from priming with 26-BB (28) showed that many of the PCB congeners produced by dechlorination either are not present in Aroclors or are present only in minor quantities. Furthermore, some of these PCB dechlorination products coelute with less chlorinated PCB congeners that are more prominent in the Aroclors. Hence, it was apparent that we would not be able to accurately quantify extensively dechlorinated PCBs by using any single Aroclor or any mixture of Aroclors as a standard. We therefore developed a quantitative standard that included known amounts of Aroclor 1260 and of 43 additional PCB congeners that were previously identified as dechlorination products of Aroclor 1260 in Woods Pond sediment (2, 28). In addition, several mono-, di-, and trichlorobiphenyls were included because they are frequently observed as PCB dechlorination products in other systems (12, 14, 24). PCB congener assignments were made as previously described (19) and reported for Woods Pond sediment (1, 32). The concentrations of the individual components of Aroclor 1260 in our standard were calculated from previously determined weight percent distributions for the congeners in Aroclor 1260 (19, 28). Our customized standard permits quantitation of 84 PCB peaks, including all significant peaks detected in our samples (28).
The GC-ECD data were collected with Dionex AI-450 chromatography software (Dionex Corp., Sunnyvale, Calif.). The PCBs were quantified by means of a four-point external calibration for the customized PCB standard (219 to 3,509 μg/liter) with a quadratic fit forced through zero. We calculated the mole percent value for each individual peak, the distribution of ortho, meta, and para chlorines per biphenyl, the total number of chlorines per biphenyl, and the PCB homolog distribution. We calculated the effect of the primed dechlorination on PCB persistence in humans from half-life values in humans for the individual PCB congeners (2). The half-life values were based on Brown’s table of relative human accumulations (over 130 years) for 136 PCB congeners (11).
RESULTS
Quantitation of the PCB dechlorination primed by 26-BB.
Dehalogenation of 26-BB to 2-BB and then biphenyl began within the first week of incubation and was essentially complete within 60 days (6). Most (∼70 mol%) of the PCBs in Woods Pond sediment have six or more chlorines; hence, the mole percent fraction of hexa- through nonachlorobiphenyls provides a very effective way to assess the extent of dechlorination of the Aroclor 1260 residue. No significant PCB dechlorination occurred in either live (Fig. 2A) or autoclaved controls, but PCB congeners with six or more chlorines were rapidly dechlorinated in samples primed with 26-BB (450 μM) (Fig. 2A). The hexachlorobiphenyls decreased most rapidly, followed by the hepta- and then the octachlorobiphenyls (Fig. 2B). The pentachlorobiphenyls and the trichlorobiphenyls increased slightly. However, the primary products were tetrachlorobiphenyls, which increased to 60 mol% of the total PCBs (Fig. 2B). Most of the dechlorination occurred in the first 2 months, but dechlorination continued at a slower rate throughout the rest of the incubation period even though the 26-BB had been depleted. After 179 days, the dechlorination had decreased the fraction of PCBs having six or more chlorines by 81%.
Specificity of the PCB dechlorination primed by 26-BB.
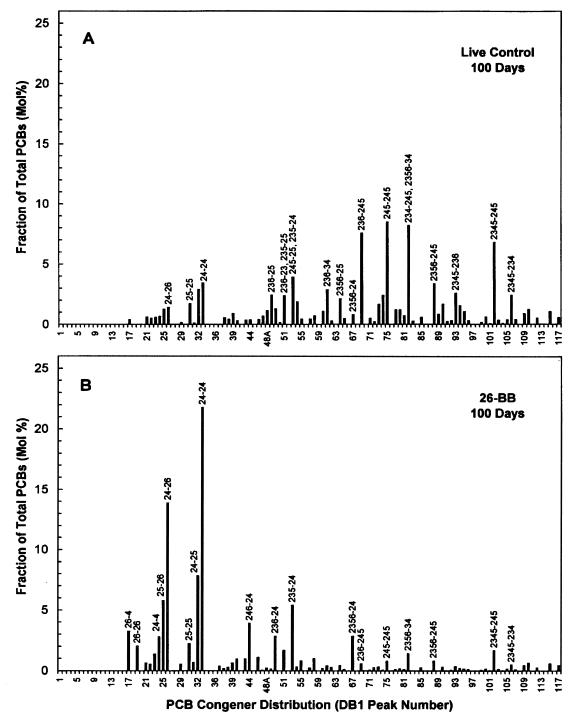
A comparison of the PCB congener distribution profiles in the live controls and in samples primed with 26-BB revealed the full impact of the dechlorination (Fig. 3). Many of the major hexa- and heptachlorobiphenyls were almost completely removed, and there were corresponding large increases in four tetrachlorobiphenyls, 24-24-CB, 24-26-CB, 24-25-CB, and 25-26-CB, and smaller increases in 26-4-CB, 24-4-CB, and 26-26-CB. There were also decreases in the amounts of certain pentachlorobiphenyls (e.g., 236-25-CB, 245-24-CB, and 236-34-CB), but several other pentachlorobiphenyl peaks increased, e.g., 246-24-CB (Fig. 4A).
FIG. 3.
PCB congener distribution of the Aroclor 1260 residue in Woods Pond sediment after 100 days in live controls (A) and in samples primed with 26-BB (450 μM) (B). The PCB congener designations indicate the positions of the chlorine atoms on each phenyl ring, and the hyphen represents separation of the rings. The data shown are averages for triplicate samples.
We had anticipated that the ortho dehalogenation of 26-BB might prime ortho dechlorination of the PCBs, but this did not occur. A comparison of the chlorophenyl substitution patterns of the dechlorination substrates and products revealed that the primed dechlorination removed primarily flanked meta chlorines, yielding ortho, para-substituted products. The dechlorination converted 234-, 245-, and 2345-chlorophenyl groups to 24-chlorophenyl groups; 2346-, and 23456-chlorophenyl groups to 246-chlorophenyl groups; and 236-chlorophenyl groups to 26-chlorophenyl groups. Four major hexa- and heptachlorobiphenyls, 245-245-CB, 245-234-CB, 2345-245-CB, and 2345-234-CB, were almost completely dechlorinated to 24-24-CB, explaining why this product was so prominent. These characteristics identify the dechlorination process primed by 26-BB as Dechlorination Process N (3, 24, 32).
PCB dechlorination primed by 26-BB: reproducibility and concentration effect.
We repeated our experiments with 26-BB many times and determined that the results were highly reproducible. We also tested the effect of 26-BB at concentrations ranging from 50 to 1,000 μM (Fig. 5). The concentration of 26-BB affected the rate and final extent of PCB dechlorination; the optimal concentration was 200 to 500 μM. Higher concentrations did not significantly increase dechlorination.
FIG. 5.
Effect of the concentration of 26-BB used as primer on the time course and extent of dechlorination. The samples contained 26-BB at the following concentrations: 0 μM (live control) (□), 50 μM (▪), 100 μM (▵), 200 μM (▴), 500 μM (○), and 1,000 μM (•). No malate was added. The data shown are means for duplicate samples. Hexa- through Nona-CBs, hexa-through nonachlorobiphenyls.
Specificity of the PCB dechlorination primed by 4-4-BB.
Dehalogenation of 4-4-BB to 4-BB and then biphenyl was first observed at 2 weeks and was essentially complete in 2 months (6). Figure 4 compares the PCB substrates and products of the dechlorination processes primed by 4-4-BB and 26-BB. This figure shows that the dechlorination primed by 4-4-BB had a narrower specificity and generated a different set of products than the dechlorination primed by 26-BB. Most of the major hexa- and heptachlorobiphenyls were substrates for the dechlorination primed by 4-4-BB, but they were not depleted as extensively as they were in the dechlorination primed by 26-BB. Many of the less prominent hexa- and heptachlorobiphenyls were not substrates for the dechlorination primed by 4-4-BB. The main products were 25-25-CB and 235-25-CB (Fig. 4B). Significant amounts of 23-25-CB, 24-25-CB, 25-3-CB, 24-3-CB, and 235-236-CB were also produced. A comparison of the substrates and products showed that this dechlorination process removed the flanked para chlorines on 34-, 234-, 245-, and 2345-chlorophenyl groups and converted them to 3-, 23-, 25-, and 235-chlorophenyl groups, respectively. This dechlorination matches Dechlorination Process P, which was previously primed in Woods Pond sediment by adding 25-34-CB (1).
Relative effectiveness of chlorinated and brominated biphenyl congeners for priming Dechlorination Processes P and N.
Dechlorination Processes P and N can also be primed with certain PCB congeners, and 25-34-CB and 23456-CB are among the most effective (1, 32). We were not able to evaluate the brominated analogs of these PCB congeners because they are not commercially available. Furthermore, most of the chlorinated analogs of the brominated biphenyl congeners that are available, including 26-CB and 4-4-CB, are not dechlorinated in this sediment and do not prime PCB dechlorination (6, 32). Therefore, in order to evaluate the relative effectiveness of chlorinated and brominated biphenyls as primers, we compared the extent of PCB dechlorination primed by 25-34-CB and 23456-CB with that primed by 4-4-BB and 26-BB. We compared the extent of dechlorination primed by 25-34-CB with the extent of dechlorination primed by 4-4-BB by examining chromatograms for various time points in several different experiments performed with each congener. The dechlorination process primed by 25-34-CB first began at ∼42 days and progressed steadily until 84 days. The data obtained at 111, 140, and 220 days reveal that the dechlorination progressed very little after 84 days. In contrast, the dechlorination primed by 4-4-BB was more extensive at 43 days than the dechlorination primed by 25-34-CB at 84, 140, or 220 days. The results were highly reproducible, both within and between experiments (for example, note the small variations in the data from duplicate incubations [1] [Tables 1 and 2]).
TABLE 1.
Effect of various halogenated biphenyl primers on chlorine removal from the partially dechlorinated Aroclor 1260 in Woods Pond sediment
| Position | Avg no. of chlorines per Biphenyl at T0 | % Decrease resulting from primed dechlorinationa
|
||||
|---|---|---|---|---|---|---|
| Process P
|
Process N
|
|||||
| 25-34-CB (84 days)b | 4-4-BB (120 days) | 23456-CB (141 days)c | 23456-CB, third generation (133 days)d | 26-BB (125 days) | ||
| ortho | 2.37 | — | — | — | — | — |
| meta | 2.18 | — | — | 30.2 ± 0.2 | 48.4 | 56.5 ± 0.2 |
| para | 1.32 | 16.5 ± 0.5 | 32.7 ± 0.1 | 3.3 ± 0.1 | 3.1 | 7.1 ± 0.4 |
| Total | 5.87 | 3.8 ± 0.2 | 7.4 ± 0.3 | 11.5 ± 0.1 | 19.5 | 22.1 ± 0.1 |
The values are means and ranges for duplicate samples. All decreases shown are significant (P ≤ 0.05) as determined by a t test. The dash (—) indicates no significant change. All primers were used at a final concentration of 350 μM.
Data from reference 1.
Data from reference 32.
Data from a single third-generation enrichment with 23456-CB (8) are shown for comparison.
TABLE 2.
Effect of various halogenated biphenyl primers on the highly chlorinated PCBs in the Aroclor 1260 residue in Woods Pond sediment
| PCB homolog | Initial mol% | % Decrease resulting from primed dechlorinationa
|
||||
|---|---|---|---|---|---|---|
| Process P
|
Process N
|
|||||
| 25-34-CB (84 days)b | 4-4-BB (120 days) | 23456-CB (141 days)c | 23456-CB, third generation (133 days)d | 26-BB (125 days) | ||
| Hexachlorobiphenyls | 39.6 | 21.9 ± 0.9 | 34.9 ± 0.3 | 47.2 ± 0.5 | 64.2 | 76.9 ± 0.5 |
| Heptachlorobiphenyls | 24.3 | 7.9 ± 0.4 | 22.8 ± 0.9 | 43.9 ± 0.1 | 76.2 | 78.4 ± 0.6 |
| Octachlorobiphenyls | 5.2 | 1.7 ± 0.1 | 11.2 ± 1.7 | 20.7 ± 0.0 | 53.0 | 61.4 ± 1.1 |
| Hexa- to nonachlorobiphenyls | 69.8 | 14.8 ± 0.6 | 28.4 ± 0.6 | 43.0 ± 0.2 | 67.8 | 75.6 ± 0.2 |
The values are means and ranges based on data from duplicate samples. All decreases shown are significant (P ≤ 0.05) as determined by a t test. All primers were used at a final concentration of 350 μM.
Data from Bedard et al. (1).
Data from Van Dort et al. (32).
Data from a single third-generation enrichment with 23456-CB (8) are shown for comparison.
The dechlorination primed by 4-4-BB and 26-BB had a greater impact on the most highly chlorinated PCBs than the dechlorination primed by 25-34-CB and 23456-CB, respectively. In terms of chlorine removal, the PCB dechlorination primed by the brominated biphenyl congeners was nearly twice as effective as the PCB dechlorination primed by the PCB congeners (Table 1). Compared to 25-34-CB, 4-4-BB primed twice as much dechlorination of the hexa- through nonachlorobiphenyls and five times as much dechlorination of hepta- and octachlorobiphenyls (Table 2). Compared to 23456-CB, 26-BB primed nearly twice as much dechlorination of the hexa- through nonachlorobiphenyls and three times as much dechlorination of octachlorobiphenyls. In fact, the extent of dechlorination resulting from a single addition of 26-BB was approached only after three generations of enrichment with 23456-CB (Tables 1 and 2) (8). Based on our experience with these sediments, these differences are too great to be explained by differences between sediment batches, and we attribute them instead to the higher priming efficiency of the brominated biphenyls.
Process N had a greater impact on the Aroclor 1260 residue in Woods Pond than Process P had. This is partly because Process N has a broader substrate range, but specificity is also important. Process N removes predominantly flanked meta chlorines, whereas Process P removes flanked para chlorines, and there are almost twice as many meta chlorines as para chlorines in Aroclor 1260 (2.57 meta chlorines per biphenyl versus 1.35 para chlorines per biphenyl) (2). Our data show that the Process N dechlorination primed by 26-BB removed 56% of the meta chlorines, but it also removed 7% of the para chlorines (Table 1). Most likely, these para chlorines were removed from 345-, 2345-, and 23456-chlorophenyl groups, because the doubly flanked para chlorines on these rings are more susceptible to microbial dechlorination than singly flanked or unflanked chlorines (33). The Process N dechlorination primed by 26-BB was nearly three times more effective than the Process P dechlorination primed by 4-4-BB in terms of total chlorine removal (Table 1) and impact on the hexa- through nonachlorobiphenyls (Table 2).
PCB dechlorination primed by other brominated biphenyls.
Most of the other brominated biphenyls also primed PCB dechlorination (Table 3). The brominated biphenyls were completely dehalogenated to biphenyl by the pathways shown (6). Generally, meta and para bromines were removed before ortho bromines. In contrast, the chlorinated counterparts of nine of these congeners (2-CB, 3-CB, 4-CB, 24-CB, 25-CB, 26-CB, 2-2-CB, 4-4-CB, and 25-3-CB) were not dechlorinated and did not prime PCB dechlorination (summarized in reference 6).
TABLE 3.
Semiquantitative assessment of the specificity and impact of the PCB dechlorination primed by various brominated biphenyl congenersa
| Bromobiphenyl congener | Bromobiphenyl dehalogenation pathwayb | Dehalogenation specificity
|
Relative impact of primed PCB dechlorinatione | |
|---|---|---|---|---|
| First bromine lossc | PCB dechlorination primedd | |||
| 2 | → BPf | ortho | Flanked meta, N | 30 |
| 26 | → 2 → BP | ortho | Flanked meta, N | 100 |
| 2-2 | → 2 → BP | ortho | ?g | 0 |
| 3 | → BP | meta | Flanked meta, N | 60h |
| 25 | → 2 → BP | meta | Flanked meta, N | 80 |
| 25-2 | → 25 → 2 → BP | meta | Flanked meta, N | 80 |
| 25-3 | → 25 → 2 → BP | meta | Flanked meta, N | 120 |
| → 2-3 → 2 → BP | meta | |||
| 25-4 | → 2-4 → 2 → BP | meta | Flanked meta, N | 120 |
| → 25 → 2 → BP | para | Flanked para, P | ||
| 245 | → 24 → 2 → BP | meta | Flanked meta, N | 100 |
| → 25 → 2 → BP | para | |||
| 345 | → 34 → 4 → BP | meta | Flanked meta, N | 80 |
| → 35 → 3 → BP | para | |||
| 4 | → BP | para | Flanked para, P | 35 |
| 24 | → 2 → BP | para | Flanked para, P | 35 |
| 4-4 | → 4 → BP | para | Flanked para, P | 35 |
| 246 | → 26 → 2 → BP | para | Flanked para, P | 10 |
| → 24 → 4 → BP | ortho | |||
All congeners were tested at a concentration of 350 μM.
Evidence for the dehalogenation pathways shown is reported elsewhere (6).
Position from which the first bromine of the brominated biphenyl was removed.
The flanked meta dechlorination is Process N dechlorination. The flanked para dechlorination is Process P dechlorination. Both dechlorination processes occurred together when 25-4-BB was used as a primer.
The impact on dechlorination of the Aroclor 1260 residue in Woods Pond sediment was estimated relative to the dechlorination primed by 26-BB at 125 days, as measured by decreases in the number of total chlorines per biphenyl and in the fraction of hexa- through nonachlorobiphenyls (Tables 1 and 2). See text for details.
BP, biphenyl.
We observed small decreases in several penta- and hexachlorobiphenyls and small increases in several tetrachlorobiphenyls as a result of incubation with 2-2-CB, but these changes did not correspond to any known pattern of dechlorination. See text for details.
This value reflects the activity of a single sample. No dechlorination was primed in the duplicate sample.
Most of the brominated biphenyl congeners primed Process N dechlorination, but several primed Process P dechlorination (Table 3). One congener, 25-4-BB, primed both dechlorination processes simultaneously. This was evident from substantial increases in the amounts of 25-25-CB and 235-25-CB (products characteristic of Process P), as well as increases in dechlorination products characteristic of Process N. None of the congeners primed ortho dechlorination or unflanked para dechlorination (Process LP) even though both of these activities have been observed in Woods Pond sediment incubated with 246-CB (8, 33–37).
The only congener that did not prime significant dechlorination of the hexa- through nonachlorobiphenyls was 2-2-BB. This congener required a much longer acclimation time (18 weeks) prior to dehalogenation than any of the other brominated biphenyls (6). We observed modest changes in a few congeners in the samples amended with 2-2-BB; for example, there were small decreases in the amounts of 235-245-CB and 235-25-CB and small increases in the amounts of 24-25-CB, 25-25-CB, and 23-25-CB. These changes do not match any known pattern of dechlorination. We considered the changes too minor to justify identification of a new pattern of dechlorination, especially since we lacked the quantitative data necessary to perform a mass balance of substrates and products.
Table 3 shows the results of a semiquantitative assessment of the impact of the PCB dechlorination primed by each congener relative to the dechlorination primed by 26-BB. The priming activity of each congener was first scored relative to the priming activity of 26-BB or 4-4-BB, depending on whether the congener primed Process N or Process P dechlorination (see Materials and Methods). The priming activity of 26-BB was arbitrarily defined as 100, and that of 4-4-BB was set at 35, because the dechlorination of Aroclor 1260 primed by 4-4-BB was ∼33 to 38% as effective as that primed by 26-BB when measured in terms of the relative decrease in total chlorines and in hexa- through nonachlorobiphenyls (Tables 1 and 2). Scores relative to 4-4-BB were scaled relative to 26-BB by multiplying by a factor of 0.35.
The most effective primers were 25-3-BB and 25-4-BB. Other strong primers were 26-BB and 245-BB, followed by 25-BB, 345-BB, and 25-2-BB. The monobrominated biphenyls and 4-4-BB, 24-BB, and 246-BB were considerably less effective (Table 3).
The dechlorination resulting from the combination of Processes P and N (primed by 25-4-BB) was no more extensive than the dechlorination resulting from Process N alone (primed by 25-3-BB) (Table 3). Dechlorination Processes P and N do not complement each other because neither process can dechlorinate the end products of the other. In fact, since they compete for the same substrates, priming both dechlorination processes together may result in removal of fewer chlorines because Process P removes fewer chlorines than Process N (e.g., Fig. 1).
Impact of primed dechlorination on PCB persistence in humans.
Almost one-half of the total PCB content of Aroclor 1260 consists of congeners that have been reported to be highly persistent in humans; i.e., they have estimated half-lives in humans of more than 10 years (2, 11). The dechlorination that has occurred in situ at Woods Pond over the last few decades has reduced this fraction of PCBs to 32.2 mol%, but the dechlorination primed by 26-BB further reduced the fraction of highly persistent PCBs in the sediment to 7.7 mol% after 100 days and to 5.8 mol% after 179 days. These values correspond to impressive decreases of 76 and 82%, respectively. Likewise, the fraction of PCBs reported to be moderately persistent in humans (i.e., those with half-lives of 1 to 10 years in humans) decreased from 14.3 to 5.5 mol% in 100 days. Thus, the dechlorination primed by 26-BB markedly reduced the proportion of the PCBs that are most persistent in humans. After the primed dechlorination (100 days), 85 mol% of the residual PCBs had half-lives in humans of less than 1 year.
DISCUSSION
Correlation between the halogen configuration of the brominated biphenyl primer and the specificity of the PCB dechlorination primed.
Our studies of PCB dechlorination in Woods Pond sediment demonstrated that the halogen configuration of a PCB congener determines whether the congener will be dechlorinated and which chlorine(s) will be removed (32). Some chlorophenyl rings (e.g., 2-, 3-, 4-, 23-, 25-, and 26-chlorophenyl rings) were not dehalogenated. At a given temperature, the halogen configuration also determines which PCB congeners will prime PCB dechlorination and which dechlorination process(es) they will prime (1, 8, 32, 37). Only PCBs substituted at carbons 2, 3, and at least one other site prime Process N (32), and congeners with 34- or 245-chlorophenyl rings prime Process P (1, 32). Only one congener, 246-CB, primes Process LP (8, 37).
The relative reactivity preferences for brominated and chlorinated biphenyls are similar; meta and para halogens are removed first, and ortho halogens are more recalcitrant (6). However, in contrast to the PCBs, every one of the brominated biphenyls that we studied was completely dehalogenated to biphenyl by the microorganisms in Woods Pond sediment (6). Evidently, the specificity for dehalogenation is much less stringent for brominated biphenyls than for chlorinated biphenyls. Consequently, it is not surprising that brominated biphenyls also exhibited relaxed specificity for priming PCB dechlorination.
All but one of the brominated biphenyls that we tested primed either Process N or Process P dechlorination even though the substitution patterns of these compounds differed considerably (Table 3). Nevertheless, there was some correlation between the halogen configuration of the brominated biphenyl primer and the specificity of the PCB dechlorination process that it activated. Congeners with only para bromines, or only ortho and para bromines, primed Process P dechlorination, which removes flanked para chlorines. Congeners with meta bromines always primed Process N dechlorination (predominantly flanked meta dechlorination), regardless of whether ortho or para bromines were present. Two congeners that contain only ortho bromines, 2-BB and 26-BB, also primed Process N dechlorination. Although the latter finding is not completely understood, it is consistent with our previous studies which showed that PCB congeners must have at least one ortho chlorine to prime dechlorination Process N and that a second ortho chlorine on the same ring enhances and sustains the priming activity (32).
One congener, 25-4-BB, that contains meta and para bromines, primed both Processes P and N, whereas the other congeners that contain both meta and para bromines (i.e., 245-BB and 345-BB) primed only Process N. A key difference is that the para bromine on 25-4-BB is unflanked, but the para bromines on the other two congeners are each flanked by at least one bromine. It is significant that the four other congeners that primed Process P also have an unflanked para bromine (Table 3). Williams noted that in PCBs, chlorines flanked by one or two chlorines are usually more easily dehalogenated than unflanked chlorines (33). Similar observations have been made for other chlorinated aromatic compounds (18). If the same applies for brominated biphenyls, flanked para bromines may be so easily dehalogenated that they do not trigger a specific para dehalogenating activity.
Apparently, the first dehalogenation step for each brominated biphenyl congener determined which PCB dechlorination process(es) would be primed. We expected that the intermediate dehalogenation products of several congeners would prime an additional dechlorination process, but this did not happen. For example, 24-BB and 4-BB primed Process P when they were added by themselves, but not when they were generated as products of 245-BB and 345-BB, respectively (Table 3). These intermediates did not accumulate significantly before dehalogenation, suggesting that they were dehalogenated by the same microorganisms that dehalogenated the parent congeners.
Evidence that the microorganisms that dehalogenate PCBs also dehalogenate brominated biphenyls.
Previously, we proposed that PCB-dechlorinating microorganisms in Woods Pond might also dehalogenate brominated biphenyls, albeit with relaxed specificity (6). The following four lines of evidence from the experiments reported here support this hypothesis. (i) The brominated biphenyls primed the same highly specific PCB dechlorination processes (N and P) that are primed by PCB congeners. (ii) The primed PCB dechlorination began shortly after the onset of brominated biphenyl dehalogenation. (iii) The specificity of the PCB dechlorination process that was activated correlated loosely with the halogen substitution on the brominated biphenyl primer (Table 3). (iv) The concentration of the brominated biphenyl primer affected the rate and extent of PCB dechlorination (Fig. 5). However, definitive proof that the same microorganisms dehalogenate PCBs and brominated biphenyls will require isolation of the microorganisms responsible.
Proposed explanations for the priming activity of halogenated biphenyls.
We proposed previously that high concentrations of halogenated biphenyls prime PCB dechlorination because they are good substrates for dehalogenases and support the growth of PCB dechlorinators, perhaps by acting as electron acceptors (1, 3, 7, 8). Recently, Wu demonstrated that the concentrations of PCB-dechlorinating microorganisms increased nearly 1,000-fold after two additions of 26-BB (350 and 700 μmol per liter of slurry) (34). Proof that the halogenated biphenyls serve as electron acceptors will require experiments that demonstrate that the dehalogenation of these compounds is coupled to the oxidation of a specific electron donor(s), and the identification of appropriate electron donors will probably require sediment-free enrichment cultures. However, we propose that the following interpretation of our data can serve as a working hypothesis to guide research until pure or highly enriched cultures of the PCB-dechlorinating microorganisms in Woods Pond become available. (i) The first step in the dehalogenation of a brominated biphenyl primes PCB dechlorination and determines which dechlorination process (and consequently which of two different dehalogenating populations) will be activated. (ii) One population of microorganisms is predominantly meta dechlorinating. This population initiates ortho and meta dehalogenation of brominated biphenyls and Process N dechlorination of PCBs. Once it has been activated, this population can also remove para bromines, perhaps because the specificity for debromination is not very stringent. This is consistent with the observation that the congeners produced as intermediates do not accumulate significantly before dehalogenation. (iii) A second population is predominantly para dechlorinating. This population initiates para dehalogenation of congeners that have unflanked para bromines and Process P dechlorination of PCBs. Once it has been activated, this population can also remove ortho bromines (e.g., the 2-BB generated from dehalogenation of 24-BB).
Implications for bioremediation.
Process N converts many of the PCBs in Woods Pond sediment to congeners containing 24- and 246-chlorophenyl groups. Process LP primarily targets the unflanked para chlorines on these two chlorophenyl groups (8, 37, 38). Consequently, when Process LP follows Process N, the combined action of these complementary dechlorination processes converts many of the hexa- through nonachlorobiphenyls in Aroclor 1260 to di- and trichlorobiphenyls (e.g., Fig. 1B) (8, 27, 37). None of the brominated biphenyls primed Process LP, but other halogenated compounds may be able to do so (27).
We did not observe anaerobic degradation of the biphenyl produced by dehalogenation of the brominated biphenyls. However, recent experiments have demonstrated that microorganisms in a variety of aquatic sediments from widely distributed locations can degrade benzene anaerobically (20). In addition, anaerobic degradation of naphthalene has been reported at several different sites (9, 15, 39). Therefore, it is possible that the capacity for anaerobic degradation of biphenyl is also widespread because biphenyl is structurally related to both benzene and naphthalene.
Several of the brominated biphenyls primed extensive dechlorination of the aged PCBs in the sediment even though the >200-fold excess of hydrocarbon oil in the sediment (7,000 μg/g) might have been expected to limit bioavailability. For example, the dechlorination primed by 26-BB decreased the proportion of hexa- through nonachlorobiphenyls in the Aroclor 1260 residue by 75%, from ∼70 mol% of the total PCBs to ∼17.5 mol%, in 125 days (Table 2). Likewise, the dechlorination converted ∼75% of the PCBs that are most persistent in humans to less persistent forms. It is also likely that the dechlorination reduced potential risks associated with the biological activity of PCBs mediated through the aryl hydrocarbon receptor (AhR), because the adjacent meta and para chlorines present in all PCB congeners that are AhR ligands make them especially susceptible to microbial dechlorination (25, 26).
We are not certain whether priming will work in all PCB-contaminated sediments or, if it does work, that the same PCB dechlorination processes will be primed. Most likely, this will depend on the indigenous microorganisms and the contamination history of each sediment. However, priming was effective in PCB-contaminated soil inoculated with microorganisms from Woods Pond. Stokes demonstrated that 26-BB primed rapid and extensive Process N dechlorination of the Aroclor 1260 (180 μg/g) in clay soil from a site in California when the soil was slurried and then inoculated with Woods Pond sediment (10% [final volume] wet sediment) (30).
These results represent a major step toward identifying an effective method for accelerating PCB dechlorination in situ because they demonstrate that compounds other than PCBs can prime extensive microbial dechlorination of PCBs. We expect that there are naturally occurring compounds that have the same effect and hope to identify such compounds.
ACKNOWLEDGMENTS
We thank Jim Cella and Elliot Shanklin for synthesizing highly purified 26-BB and Lynn Smullen and Rosanna Stokes for assistance with the quantitative PCB analyses.
REFERENCES
- 1.Bedard D L, Bunnell S C, Smullen L A. Stimulation of microbial para-dechlorination of polychlorinated biphenyls that have persisted in Housatonic River sediment for decades. Environ Sci Technol. 1996;30:687–694. [Google Scholar]
- 2.Bedard D L, May R J. Characterization of the polychlorinated biphenyls in the sediments of Woods Pond: evidence for microbial dechlorination of Aroclor 1260 in situ. Environ Sci Technol. 1996;30:237–245. [Google Scholar]
- 3.Bedard D L, Quensen J F., III . Microbial reductive dechlorination of polychlorinated biphenyls. In: Young L Y, Cerniglia C E, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: Wiley-Liss Div., John Wiley & Sons, Inc.; 1995. pp. 127–216. [Google Scholar]
- 4.Bedard D L, Smullen L A, May R J. Abstracts of the 1996 International Symposium on Subsurface Microbiology. Zürich, Switzerland: Swiss Society of Microbiology and Institute of Plant Biology, University of Zürich; 1996. Microbial dechlorination of highly chlorinated PCBs in the Housatonic River, abstr. 81; p. 117. [Google Scholar]
- 5.Bedard D L, Van Dort H M. The role of microbial PCB dechlorination in natural restoration and bioremediation. In: Sayler G S, Sanseverino J, Davis K, editors. Biotechnology in the sustainable environment. New York, N.Y: Plenum Publishing Corp.; 1997. pp. 65–71. [Google Scholar]
- 6.Bedard D L, Van Dort H M. Complete reductive dehalogenation of brominated biphenyls by anaerobic microorganisms in sediment. Appl Environ Microbiol. 1998;64:940–947. doi: 10.1128/aem.64.3.940-947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedard D L, Van Dort H M, Bunnell S C, Principe J M, DeWeerd K A, May R J, Smullen L A. Anaerobic dehalogenation and its environmental implications. Athens, Ga: Office of Research and Development, U.S. Environmental Protection Agency; 1993. Stimulation of reductive dechlorination of Aroclor 1260 contaminant in anaerobic slurries of Woods Pond sediment; pp. 19–21. [Google Scholar]
- 8.Bedard D L, Van Dort H M, May R J, Smullen L A. Enrichment of microorganisms that sequentially meta-, para-dechlorinate the residue of Aroclor 1260 in Housatonic River sediment. Environ Sci Technol. 1997;31:3308–3313. [Google Scholar]
- 9.Bedessem M E, Swoboda-Colberg N G, Colberg P J S. Naphthalene mineralization coupled to sulfate reduction in aquifer-derived enrichments. FEMS Microbiol Lett. 1997;152:213–218. [Google Scholar]
- 10.Berkaw M, Sowers K R, May H D. Anaerobic ortho dechlorination of polychlorinated biphenyls by estuarine sediments from Baltimore Harbor. Appl Environ Microbiol. 1996;62:2534–2539. doi: 10.1128/aem.62.7.2534-2539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown J F., Jr Determination of PCB metabolic, excretion, and accumulation rates for use as indicators of biological response and relative risk. Environ Sci Technol. 1994;28:2295–2305. doi: 10.1021/es00062a013. [DOI] [PubMed] [Google Scholar]
- 12.Brown J F, Jr, Bedard D L, Brennan M J, Carnahan J C, Feng H, Wagner R E. Polychlorinated biphenyl dechlorination in aquatic sediments. Science. 1987;236:709–712. doi: 10.1126/science.236.4802.709. [DOI] [PubMed] [Google Scholar]
- 13.Brown J F, Jr, Wagner R E. PCB movement, dechlorination, and detoxication in the Acushnet Estuary. Environ Toxicol Chem. 1990;9:1215–1233. [Google Scholar]
- 14.Brown J F, Jr, Wagner R E, Feng H, Bedard D L, Brennan M J, Carnahan J C, May R J. Environmental dechlorination of PCBs. Environ Toxicol Chem. 1987;6:579–593. [Google Scholar]
- 15.Coates J D, Woodward J, Allen J, Philp P, Lovley D R. Anaerobic degradation of polycyclic aromatic hydrocarbons and alkanes in petroleum-contaminated marine harbor sediments. Appl Environ Microbiol. 1997;63:3589–3593. doi: 10.1128/aem.63.9.3589-3593.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Environmental Protection Agency. Test methods for evaluating solid waste: physical/chemical methods. 3rd ed. 1B. Publication 530/SW-846. Washington, D.C: Environmental Protection Agency; 1986. EPA Method 3540. Soxhlet extraction. Chapter 4, Section 4.2.1. [Google Scholar]
- 17.Environmental Protection Agency. Test methods for evaluating solid waste: physical/chemical methods. 3rd ed. 1B. Publication 530/SW-846. Washington, D.C: Environmental Protection Agency; 1986. EPA Method 3660. Sulfur cleanup, Procedure 7.1, removal of sulfur using copper, Chapter 4, Section 4.2.2. [Google Scholar]
- 18.Fathepure B Z, Tiedje J M, Boyd S A. Reductive dechlorination of hexachlorobenzene to tri- and dichlorobenzenes in anaerobic sewage sludge. Appl Environ Microbiol. 1988;54:327–330. doi: 10.1128/aem.54.2.327-330.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frame G M, Wagner R E, Carnahan J C, Brown J F, Jr, May R J, Smullen L A, Bedard D L. Comprehensive, quantitative, congener-specific analyses of eight Aroclors and complete PCB congener assignments on DB-1 capillary GC columns. Chemosphere. 1996;33:603–623. [Google Scholar]
- 20.Kazumi J, Caldwell M E, Suflita J M, Lovley D R, Young L Y. Anaerobic degradation of benzene in diverse anoxic environments. Environ Sci Technol. 1997;31:813–818. [Google Scholar]
- 21.Kim J, Rhee G-Y. Population dynamics of polychlorinated biphenyl-dechlorinating microorganisms in contaminated sediments. Appl Environ Microbiol. 1997;63:1771–1776. doi: 10.1128/aem.63.5.1771-1776.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lake J L, Pruell R J, Osterman F A. An examination of dechlorination processes and pathways in New Bedford Harbor sediments. Mar Environ Res. 1992;33:31–47. [Google Scholar]
- 23.Morris P J, Quensen III J F, Tiedje J M, Boyd S A. Reductive debromination of the commercial polybrominated biphenyl mixture Firemaster BP6 by anaerobic microorganisms from sediments. Appl Environ Microbiol. 1992;58:3249–3256. doi: 10.1128/aem.58.10.3249-3256.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quensen J F, III, Boyd S A, Tiedje J M. Dechlorination of four commercial polychlorinated biphenyl mixtures (Aroclors) by anaerobic microorganisms from sediments. Appl Environ Microbiol. 1990;56:2360–2369. doi: 10.1128/aem.56.8.2360-2369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quensen J F, III, Mousa M A, Boyd S A, Sanderson J T, Froese K L, Giesy J P. Reduction of Ah receptor mediated activity of PCB mixtures due to anaerobic microbial dechlorination. Environ Toxicol Chem. 1998;17:806–813. [Google Scholar]
- 26.Quensen J F, III, Tiedje J M, Boyd S A, Enke C, Lopshire R, Giesy J, Mora M, Crawford R, Tillit D. International Symposium on Soil Decontamination Using Biological Processes. Frankfurt am Main, Germany: Dechema; 1992. Evaluation of the suitability of reductive dechlorination for the bioremediation of PCB-contaminated soils and sediments; pp. 91–100. [Google Scholar]
- 27.Smullen L A, Bedard D L. Abstracts of the 95th General Meeting of the American Society for Microbiology 1995. Washington, D.C: American Society for Microbiology; 1995. Stimulation of two successive stages of microbial dechlorination of Aroclor 1260 in anaerobic pond sediment, abstr. Q-82; p. 414. [Google Scholar]
- 28.Smullen L A, DeWeerd K A, Bedard D L, Fessler W A, Carnahan J C, Wagner R E. Twelfth progress report, Research and Development Program for the Destruction of PCBs. Schenectady, N.Y: General Electric Company Corporate Research and Development; 1993. Development of a customized congener specific PCB standard for quantification of Woods Pond sediment PCBs; pp. 45–65. [Google Scholar]
- 29.Sokol R C, Kwon O-S, Bethoney C M, Rhee G-Y. Reductive dechlorination of polychlorinated biphenyls in St. Lawrence River sediments and variations in dechlorination characteristics. Environ Sci Technol. 1994;28:2054–2064. doi: 10.1021/es00061a013. [DOI] [PubMed] [Google Scholar]
- 30.Stokes, R. W. (GE Corporate Research and Development, Schenectady, N.Y.) Personal communication.
- 31.Van Dort H M, Bedard D L. Reductive ortho and meta dechlorination of a polychlorinated biphenyl congener by anaerobic microorganisms. Appl Environ Microbiol. 1991;57:1576–1578. doi: 10.1128/aem.57.5.1576-1578.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Dort H M, Smullen L A, May R J, Bedard D L. Priming meta-dechlorination of polychlorinated biphenyls that have persisted in Housatonic River sediments for decades. Environ Sci Technol. 1997;31:3300–3307. [Google Scholar]
- 33.Williams W A. Microbial reductive dechlorination of trichlorobiphenyls in anaerobic slurries. Environ Sci Technol. 1994;28:630–635. doi: 10.1021/es00053a015. [DOI] [PubMed] [Google Scholar]
- 34.Wu Q. Ph.D. dissertation. Athens: The University of Georgia; 1996. [Google Scholar]
- 35.Wu Q, Bedard D L, Wiegel J. Influence of incubation temperature on the microbial reductive dechlorination of 2,3,4,6-tetrachlorobiphenyl in two freshwater sediments. Appl Environ Microbiol. 1996;62:4174–4179. doi: 10.1128/aem.62.11.4174-4179.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Q, Bedard D L, Wiegel J. Effect of incubation temperature on the route of microbial reductive dechlorination of 2,3,4,6-tetrachlorobiphenyl in polychlorinated biphenyl (PCB)-contaminated and PCB-free freshwater sediments. Appl Environ Microbiol. 1997;63:2836–2843. doi: 10.1128/aem.63.7.2836-2843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Q, Bedard D L, Wiegel J. Temperature determines the pattern of anaerobic microbial reductive dechlorination of Aroclor 1260 primed by 2,3,4,6-tetrachlorobiphenyl in Woods Pond sediment. Appl Environ Microbiol. 1997;63:4818–4825. doi: 10.1128/aem.63.12.4818-4825.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Q, Wiegel J. Two anaerobic polychlorinated biphenyl-dehalogenating enrichments that exhibit different para-dechlorination specificities. Appl Environ Microbiol. 1997;63:4826–4832. doi: 10.1128/aem.63.12.4826-4832.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Young L Y. Carboxylation as an initial reaction in the metabolism of naphthalene and phenanthrene by sulfidogenic consortia. Appl Environ Microbiol. 1997;63:4759–4764. doi: 10.1128/aem.63.12.4759-4764.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]