Abstract
Background
This study was to probe into the relationship between the neutrophil-to-lymphocyte ratio (NLR) and both in-hospital and long-term heart failure risk in patients with acute myocardial infarction (AMI).
Methods
990 patients with AMI, including 386 with non-ST-segment elevation myocardial infarction (NSTEMI) and 604 with segment elevation myocardial infarction (STEMI) were recruited between January 2019 and March 2022. The in-hospital acute heart failure (AHF) and arrhythmia events were recorded.
Results
The NLR was significantly greater in the AHF group in STEMI and NSTEMI patients, with a higher frequency of arrhythmia in comparison to the non-AHF group. A high NLR was related to a high level of myocardial injury markers, accompanied with more AHF and arrhythmia events. Multivariate logistic regression analyses revealed that high NLR is independently linked with increased in-hospital AHF and arrhythmia risk. Receiver operating characteristic curve analyses revealed that the prognostic value of NLR for in-hospital AHF was 0.704 in STEMI patients and 0.766 in NSTEMI patients. However, during a median follow-up of 28 months with 32 heart failure patients, there was no significant difference between the low NLR group (n = 18) and the high NLR group (n = 14). Further analysis showed that the two groups did not significantly differ in the occurrence of heart failure within 12 months of discharge.
Conclusion
Our results indicate that NLR is an independent risk factor of in-hospital AHF in AMI patients. However, NLR has no value in predicting long-term heart failure.
Keywords: NLR, AMI, STEMI, NSTEMI, in-hospital AHF, arrhythmia
Introduction
According to the 2021 China Cardiovascular Health and Disease Report, about 330 million patients have been suffered from cardiovascular and cerebrovascular diseases in China, including about 24 million patients with coronary heart disease and stroke. Acute myocardial infarction (AMI) has a high mortality rate, and although interventional treatment can markedly reduce the mortality rate, AMI prognosis remains relatively poor, and the incidence of acute heart failure (AHF) is high (1).
The neutrophil-to-lymphocyte ratio (NLR) is a well-established indicator in the study of cardiovascular disease prognostic models. A recent study found that the NLR is an important predictor of in-hospital death in AMI patients (2). In addition, NLR had predictive value in heart failure readmissions and prognosis in patients undergoing transcatheter aortic valve replacement (3). However, there is currently a lack of research on the potential use of NLR to predict the occurrence of in-hospital and long-term heart failure in patients with AMI. Although NLR does not directly reflect changes in cardiac inflammation and metabolic environment, it can be used to evaluate the prognosis of patients early on upon initial presentation to the emergency department. Furthermore, when combining NLR with classical markers of myocardial injury, the predictive value can be further improved. Moreover, due to the easy acquisition of NLR and the low cost to patients, a relatively complete cohort data can be obtained during follow-up.
During the course of clinical management of patients, a reciprocal causal relationship between heart failure and malignant arrhythmias was observed, in which both can be induced by the inflammatory environment. Additionally, major adverse cardiovascular events (MACE) in AMI patients following discharge represent a crucial research area that requires additional attention. It is found that NLR available at the time of admission could be applied for the evaluation of short-term outcomes in patients with cardiogenic shock as a complication of AMI, and higher NLR is an independent risk factor for elevated 30-day all-cause mortality and MACE (4). Besides, a study found that postoperative NLR (within 24 h) may be more effective in predicting the incidence of MACE in NSTEMI patients within one year after elective percutaneous coronary intervention (5). However, we found that most prognostic studies on NLR are confined to a duration of less than one year, and there is a lack of evidence for longer-term prognostic evaluations beyond this timeframe.
In the present study, the correlation between NLR and in-hospital AHF in patients with AMI was investigated. Moreover, we assessed the predictive utility of NLR for arrhythmia during hospitalization and MACE during the follow-up period.
Patients and methods
Participants
This study was carried out in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Clinical Investigation of The Affiliated Changzhou No.2 People's Hospital of Nanjing Medical University (KY314-01). Additionally, the study was registered in the China Clinical Trial Registration Center (ChiCTR2300067892). The clinical informed consent was collected from all subjects and/or their legal guardian(s), in ethics and consent section under declaration. We included a total of 1,058 AMI patients, and after exclusion criteria, 990 patients were ultimately included. A total of 990 patients with AMI were admitted in the study, including 386 with NSTEMI and 604 with STEMI, all of whom were admitted to the Affiliated Changzhou No. 2 People's Hospital of Nanjing Medical University between January 2019 and March 2022. The primary outcome measure was in-hospital AHF, and the secondary outcome measures were in-hospital arrhythmia and MACE.
The following inclusion criteria were used: (1) age between 18 and 80 years, (2) admitting diagnosis of AMI (STEMI and NSTEMI). Diagnostic criteria of STEMI and NSTEMI have been described in the previous study (6). Diagnostic criteria of STEMI: (1) chest pain history; (2) continuous elevation of ST segments (≥ 0.1 mV for more than 30 min, or V2 or V3 ≥ mV in two or more adjacent ECG leads), or new onset of left bundle branch block; (3) myocardial injury markers increased beyond the 99th percentile of the laboratory reference limit. Diagnostic criteria of NSTEMI: (1) a history of chest pain; (2) in leads with R wave dominant or R/S >1, a new horizontal or downwardly inclined ST segment depression (≥0.05 mV or T wave inversion ≥0.1 mV in two adjacent leads); (3) myocardial injury markers increased beyond the 99th percentile of the laboratory reference limit. The following exclusion criteria were used: (1) known history of heart failure or arrhythmia, (2) malignant tumor, (3) pregnancy, (4) severe liver dysfunction (decompensated liver cirrhosis, liver failure, etc), (5) severe hematological disorders (acute and chronic leukemia, lymphoma, etc), (6) history of coronary artery bypass grafting, (7) cardiogenic shock, (8) mechanical ventilation, and (9) mechanical circulatory support. The inclusion and follow-up process of this study population is consistent with previous studies (6, 7).
Data retrieval and definitions
The Information related to demographics and clinical data during the patient's hospital stay was obtained from an electronic medical record system of hospital. The diagnosis of AHF was based on typical symptoms, signs, and laboratory examination, such as orthopnea, acute pulmonary edema, and B-type natriuretic peptide (BNP) levels, which was consistent with previous study (6). The definition of Arrhythmia is single or multiple episodes of atrial fibrillation, atrial flutter, ventricular fibrillation, or ventricular flutter, which was also consistent with previous study(6). The Gensini score was calculated to assess the severity of coronary artery disease, according to a previously described protocol (8).
Follow-up
786 AMI patients recruited from January 2019 to August 2021 were followed up. Patients were interviewed one month after discharge and every three months afterward. General information, inpatient data, and medication situation were collected at each interview. Based on the information gathered during follow-up, outpatient visit was suggested if necessary. The study recorded MACE, which included all-cause mortality, heart failure, nonfatal MI, nonfatal stroke, and unplanned repeat revascularization (URR), and this information has been previously described in another study (7). The definition of heart failure during follow-up is consistent with that during hospitalization. Nonfatal MI was defined as new pathological Q waves in ≥2 contiguous electrocardiogram leads. Patients with acute ischemic cerebral vascular events were marked as stroke. Finally, URR was defined as any non-staged revascularization after Percutaneous Coronary Intervention (PCI).
Statistical analyses
All continuous data have been normality tested. Normally distributed data were represented by the mean ± standard deviation, while skewed continuous data were represented by the median (interquartile range). Student's t-test or the Mann–Whitney U-test was performed to compare continuous variables between two groups, and the chi-squared test was used to compare categorical variables between two groups. Univariate and multivariate logistic regression analyses were conducted to assess the predictive value of the NLR for in-hospital AHF or arrhythmia risk. Receiver operating characteristic (ROC) curve analysis was performed to measure the cutoff value of the NLR for AHF during hospitalization. All tests were two-tailed, and p values <0.05 were considered significant. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, N.Y., USA).
Results
The clinical characteristics of the 990 patients who participated in the study were presented in Table 1. The AHF events was recorded in 38 out of 604 STEMI patients, and in 51 out of 386 NSTEMI patients. Among the STEMI group, patients who had AHF were significantly older than those without AHF (64.9 ± 12.3 vs. 60.4 ± 14.2, P = 0.038). In both STEMI and NSTEMI patients, the proportion of concurrent arrhythmia events in the AHF patients was significantly higher than that in the non-AHF patients. Furthermore, The NLR was notably higher in the AHF group compared with the non-AHF group (10.5 ± 6.9 vs. 6.6 ± 5.6, P = 0.001).
Table 1.
Baseline characteristics of the study population.
| Characteristics | STEMI | P-value | NSTEMI | P-value | ||
|---|---|---|---|---|---|---|
| Without AHF (n = 566) | With AHF (n = 38) | Without AHF (n = 335) | With AHF (n = 51) | |||
| Age (years) | 60.4 ± 14.2 | 64.9 ± 12.3 | 0.038 | 63.3 ± 13.1 | 65.6 ± 14.7 | 0.296 |
| Sex, male, n (%) | 459 (81.1) | 31 (81.6) | 0.941 | 241 (71.9) | 33 (64.7) | 0.289 |
| BMI (kg/m2) | 25.0 ± 4.1 | 25.1 ± 3.0 | 0.788 | 24.5 ± 4.2 | 24.2 ± 3.5 | 0.628 |
| Smoking, n (%) | 275 (50.9) | 22 (59.5) | 0.315 | 146 (46.8) | 19 (38.8) | 0.295 |
| Hypertension, n (%) | 342 (60.4) | 28 (73.7) | 0.104 | 218 (65.1) | 37 (72.5) | 0.294 |
| Diabetes, n (%) | 133 (23.5) | 14 (36.8) | 0.064 | 91 (27.2) | 18 (35.3) | 0.230 |
| In-hospital arrhythmia, n (%) | 28 (4.9) | 17 (44.7) | <0.001 | 6 (1.8) | 24 (47.1) | <0.001 |
| Gensini score | 49 (35–81) | 53 (37–81) | 0.690 | 29 (9–48) | 60 (46–84) | < 0.001 |
| Length of stay (days) | 8.2 ± 2.9 | 8.0 ± 2.7 | 0.657 | 7.4 ± 2.6 | 8.5 ± 3.2 | 0.023 |
| Biochemical test | ||||||
| NEUT (*109) | 7.7 ± 3.1 | 9.9 ± 4.5 | 0.005 | 7.3 ± 3.2 | 9.3 ± 4.1 | 0.001 |
| LYM (*109) | 1.5 ± 0.8 | 1.2 ± 0.6 | 0.012 | 1.5 ± 1.1 | 1.4 ± 0.8 | 0.536 |
| NLR | 6.6 ± 5.6 | 10.5 ± 6.9 | 0.001 | 6.3 ± 4.9 | 9.0 ± 7.2 | 0.012 |
| UA (umol/L) | 328 (272–392) | 344 (287–418) | 0.417 | 335 (277–410) | 353 (309–418) | 0.135 |
| LDL-C (mmol/L) | 2.74 ± 0.84 | 2.82 ± 1.00 | 0.671 | 2.54 ± 0.92 | 2.79 ± 1.37 | 0.209 |
| HDL-C (mmol/L) | 1.03 ± 0.28 | 1.09 ± 0.33 | 0.330 | 1.05 ± 0.29 | 0.97 ± 0.22 | 0.020 |
| TC (mmol/L) | 4.5 ± 1.2 | 4.6 ± 1.1 | 0.327 | 4.3 ± 1.2 | 4.5 ± 1.5 | 0.328 |
| TG (mmol/L) | 1.47 (1.04–2.10) | 1.75 (1.12–2.14) | 0.518 | 1.52 (1.13–2.16) | 1.45 (1.17–1.90) | 0.779 |
| TyG | 9.0 ± 0.7 | 9.1 ± 0.8 | 0.382 | 9.0 ± 0.7 | 9.1 ± 0.7 | 0.301 |
| TG/HDL | 2.0 ± 3.1 | 2.0 ± 1.9 | 0.974 | 2.1 ± 2.6 | 2.2 ± 2.9 | 0.807 |
| CPK (U/L) | 950 (312–2003) | 1,132 (528–1,791) | 0.517 | 154 (76–376) | 236 (116–879) | 0.010 |
| CK-MB (U/L) | 81 (35–167) | 102 (47–144) | 0.598 | 22 (16–42) | 31 (19–78) | 0.008 |
| HBDH (U/L) | 483 (276–824) | 493 (349–718) | 0.662 | 197 (151–324) | 274 (188–444) | 0.001 |
| BNP (pg/ml) | 291 (81–1,165) | 394 (79–1,445) | 0.703 | 456 (149–1,610) | 913 (284–3,193) | 0.006 |
| HbA1c (%) | 6.5 ± 1.5 | 6.9 ± 1.7 | 0.198 | 6.5 ± 1.5 | 6.8 ± 1.7 | 0.262 |
| Ccr (ml/min) | 69 ± 43 | 71 ± 33 | 0.775 | 63 ± 31 | 59 ± 29 | 0.466 |
| Ultrasonic cardiogram | ||||||
| LA (mm) | 3.9 ± 0.5 | 3.9 ± 0.4 | 0.825 | 3.9 ± 0.5 | 4.0 ± 0.5 | 0.198 |
| LV (mm) | 5.3 ± 2.2 | 5.2 ± 0.6 | 0.571 | 5.2 ± 0.6 | 5.3 ± 0.6 | 0.142 |
| EF (%) | 50 ± 9 | 52 ± 8 | 0.159 | 55 ± 9 | 50 ± 13 | 0.020 |
| Pharmacological intervention | ||||||
| Double antiplatelet, n (%) | 521 (92.4) | 37 (97.4) | 0.694 | 291 (87.4) | 48 (94.1) | 0.432 |
| Anticoagulation, n (%) | 19 (3.4) | 0 (0) | 0.493 | 11 (3.3) | 2 (3.9) | 0.829 |
| β-block, n (%) | 340 (60.1) | 21 (55.3) | 0.550 | 176 (52.5) | 29 (56.9) | 0.578 |
| Statin, n (%) | 559 (98.8) | 37 (97.4) | 0.382 | 331 (98.8) | 51 (100) | 0.497 |
| ACEI/ARB, n (%) | 176 (31.1) | 12 (31.6) | 0.956 | 106 (31.6) | 14 (27.5) | 0.538 |
| ARNI, n (%) | 150 (26.5) | 10 (26.3) | 0.944 | 107 (32.3) | 8 (16.0) | 0.019 |
| SGLT2i, n (%) | 131 (23.2) | 5 (13.1) | 0.151 | 93 (27.8) | 5 (9.8) | 0.006 |
| MRA, n (%) | 114 (20.1) | 9 (23.7) | 0.611 | 50 (14.9) | 16 (31.4) | 0.004 |
BMI, body mass index; AHF, acute heart failure; NLR, neutrophil-to-lymphocyte ratio; UA, uric acid; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; BNP, brain natriuretic peptide; CPK, creatine phosphokinase; CK-MB, creatine kinase-MB; HBDH, hydroxybutyrate dehydrogenase; Ccr, creatinine clearance rate; LA, left atrium; LV, left ventricle; LVEF, left ventricular ejection factor; ARNI, angiotensin receptor-neprilysin inhibitor; SGLT2i, sodium-glucose cotransporter 2 inhibitor; MRA, mineralocorticoid receptor antagonist.
Values are shown as the means ± SD, median (interquartile range) or percentage.
In NSTEMI patients, the AHF group had a significantly higher Gensini score compared to the non-AHF group (60 (46–84) vs. 29 (9–48), P < 0.001). In NSTEMI patients, the AHF group had lower levels of HDL-C compared to the non-AHF group (0.97 ± 0.22 vs. 1.05 ± 0.29, P = 0.020), and higher levels of myocardial injury and heart failure markers, including creatine phosphokinase (CPK, 236 (116–879) vs. 154 (76–376), P = 0.010), creatine kinase-MB (CK-MB, 31 (19–78) vs. 22 (16–42), P = 0.008), hydroxybutyrate dehydrogenase (HBDH, 274 (188–444) vs. 197 (151–324), P = 0.001), and B-type natriuretic peptide (BNP, 913 (284–3,193) vs. 456 (149–1,610), P = 0.006). Furthermore, patients with AHF had lower Ejection Fraction (EF) values than those without AHF (50 ± 13 vs. 55 ± 9, P = 0.020). Additionally, we examined the medications administered to patients during hospitalization. The ratios of patients receiving angiotensin receptor-neprilysin inhibitor (ARNI) and sodium-glucose cotransporter 2 inhibitor (SGLT2i) therapies in the AHF group was markedly lower than that in the non-AHF group (8 (16.0) vs. 107 (32.3), P = 0.019; 5 (9.8) vs. 93 (27.8), P = 0.006, respectively), while an opposite fact was found for mineralocorticoid receptor antagonist (MRA) treatment (16 (31.4) vs. 50 (14.9), P = 0.004) (Table 1).
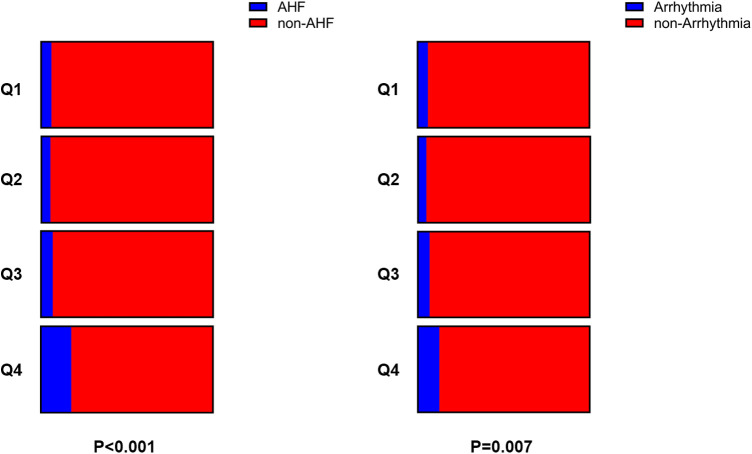
As BNP and myocardial injury indicators tests are used to assess the prognosis for in-hospital AMI patients, we grouped patients based on their NLR quartiles. The results indicated a significant increase in the levels of BNP, CPK, CK-MB and HBDH, with an increase in the NLR. Moreover, the proportion of patients with AHF and arrhythmia also increased significantly, particularly in the highest quartile (fourth quartile) group (Table 2 and Figure 1).
Table 2.
The levels of myocardial injury markers and the occurrence of AHF and arrhythmia were correlated with NLR.
| NLR | P value | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| BNP (pg/ml) | 285 (88–1,203) | 362 (100–1,323) | 419 (118–1,340) | 477 (121–1,490) | 0.003 |
| CPK (U/L) | 320 (105–1,067) | 465 (132–1,178) | 690 (171–1,672) | 536 (158–1,540) | 0.032 |
| CK-MB (U/L) | 38 (21–104) | 44 (21–109) | 60 (26–129) | 55 (21–124) | 0.015 |
| HBDH (U/L) | 298 (183–578) | 332 (187–612) | 395 (218–730) | 357 (193–621) | 0.308 |
| AHF, n (%) | 15 (6.0) | 13 (5.3) | 17 (6.9) | 44 (17.7) | <0.001 |
| Arrhythmia, n (%) | 15 (6.0) | 12 (4.9) | 17 (6.9) | 31 (12.5) | 0.007 |
BNP, brain natriuretic peptide; CPK, creatine phosphokinase; CK-MB, creatine kinase-MB; HBDH, hydroxybutyrate dehydrogenase.
Figure 1.
The proportion of patients with in-hospital AHF and arrhythmia in each of the 4 groups.
Both univariate and multivariate logistic regression analyses suggested that a high NLR was associated with an increased risk of in-hospital AHF in both STEMI (HR = 2.928, 95% CI: 1.393–6.158, P = 0.005) and NSTEMI patients (HR = 2.336, 95% CI: 1.236–4.416, P = 0.009) (Tables 3, 4). We also evaluated the potential of NLR in predicting arrhythmia occurrence during hospitalization. It was indicated that a high NLR was related with an increased risk of in-hospital arrhythmia in STEMI patients in both univariate and multivariate logistic regression analyses (HR = 2.133, 95% CI: 1.107–4.133, P = 0.024), but not in NSTEMI patients (Tables 5, 6).
Table 3.
Univariate and multivariate logistic analyses for the in-hospital AHF risk in STEMI patients.
| Parameters for AHF (STEMI) | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age (years) | 1.023 | 0.999–1.048 | <0.001 | 1.025 | 1.000–1.050 | 0.051 |
| Sex, male, n (%) | 0.969 | 0.415–2.259 | 0.941 | |||
| Smoking, n (%) | 1.413 | 0.718–2.783 | 0.317 | |||
| Hypertension, n (%) | 1.834 | 0.874–3.849 | 0.109 | |||
| Diabetes, n (%) | 1.899 | 0.955–3.776 | 0.067 | |||
| LDL-C (mmol/L) >3.4 | 0.991 | 0.443–2.218 | 0.983 | |||
| HDL-C (mmol/L) <1.04 | 0.907 | 0.466–1.765 | 0.774 | |||
| BNP (pg/ml) >400 | 1.081 | 0.560–2.085 | 0.816 | |||
| CPK (U/L) >200 | 1.331 | 0.507–3.498 | 0.562 | |||
| CK-MB (U/L) >20 | 1.084 | 0.372–3.154 | 0.883 | |||
| HBDH (U/L) >182 | 1.976 | 0.463–8.429 | 0.357 | |||
| NLR >median | 2.860 | 1.364–5.998 | 0.005 | 2.928 | 1.393–6.158 | 0.005 |
| β-block, n (%) | 0.817 | 0.422–1.584 | 0.550 | |||
| ACEI/ARB, n (%) | 1.020 | 0.503–2.068 | 0.956 | |||
| ARNI, n (%) | 0.974 | 0.462–2.053 | 0.944 | |||
| SGLT2i, n (%) | 0.501 | 0.192–1.309 | 0.158 | |||
| MRA, n (%) | 1.222 | 0.563–2.655 | 0.612 | |||
LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; BNP, brain natriuretic peptide; CPK, creatine phosphokinase; CK-MB, creatine kinase-MB; HBDH, hydroxybutyrate dehydrogenase; ARNI, angiotensin receptor-neprilysin inhibitor; SGLT2i, sodium-glucose cotransporter 2 inhibitor; MRA, mineralocorticoid receptor antagonist.
Table 4.
Univariate and multivariate logistic analyses for the in-hospital AHF risk in NSTEMI patients.
| Parameters for AHF (NSTEMI) | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age (years) | 1.013 | 0.991–1.036 | <0.001 | 1.003 | 0.980–1.027 | 0.795 |
| Sex, male, n (%) | 1.398 | 0.751–2.604 | 0.290 | |||
| Smoking, n (%) | 0.720 | 0.389–1.334 | 0.296 | |||
| Hypertension, n (%) | 1.418 | 0.747–2.730 | 0.295 | |||
| Diabetes, n (%) | 1.463 | 0.785–2.726 | 0.231 | |||
| LDL-C (mmol/L) >3.4 | 1.190 | 0.563–2.513 | 0.649 | |||
| HDL-C (mmol/L) <1.04 | 1.526 | 0.820–2.840 | 0.182 | |||
| BNP (pg/mL) >400 | 1.690 | 0.900–3.172 | 0.102 | |||
| CPK (U/L) >200 | 1.439 | 0.797–2.597 | 0.227 | |||
| CK-MB (U/L) >20 | 1.681 | 0.886–3.190 | 0.112 | |||
| HBDH (U/L) >182 | 2.603 | 1.316–5.149 | 0.006 | 2.240 | 1.106–4.534 | 0.025 |
| NLR >median | 2.155 | 1.167–3.978 | 0.014 | 2.336 | 1.236–4.416 | 0.009 |
| β-block, n (%) | 1.183 | 0.653–2.144 | 0.579 | |||
| ACEI/ARB, n (%) | 0.814 | 0.422–1.570 | 0.539 | |||
| ARNI, n (%) | 0.399 | 0.181–0.879 | 0.156 | |||
| SGLT2i, n (%) | 0.282 | 0.109–0.731 | 0.009 | 0.326 | 0.118–0.900 | 0.031 |
| MRA, n (%) | 0.597 | 0.337–1.041 | 0.005 | 0.872 | 0.342–2.143 | 0.007 |
LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; BNP, brain natriuretic peptide; CPK, creatine phosphokinase; CK-MB, creatine kinase-MB; HBDH, hydroxybutyrate dehydrogenase; ARNI, angiotensin receptor-neprilysin inhibitor; SGLT2i, sodium-glucose cotransporter 2 inhibitor; MRA, mineralocorticoid receptor antagonist.
Table 5.
Univariate and multivariate logistic analyses for the in-hospital arrhythmia risk in STEMI patients.
| Parameters for arrhythmia (STEMI) | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age (years) | 1.034 | 1.010–1.057 | 0.005 | 1.024 | 0.998–1.051 | 0.068 |
| Sex, male, n (%) | 2.323 | 1.205–4.480 | 0.012 | 1.103 | 0.408–2.979 | 0.847 |
| Smoking, n (%) | 0.630 | 0.338–1.177 | 0.147 | |||
| Hypertension, n (%) | 1.437 | 0.748–2.762 | 0.277 | |||
| Diabetes, n (%) | 2.222 | 1.186–4.164 | 0.013 | 1.993 | 1.047–3.791 | 0.036 |
| LDL-C (mmol/L) >3.4 | 1.568 | 0.797–3.085 | 0.192 | |||
| HDL-C (mmol/L) <1.04 | 0.742 | 0.403–1.365 | 0.337 | |||
| BNP (pg/ml) >400 | 2.055 | 1.091–3.868 | 0.026 | 1.629 | 0.826–3.210 | 0.159 |
| CPK (U/L) >200 | 1.083 | 0.469–2.500 | 0.851 | |||
| CK-MB (U/L) >20 | 1.325 | 0.460–3.822 | 0.602 | |||
| HBDH (U/L) >182 | 1.528 | 0.458–5.092 | 1.528 | |||
| NLR >median | 2.022 | 1.064–3.840 | 0.032 | 2.133 | 1.107–4.133 | 0.024 |
| β-block, n (%) | 0.826 | 0.448–1.523 | 0.540 | |||
| ACEI/ARB, n (%) | 0.696 | 0.345–1.406 | 0.313 | |||
| ARNI, n (%) | 1.119 | 0.572–2.191 | 0.742 | |||
| SGLT2i, n (%) | 0.846 | 0.397–1.804 | 0.666 | |||
| MRA, n (%) | 0.829 | 0.376–1.829 | 0.643 | |||
LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; BNP, brain natriuretic peptide; CPK, creatine phosphokinase; CK-MB, creatine kinase-MB; HBDH, hydroxybutyrate dehydrogenase; ARNI, angiotensin receptor-neprilysin inhibitor; SGLT2i, sodium-glucose cotransporter 2 inhibitor; MRA, mineralocorticoid receptor antagonist.
Table 6.
Univariate and multivariate logistic analyses for the in-hospital arrhythmia risk in NSTEMI patients.
| Parameters for arrhythmia (NSTEMI) | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age (years) | 1.010 | 0.982–1.039 | 0.499 | |||
| Sex, male, n (%) | 1.245 | 0.563–2.752 | 0.588 | |||
| Smoking, n (%) | 0.706 | 0.324–1.542 | 0.383 | |||
| Hypertension, n (%) | 1.756 | 0.733–4.207 | 0.206 | |||
| Diabetes, n (%) | 1.780 | 0.140–1.780 | 0.140 | |||
| LDL-C (mmol/L) >3.4 | 0.716 | 0.241–2.122 | 0.546 | |||
| HDL-C (mmol/L) <1.04 | 2.534 | 1.060–6.058 | 0.037 | 2.388 | 0.989–5.766 | 0.053 |
| BNP (pg/ml) >400 | 1.490 | 0.678–3.276 | 0.321 | |||
| CPK (U/L) >200 | 0.926 | 0.437–1.964 | 0.842 | |||
| CK-MB (U/L) >20 | 0.984 | 0.460–2.105 | 0.966 | |||
| HBDH (U/L) >182 | 1.739 | 0.774–3.903 | 0.180 | |||
| NLR >median | 1.660 | 0.777–3.547 | 0.191 | |||
| β-block, n (%) | 0.868 | 0.412–1.830 | 0.711 | |||
| ACEI/ARB, n (%) | 1.114 | 0.505–2.458 | 0.790 | |||
| ARNI, n (%) | 0.458 | 0.170–1.233 | 0.017 | 0.510 | 0.167–1.559 | 0.011 |
| SGLT2i, n (%) | 0.715 | 0.283–1.803 | 0.477 | |||
| MRA, n (%) | 1.527 | 0.626–3.722 | 0.352 | |||
LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; BNP, brain natriuretic peptide; CPK, creatine phosphokinase; CK-MB, creatine kinase-MB; HBDH, hydroxybutyrate dehydrogenase; ARNI, angiotensin receptor-neprilysin inhibitor; SGLT2i, sodium-glucose cotransporter 2 inhibitor; MRA, mineralocorticoid receptor antagonist.
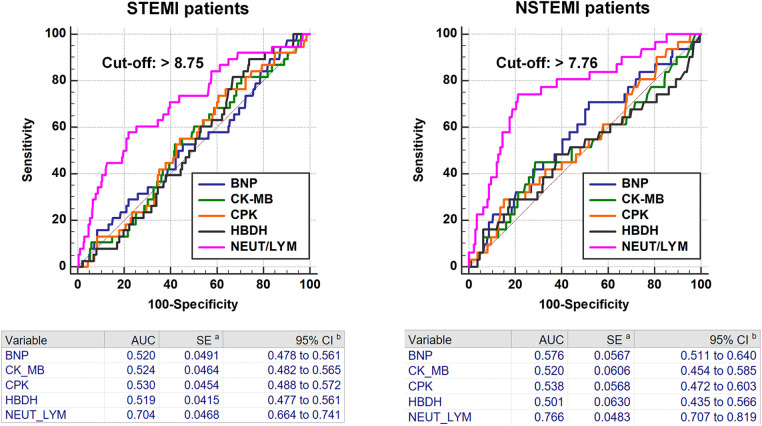
The ROC curve analyses indicated that NLR had areas under the curve of 0.704 (95% CI: 0.664–0.741, P = 0.002, cut-off value: >8.75) in STEMI patients and 0.766 (95% CI: 0.707–0.819, P < 0.001, cut-off value: >7.76) in NSTEMI patients for predicting in-hospital AHF occurrence (Figure 2).
Figure 2.
ROC curve analyses of BNP, CK-MB, CPK, HBDH and NLR for in-hospital AHF.
Finally, we investigated the association of the NLR and long-term AHF occurrence. As presented in Table 7, the prevalence of overall death, heart failure, non-fatal MI, non-fatal stroke and URR had no significant difference between the low and high NLR groups. Since NLR is a short-term inflammation indicator, we then analyzed the correlation between the NLR and AHF occurrence within 12 months of discharge. Similarly, there was no significant difference observed in overall mortality, heart failure, non-fatal MI, non-fatal stroke and URR between the low and high NLR groups during the 12-month period following discharge (Supplementary Table S1).
Table 7.
Adverse cardiovascular events during follow-up.
| Adverse Cardiovascular events | NLR <median | NLR >or = median | P value |
|---|---|---|---|
| MACE, n (%) | 64 (12.4) | 66 (11.8) | 0.799 |
| Overall death, n (%) | 4 (1.0) | 6 (1.0) | 0.968 |
| Heart failure, n (%) | 33 (4.7) | 37 (3.5) | 0.722 |
| Non-fatal MI, n (%) | 38 (5.2) | 43 (6.0) | 0.600 |
| Non-fatal stroke, n (%) | 8 (2.1) | 7 (1.0) | 0.825 |
| URR, n (%) | 55 (9.0) | 54 (10.3) | 0.751 |
MACE, major adverse cardiovascular events; MI, myocardial infarction; URR, unplanned repeat revascularization.
The MACE was defined as the composite of overall death, heart failure, non-fatal MI, non-fatal stroke, and URR.
Discussion
The study was to assess the association between NLR and in-hospital heart failure in AMI patients. The findings revealed that AHF patients had a significantly higher NLR with a higher incidence of arrhythmia compared to the non-AHF group. In addition, elevated NLR was independently associated with increased risk of in-hospital AHF and arrhythmia. NLR was predictive of in-hospital AHF in AMI patients, however, NLR was not a predictor of long-term heart failure in AMI patients.
Following myocardial infarction, necrosis of the myocardium can lead to an inflammatory storm that can further disrupt the homeostasis of the myocardial environment, potentially resulting in adverse cardiovascular events (9). In this context, the neutrophil-to-lymphocyte ratio is a readily available, inexpensive, and credible indicator of systemic inflammation, and thus has been evaluated in our study as a potential biomarker for predicting in-hospital and long-term cardiovascular outcomes.
Due to the standardization of clinical diagnosis and treatment, patients with suspected AMI are now subjected to laboratory investigations, including blood routine tests, myocardial enzyme profiling, and coagulation tests upon their initial admission to the emergency department. Furthermore, NLR has been confirmed to possess a good predictive value in evaluating the degree of sepsis and local inflammation (10, 11). Inflammatory markers, including NLR, are also influenced by the patient's hyperglycaemic state and that it also has an impact on long-term prognosis (12, 13). In this setting, NLR can be obtained at an early stage as an inflammatory indicator.
Inflammatory storms are triggered by various factors such as trauma, shock, infection, and injury (14). Firstly, mononuclear macrophages activate inflammatory cells by presenting antigens through natural immunity. These inflammatory cells then release cytokines, which enter the blood circulation and act on different cells including, white blood cells, red blood cells, platelets, and vascular endothelial cells (15, 16). This action leads to the production of platelet aggregation factor, prostaglandin, peroxidase synthase, leukotriene, nitric oxide, and other cytokines, which can increase the concentration of C-reactive protein, α2-macroglobulin, and fibrinogen while decreasing the concentration of albumin and transferrin (17, 18). Ultimately, these effects lead to high discharge and low resistance of the entire heart circulation, eventually resulting in heart failure (19, 20). Our study revealed a significant increase in NLR in the AHF group among STEMI and NSTEMI patients. Conversely, there was no significant difference in other clinical indicators between the AHF and non-AHF groups in STEMI patients. It is noteworthy that STEMI patients typically undergo emergency PCI surgery upon admission, which can offer substantial protection to the heart from injury. However, significant differences in many traditional indicators were observed between the AHF and non-AHF groups in the NSTEMI group. This could be attributed to the fact that NSTEMI patients usually have more diffuse coronary artery lesions, and interventional treatment is usually elective surgery, leading to more severe inflammation and myocardial injury in patients with AHF. Multivariate logistic regression and ROC analyses suggested that a high NLR is associated with an increased risk of in-hospital AHF. Based on the data obtained from this study, it can be concluded that a high NLR can predict the occurrence of in-hospital AHF to a certain extent.
In this study, we analyzed the predictive value of NLR in arrhythmia. Logistic regression analysis showed that a high NLR is an independent predictor of arrhythmia in STEMI patients during hospitalization. We also observed that the AHF group had a high proportion of patients who have arrhythmia in STEMI and NSTEMI patients. Arrhythmia can lead to hemodynamic disorders and exacerbate cardiac workload. In addition, the occurrence of AHF activates the sympathetic nervous system in vivo, which triggers abnormal pacemakers in the heart cavity (21). Therefore, a reciprocal cause-and-effect relationship between NLR and arrhythmia has been reported in several studies. Elevated NLR significantly increases the risk of left atrial thrombosis in non-valvular atrial fibrillation patients, where NLR is an independent risk factor. Moreover, NLR is an independent predictor of long-term outcomes in patients with atrial fibrillation (AF) (22)., while a high NLR is associated with an increased risk of new-onset AF (23). Noteworthy, the small sample size in this study may have contributed to the lack of statistical significance in the predictive value of NLR in patients with NSTEMI.
The primary purpose of this study was to investigate the effect of SGLT2i on the risk of cardiovascular and cerebrovascular adverse events in patients with AMI. Therefore, we extracted the health and medical information of patients after discharge. The protective role of SGLT2i on in-hospital outcome in AMI patients was consistent with the previous studies (24–26). In this study, there was no statistically association between the NLR ratio and the incidence of long-term heart failure. This is likely due to the fact that the NLR data were obtained from patients admitted to the emergency department and is indicative of short-term inflammation levels. Clinical cohort studies examining the association between NLR and long-term outcomes in AMI patients may provide valuable insights into the management of these patients.
Limitations
This single-center, retrospective study, was limited by a potential bias caused by confounding factors. However, univariate and multivariate logistic regression analyses were employed to mitigate the interference of such confounding factors. Besides, the results may not be generalizable to populations of different ethnic backgrounds and over 80 years of age. The small sample size is also a limitation.
Conclusion
Our study found that a high NLR was independently related with an increased risk of in-hospital AHF and arrhythmia in patients with acute myocardial infarction. Furthermore, NLR was found to be a predictive marker for in-hospital AHF in AMI patients. However, a high NLR could not predict long-term heart failure in AMI patients. These findings provide further evidence for the relationship between NLR and the prognosis of AMI patients, which is expected to play a role in the clinical management of AMI patients.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the Changzhou Health and Wellness Committee Research Project (CQ20210122), Changzhou Sci&Tech Program (CZ20230023), and Nanjing Medical University Intensive Construction Special Nursing Advantage Discipline (Yan Shao).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Clinical Investigation of The Affiliated Changzhou No.2 People's Hospital of Nanjing Medical University (KY314-01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JZ: Writing – original draft, Writing – review & editing. RY: Data curation, Writing – original draft, Writing – review & editing. YZ: Writing – review & editing, Writing – original draft, Data curation. YS: Data curation, Writing – original draft. YJ: Supervision, Writing – original draft. FW: Supervision, Writing – original draft, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1275713/full#supplementary-material
References
- 1.Song L, Zhao X, Chen R, Li J, Zhou J, Liu C, et al. Association of PCSK9 with inflammation and platelet activation markers and recurrent cardiovascular risks in STEMI patients undergoing primary PCI with or without diabetes. Cardiovasc Diabetol. (2022) 21(1):80. 10.1186/s12933-022-01519-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji Z, Liu G, Guo J, Zhang R, Su Y, Carvalho A, et al. The neutrophil-to-lymphocyte ratio is an important indicator predicting in-hospital death in AMI patients. Front Cardiovasc Med. (2021) 8:706852. 10.3389/fcvm.2021.706852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khalil C, Pham M, Sawant AC, Sinibaldi E, Bhardwaj A, Ramanan T, et al. Neutrophil-to-lymphocyte ratio predicts heart failure readmissions and outcomes in patients undergoing transcatheter aortic valve replacement. Indian Heart J. (2018) 70(Suppl 3):S313–8. 10.1016/j.ihj.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sasmita BR, Zhu Y, Gan H, Hu X, Xue Y, Xiang Z, et al. Prognostic value of neutrophil-lymphocyte ratio in cardiogenic shock complicating acute myocardial infarction: a cohort study. Int J Clin Pract. (2021) 75(10):e14655. 10.1111/ijcp.14655 [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Wang J, Cao D, Han L. Correlation of neutrophil-to-lymphocyte ratio with the prognosis of non-ST-segment elevation in patients with acute coronary syndrome undergoing selective percutaneous coronary intervention. J Int Med Res. (2020) 48(10):300060520959510. 10.1177/0300060520959510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y, Zhang JL, Jin H, Ji Y, Wang FF. The effect of SGLT2i on in-hospital acute heart failure risk in acute myocardial infarction patients-a retrospective study. Front Cardiovasc Med. (2023) 10:1158507. 10.3389/fcvm.2023.1158507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Y, Zhang JL, Yan XJ, Sun L, Ji Y, Wang FF. Effect of dapagliflozin on the prognosis of patients with acute myocardial infarction undergoing percutaneous coronary intervention. Cardiovasc Diabetol. (2022) 21(1):186. 10.1186/s12933-022-01627-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu HH, Cao YX, Li S, Guo YL, Zhu CG, Wu NQ, et al. Impacts of prediabetes mellitus alone or plus hypertension on the coronary severity and cardiovascular outcomes. Hypertension (Dallas, Tex: 1979). (2018) 71(6):1039–46. 10.1161/HYPERTENSIONAHA.118.11063 [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Rong H, Wu W, Huang T, Xu J. Application value of NT-proBNP combined with NLR in evaluation of Major adverse cardiac events in elderly patients with chronic heart failure. Emerg Med Int. (2022) 2022:3689445. 10.1155/2022/3689445 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Li D, Li J, Zhao C, Liao X, Liu L, Xie L, et al. Diagnostic value of procalcitonin, hypersensitive C-reactive protein and neutrophil-to-lymphocyte ratio for bloodstream infections in pediatric tumor patients. Clin Chem Lab Med. (2023) 61(2):366–76. 10.1515/cclm-2022-0801 [DOI] [PubMed] [Google Scholar]
- 11.Karadeniz M, Ser MH, Nalbantoglu M, Tumay FB, Yilmaz N, Acikgoz S, et al. Neutrophil-to-lymphocyte ratio as a marker of inflammation in restless legs syndrome during pregnancy. Bratisl Lek Listy. (2023) 124(1):42–6. 10.4149/BLL_2023_006 [DOI] [PubMed] [Google Scholar]
- 12.Paolisso P, Foà A, Bergamaschi L, Donati F, Fabrizio M, Chiti C, et al. Hyperglycemia, inflammatory response and infarct size in obstructive acute myocardial infarction and MINOCA. Cardiovasc Diabetol. (2021) 20(1):33. 10.1186/s12933-021-01222-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paolisso P, Foà A, Bergamaschi L, Angeli F, Fabrizio M, Donati F, et al. Impact of admission hyperglycemia on short and long-term prognosis in acute myocardial infarction: MINOCA versus MIOCA. Cardiovasc Diabetol. (2021) 20(1):192. 10.1186/s12933-021-01384-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu LL, Skribek M, Harmenberg U, Gerling M. Systemic inflammatory syndromes as life-threatening side effects of immune checkpoint inhibitors: case report and systematic review of the literature. J Immunother Cancer. (2023) 11(3):e005841. 10.1136/jitc-2022-005841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lauritano D, Mastrangelo F, D'Ovidio C, Ronconi G, Caraffa A, Gallenga CE, et al. Activation of mast cells by neuropeptides: the role of pro-inflammatory and anti-inflammatory cytokines. Int J Mol Sci. (2023) 24(5):4811. 10.3390/ijms24054811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ain QU, Sarfraz M, Prasesti GK, Dewi TI, Kurniati NF. Confounders in identification and analysis of inflammatory biomarkers in cardiovascular diseases. Biomolecules. (2021) 11(10):1464. 10.3390/biom11101464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silveira Rossi JL, Barbalho SM, Reverete de Araujo R, Bechara MD, Sloan KP, Sloan LA. Metabolic syndrome and cardiovascular diseases: going beyond traditional risk factors. Diabetes Metab Res Rev. (2022) 38(3):e3502. 10.1002/dmrr.3502 [DOI] [PubMed] [Google Scholar]
- 18.Hanna A, Frangogiannis NG. Inflammatory cytokines and chemokines as therapeutic targets in heart failure. Cardiovasc Drugs Ther. (2020) 34(6):849–63. 10.1007/s10557-020-07071-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Buono MG, Moroni F, Montone RA, Azzalini L, Sanna T, Abbate A. Ischemic cardiomyopathy and heart failure after acute myocardial infarction. Curr Cardiol Rep. (2022) 24(10):1505–15. 10.1007/s11886-022-01766-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davison BA, Takagi K, Edwards C, Adams KF, Jr., Butler J, Collins SP, et al. Neutrophil-to-Lymphocyte ratio and outcomes in patients admitted for acute heart failure (as seen in the BLAST-AHF, Pre-RELAX-AHF, and RELAX-AHF studies). Am J Cardiol. (2022) 180:72–80. 10.1016/j.amjcard.2022.06.037 [DOI] [PubMed] [Google Scholar]
- 21.Chyou JY, Barkoudah E, Dukes JW, Goldstein LB, Joglar JA, Lee AM, et al. Atrial fibrillation occurring during acute hospitalization: a scientific statement from the American heart association. Circulation. (2023) 147(15):e676–98. 10.1161/CIR.0000000000001133 [DOI] [PubMed] [Google Scholar]
- 22.Wu S, Yang YM, Zhu J, Ren JM, Wang J, Zhang H, et al. Impact of baseline neutrophil-to-lymphocyte ratio on long-term prognosis in patients with atrial fibrillation. Angiology. (2021) 72(9):819–28. 10.1177/00033197211000495 [DOI] [PubMed] [Google Scholar]
- 23.Berkovitch A, Younis A, Grossman Y, Segev S, Kivity S, Sidi Y, et al. Relation of neutrophil to lymphocyte ratio to risk of incident atrial fibrillation. Am J Cardiol. (2019) 123(3):396–401. 10.1016/j.amjcard.2018.10.036 [DOI] [PubMed] [Google Scholar]
- 24.Paolisso P, Bergamaschi L, Santulli G, Gallinoro E, Cesaro A, Gragnano F, et al. Infarct size, inflammatory burden, and admission hyperglycemia in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: a multicenter international registry. Cardiovasc Diabetol. (2022) 21(1):77. 10.1186/s12933-022-01506-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cesaro A, Gragnano F, Paolisso P, Bergamaschi L, Gallinoro E, Sardu C, et al. In-hospital arrhythmic burden reduction in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: insights from the SGLT2-I AMI PROTECT study. Front Cardiovasc Med. (2022) 9:1012220. 10.3389/fcvm.2022.1012220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paolisso P, Bergamaschi L, Gragnano F, Gallinoro E, Cesaro A, Sardu C, et al. Outcomes in diabetic patients treated with SGLT2-inhibitors with acute myocardial infarction undergoing PCI: the SGLT2-I AMI PROTECT registry. Pharmacol Res. (2023) 187:106597. 10.1016/j.phrs.2022.106597 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.