Abstract
Objectives
Multiparametric magnetic resonance imaging (mpMRI) surveillance post focal cryotherapy (FT) of prostate cancer is challenging as post treatment artefacts alter mpMRI findings. In this initial experience, we assessed diagnostic performance of mpMRI in detecting clinically significant prostate cancer (csPCa) after FT.
Materials and methods
This single-centre phase II prospective clinical trial recruited 28 men with localized csPCa for FT between October 2019 and April 2021. 12-months post FT mpMRI were performed prior to biopsy and sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of all mpMRI positive subjects were analysed. Chi square goodness of fit test correlated biopsy positive PIRADS3 (P3) and PIRADS4/5 lesions with histology grade group. One way ANOVA test assessed performance of ADC values in differentiating csPCa, non csPCa and benign lesions.
Results
Sensitivity, specificity, PPV and NPV of mpMRI were 100%, 14.28%, 53.84% and 100% for subjects with histologically proven cancer. Correlation of PIRADS v2.1 scores with histologically proven prostate cancer was statistically significant (p < 0.5). Correlation of P3 lesions with non-csPCa was statistically significant (p < 0.02535). Higher ADC value was associated with benign histology (adjusted odds ratio OR 0.97, 95% confidence interval: 0.94, 0.99) (p = 0.008). Among the malignant lesions, higher ADC value was associated with non-csPCa (adjusted OR: 0.97; 95% CI: 0.95, 0.99) (p = 0.032).
Conclusion
mpMRI is highly sensitive in detecting residual cancer. ADC values and PIRADS scores may be of value in differentiating csPCa from non-csPCa with a potential for risk stratification of men requiring re-biopsy versus non-invasive surveillance of remnant prostate.
keywords: Prostate, Prostate cancer, Multiparametric MRI, Focal cryotherapy, Prostate cancer recurrence
1. Introduction
Low risk prostate cancer patients with predominantly grade group 1 disease may be closely monitored with active surveillance strategies [1]. However, for intermediate and high risk prostate cancer, whole gland therapy including radical prostatectomy or radiation with or without systemic therapy remains the standard of care. Whole gland therapy, unfortunately, is associated with significant debilitating morbidities affecting the quality of life of these patients which include urinary incontinence, erectile dysfunction and impotence[2], [3]. Focal therapy of prostate cancer provides a promising middle ground for intermediate and high risk localized prostate cancer where urologists and radiologists may treat the portion of prostate gland harboring clinically significant prostate cancer and actively monitor the rest of the gland for potential recurrence.
Cryotherapy, though originally used for whole gland treatment, has recently been adopted for focal cryotherapy (FT) [4]. Contrary to focal therapy techniques which employ thermal energy or electric currents to destroy cancer cells, cryotherapy treats cancer by freeze and thaw cycles to induce cell death. Cancers are treated by several cycles of rapid freezing and thawing with needles placed into the prostate via a transperineal approach [5], [6]. Cryotherapy induces coagulative necrosis within the gland, drastically altering the gland architecture and mpMRI findings, confounding the analysis [7]. Therapy induces changes in the prostate may overlap with tumor appearance [8]. Tokuda et al. reported on time course changes in mpMRI in a small cohort of 16 patients following focal cryotherapy and found dynamic signal changes around the ablation sites in all sequences especially in diffusion weighted imaging (DWI) and dynamic contrast enhanced MRI (DCE MRI) which significantly improved 11–17 months post treatment [9]. It is therefore important to consider the post treatment effects on surveillance mpMRI in determining adequate treatment response and assessment of possible recurrence. Unlike radical prostatectomy where histopathology assessment of resected surgical specimens provides definitive status of treatment margins, imaging surveillance offers assessment of treatment efficacy after cryotherapy as it allows visualization of post treatment changes and evaluation of recurrence. As mpMRI is extremely important in detection of recurrent tumor, an integrated approach incorporating mpMRI for imaging surveillance has been recommended and is a crucial tool in determining treatment outcome post FT [10].
In this initial experience, we report our findings of surveillance mpMRI performed one year after FT in a cohort of patients with intermediate and high risk prostate cancer recruited in a single-centre phase II prospective clinical trial. Using PIRADS v2.1 criteria, we assess the diagnostic performance of mpMRI in detecting clinically significant prostate cancer in the remnant prostate after ablative treatment, using transperineal MRI-ultrasound fusion targeted and systematic biopsy as the reference standard.
2. Materials and methods
This single-arm phase II prospective clinical trial received IRB approval and enrolled patients with localized, non-metastatic clinically significant prostate cancer (csPCa) for focal cryotherapy between October 2019 and April 2021. Written informed consent was obtained from all participants.
The sample size was estimated based on the primary outcome of the trial to determine the change in major functional domains after using EPIC questionnaire. Based on this, a sample size of 24 participants would need to be recruited to detect a mean difference of 5 points with a standard deviation of 6.7 in each EPIC functional domain using a paired t-test at an alpha of 0.0125 (Bonferroni correction factor of 4 to account for potential multiple intragroup comparisons to baseline) and a power of 80%. To account for 20% attrition, 28 participants were recruited.
All 28 participants underwent pre-treatment 3 T mpMRI of the prostate which were interpreted by a abdominal radiologists with 3–8 years’ of experience in reading MRI prostate using PIRADS v2.1 criteria. A maximum of 4 lesions were identified for each participant and each lesion was graded and assigned a lesion specific overall PIRADS v2.1 category. All lesions assigned PIRADS v2.1 category ≥ 3 were marked for transperineal MRI-TRUS fusion targeted and systematic biopsy. The mpMRI findings and biopsy outcomes were reviewed by a multi-disciplinary team (MDT) comprising radiologist, pathologist and treating urologist to determine eligibility. Two recent expert consensus on patient selection were used to develop our inclusion criteria [11], [12]. We included patients with PSA level < 20 ng/ml, biopsy prognostic grade GG≤ 4, mpMRI demonstrating localized tumor with no extraprostatic extension and mpMRI determined index lesion volume of ≤ 3 ml for single lesion or ≤ 1.5 ml each for multiple lesions.
At 12 months post-cryotherapy, all participants underwent post FT surveillance mpMRI followed by transperineal MRI-TRUS fusion targeted and systematic prostate re-biopsy within 1 month, comprising of (1) targeted biopsy over the previous ablation sites, (2) targeted biopsy of PIRADS v2.1 category ≥ 3 lesions detected on post treatment mpMRI in the untreated gland and (3) systematic saturation biopsy of the remaining prostate. Biopsy was performed by a single experienced urologist with 8 years’ experience in performing transperineal MRI-TRUS fusion biopsy. Targeted cores over the ablation sites and lesions were obtained followed by systematic saturation biopsy.
The current study represents primary analysis of mpMRI findings at 12 months post FT surveillance of the treatment zone and remnant prostate prior to fusion targeted and saturation biopsy. Fig. 1 outlines the study flowchart.
Fig. 1.
Flowchart of selection criteria and study participation. csPCa – clinically significant prostate cancer; mpMRI – multiparametric magnetic resonance imaging.
2.1. Multiparametric MRI data acquisition
All 28 participants underwent high field mpMRI examination without an endorectal coil on a 3-Tesla MRI (Magnetom Skyra; Siemens, Erlangen, Germany) with a 60-channel pelvic phased array coil one year post focal cryotherapy. The image acquisition protocols are shown in the Table 1. For DWI, all 4 B-values (0–50, 500,1000, 1800 s/mm2) were acquired and ADC mapping was acquired with all available B-values. All participants received gadoterate meglumine (DOTAREM ®, Guerbet KKC, Bloomington, IN, USA) as intravenous contrast, injected at a dose of 0.1 mmol/kg body weight at a rate of 2–3 ml/sec, followed by a 20 ml of saline flush.
Table 1.
IMAGING PARAMETERS.
| Pulse sequence | Plane |
TR/TE (ms) |
FOV (cm) |
Matrix | Slice thickness | Additional parameters |
|---|---|---|---|---|---|---|
| T2W - TSE | Axial, sagittal & coronal | 3650/100 | 20 | 320 × 320 | 3 mm, No interslice gap |
|
| DWI - EPI Zoomit | Axial | 6200/92 msec | 20 | 122 × 122 | 3 mm, no interslice gap | b-values 0–50, 500,1000, 1800 s/mm2 |
| DCE – VIBE DIXON TWIST | Axial | 3.81/1.24, 2.47 | 26 | 192 × 192 | 3 mm | 3.5–5 s/acquisition |
TE – echo time; TR – repetition time; FoV – field of view; T2W – T2 weighted imaging; TSE – turbo spin echo; EPI – echo planar imaging; DWI – diffusion weighted imaging; DCE – dynamic contrast enhanced imaging; VIBE DIXON TWIST - volumetric interpolated breath-hold examination time-resolved imaging with interleaved stochastic trajectories.
3. Image interpretation
The mpMRI images were reviewed on a commercial PACS workstation (Carestream, Rochester, NY, USA) by a single senior genitourinary radiologist with 8 years of experience in reading prostate mpMRI (average 500–600 mpMRI a year). All post FT mpMRI examinations were prospectively interpreted prior to biopsy. T2W (T2 weighted imaging), diffusion weighted imaging (DWI) with apparent diffusion coefficient (ADC) map and dynamic contrast enhanced (DCE) sequences were assessed scoring each lesion according to PI-RADS v2.1 criteria. ADC values of all lesions assigned PIRADS v2.1 category ≥ 3 were also prospectively obtained with small circular region of interest (ROI) prior to biopsy.
4. Data analysis
mpMRI lesions assigned PIRADS v2.1 scores of < 2 (≤P2) were designated negative and scores of 3 (P3), 4 (P4) and 5 (P5) were designated positive on mpMRI. P3 was considered low score whilst P4 and P5 were considered high scores. Participants with at least one lesion assigned ≥ P3 were designated mpMRI positive. Each mpMRI positive participant may have one or more lesions scored ≥ P3.
All 12 month post FT surveillance mpMRI findings and re-biopsy outcomes were reviewed at MDT to determine radiological-pathological concordance. A lesion is deemed concordant when a positive biopsy core was present within the MRI detected lesion or within a margin of 2.5 mm around the MRI detected lesion. All lesions scored ≥ P3 with concordant positive histological diagnosis of carcinoma on targeted biopsy were designated true positive whilst those with negative histological diagnosis were designated false positive. Carcinoma detected in systematic biopsy cores but deemed MRI occult during MDT review were designated false negative.
All biopsy cores positive for carcinoma were further categorised into clinically significant prostate cancer (csPCa) and non-clinically significant prostate cancer (non-cs PCa) based on International Society of Urological Pathology Grade Group (ISUP GG) prostate cancer grading system. All ISUP GG 1 lesions were deemed non clinically signiifcant prostate cancer and all ISUP GG ≥ 2 lesions were deemed clinically signiifcant prostate cancer (cs PCa).
The sensitivity, specificity of all mpMRI positive participants and mpMRI positive lesions were calculated. In addition, positive predictive value (PPV) and negative predictive value (NPV) of mpMRI positive participants was calculated. We correlated PI-RADS score for every lesion with biopsy findings.
Subanalysis was then performed to assess correlation of high PI-RADS scores (P4 and P5) with csPCa and low PI-RADS scores (P3) with non csPCa. Chi square test was done to determine the relationship between PI-RADS scores and biopsy findings. Chi square goodness of fit test was done to determine whether the true positive P3 lesions are likely to be non csPCa or not, likewise true positive P4/5 lesions are likely to be csPCa or not. The sensitivity, specificity, PPV and NPV for detecting csPCa was also calculated. Furthermore, one way ANOVA test was done to compare the mean ADC value among benign, non csPCa and csPCa groups. We conducted all analyses in STATA version 14.1, All tests were two-sided and used p < 0.05 as the level of statistical significance.
5. Results
5.1. Participant characteristics
The participant characteristics are shown in Table 2. A total of 28 participants with csPCa underwent post FT mpMRI followed by biopsy. Median age of the participants was 70 (IQR+9). Twenty six participants had positive mpMRI lesions on post FT mpMRI of which histologically confirmed prostate cancer in remnant gland were present in 14 of 26 participants (53.8%) whilst the remainder 12 (46.2%) were negative for cancer in remnant gland. Two out of the total 28 participants had no lesion on postFT mpMRI and this was concordant with the negative targeted and systematic biopsy outcome in these two participants.
Table 2.
Participant characteristics.
| Age (Years, Median/IQR) | 70(9) |
|---|---|
| Pre-FT PSA (Mean/SD) | 8.4(3) |
| Post-FT PSA (Mean/SD) | 5.3(4) |
| ISUP grade group | |
| 2 (number of participants /percentage %) |
19 (67.9%) |
| 3 (number of participants/percentage %) |
5 (17.8%) |
| 4 (number of participants/percentage %) | 4 (14.3%) |
| Positive post FT mpMRI (number of participants/percentage %) |
26 (100%) |
| Benign | 12 (46.2%) |
| Non-csPca | 8 (30.8%) |
| csPca | 6 (23%) |
| Negative post FT mpMRI (number of participants/percentage %) |
2 (100%) |
| Benign | 2 (100%) |
| Malignant | 0 |
| Lesions identified on post FT mpMRI (number of lesions/percentage %) | 55 (100%) |
| Benign | 32 (58.2%) |
| Non-csPca | 13 (23.6%) |
| Cs-Pca | 10 (18.2%) |
5.2. Participant-wise diagnostic performance of mpMRI post FT
For detection of histologically confirmed cancer in residual prostate 12 months post FT, the sensitivity, specificity, PPV and NPV were 100%, 14.28%, 53.84% and 100% respectively.
5.3. Lesion-wise performance of post FT mpMRI
Of the 26 post FT mpMRI postive participants, there were a total of 55 positive lesions (≥ P3) detected on mpMRI of which 23 of 55 lesions (41.8%) correlated with biopsy cores positive for cancer whilst 32 of 55 lesions (58.2%) were negative for cancer on biopsy. Sensitivity of post FT mpMRI to detect prostate cancer in residual prostate was calculated at 92%.
5.4. Leison-wise correlation of PI-RADS V2.1 scores and biopsy diagnosis
Of the 55 positive post FT mpMRI lesions, 24 of 55 lesions (43.6%) were scored P3 and 29 of 55 lesions (52.7%) were scored P4 and 2 of 55 were scored P5 (3.7%). Of the 24 P3 lesions, 5 lesions were positive on biopsy (20.83%) and of the 31 P4 and P5 lesions (P4 + P5), a total of 18 lesions (17 +1) were postive on biopsy (58.06%) (p < 0.5). Lesion-wise correlation of PI-RADS v2.1 scores and biopsy diagnosis is shown in Table 3. A few case examples of post FT mpMRI are shown in Fig. 2, Fig. 3, Fig. 4.
Table 3.
Correlation of post focal cryotherapy (FT) mpMRI positive lesions with biopsy diagnosis of all lesions.
| PI-RADS v2.1 score | Post FT mpMRI lesions | Negative biopsy (false positive) |
Positive biopsy (true positive) |
Aggressiveness of true positive lesions (csPCa vs Non csPCa) |
|---|---|---|---|---|
| P3 | 24 | 19 (79.17%) | 5 (20.83%) | csPCa – 0 Non csPCa – 5 (100%) (p < 0.02535) |
| >P3 (P4 + P5) | 31 | 13 (41.4%) | 18 (58.6%) | csPCa – 10 (55.6%) (p < 0.63735) Non csPCa – 8 (44%) |
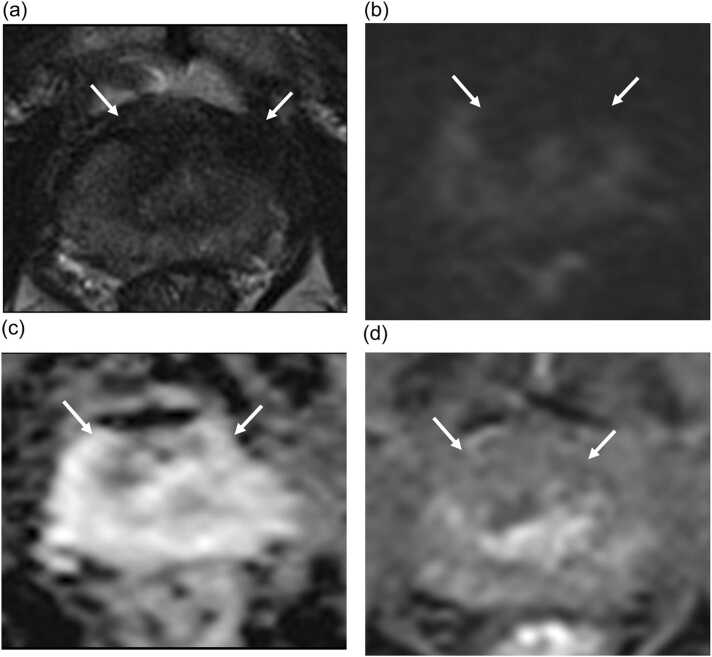
Fig. 2.
mpMRI of a 78 year old participant at 12 months post focal cryotherapy follow up. Anterior cryoablation was performed for Gleason 3 + 4 adenocarcinoma at midline anterior transition zone. Ablation site demonstrates scarring and capsular retraction on T2W image (arrow in A), no restriction diffusion on DWI/ADC images (arrows in B and C) and no enhancement on DCE image (arrow in D). Post ablation targeted biopsy of ablation site and systematic biopsy show no residual carcinoma.
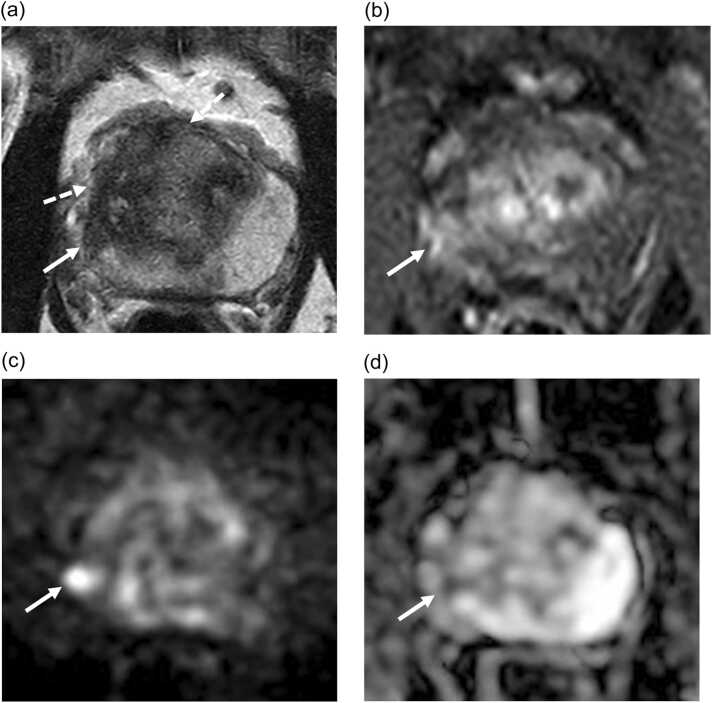
Fig. 3.
mpMRI of a 62 year old participant at 12 months post focal cryotherapy follow up demonstrating a PIRADS 4 lesion at periablation zone at the right posterolateral peripheral zone at the base of the gland seen as a focal T2W hypointensity (arrow in A) with early enhancement (arrow in B), high signal on DWI (arrow in C) and low signal on ADC map (arrow in D). This lesion was positive for cancer on biopsy (Gleason 4 +4). Area of ablation is seen as decreased T2W signal (dashed arrows) with no associated early enhancement or restricted diffusion.
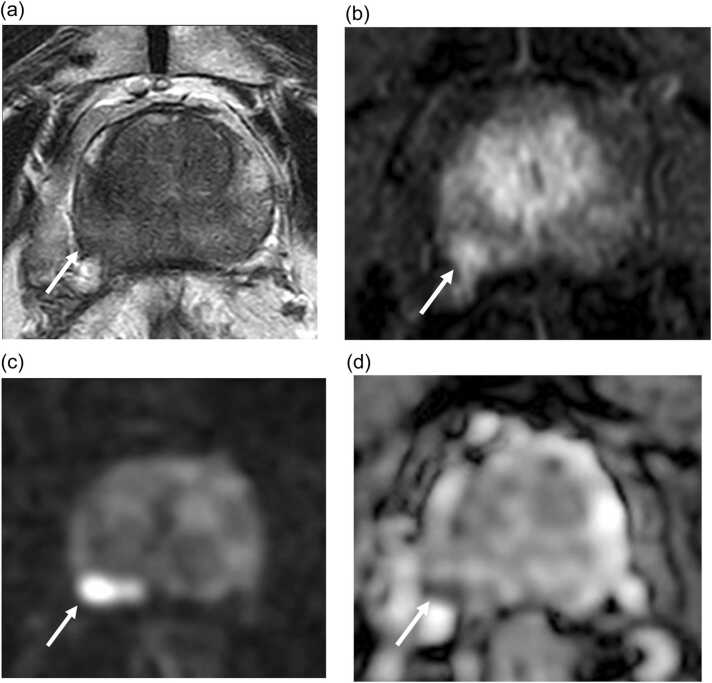
Fig. 4.
mpMRI of a 77 year old participant at 12 months post focal cryotherapy for ablation of Gleason 3 + 4 adenocarcinoma at right posterior peripheral zone demonstrates a PIRADS 5 lesion at ablation zone seen as a focal T2W hypointensity with capsular irregularity (arrow in A) with early enhancement (arrow in B), high signal on DWI (arrow in C) and low signal on ADC map (arrow in D). This lesion was positive for cancer on biopsy (Gleason 3 +4), in keeping with infield recurrence.
5.5. Post FT mpMRI performance to distinguish clinically significant prostate cancer (csPCa) and non-clinically significant prostate cancer (non csPCa)
All of the 5 true positive P3 lesions were non csPCa (100%) (p < 0.02535). Of the 18 true positive P4 and P5 lesions, 10 lesions were csPCa (55.56%) (p < 0.63735). For detection of csPCa, the sensitivity, specificity, PPV and NPV were 78.3%, 59.38%, 58.1% and 79.2% respectively.
The mean ADC values of benign group (901.7 ± 200.6) was significantly higher than non-csPCa (733.2 ± 91.3) and csPCa (589.1 ± 50.8 (p < 0.001). In the multivariate logistic analysis, we observed that higher ADC value was associated with benign histology (adjusted odds ratio OR 0.97, 95% confidence interval: 0.94, 0.99) (p = 0.008). Among the malignant lesions, higher ADC value was associated with non-csPca (adjusted OR: 0.97; 95% CI: 0.95, 0.99) (p = 0.032).
6. Discussion
There has been growing interest in targeting only the cancerous area of the prostate using FT. However, there is still a lack of evidence guiding the use of imaging and biopsy in post-FT surveillance. International consensus have recommended that at minimum, mpMRI together with fusion and systematic biopsies should be performed 6–12 months after FT [13], [14]. However, few published studies exist in which the diagnostic performance of surveillance mpMRI and PIRADS v2.1 post FT were correlated with MRI-TRUS fusion targeted and systematic saturation biopsy as reference standard. To our knowledge, this is the first such prospective study in a phase II clinical trial.
As many as one in five post focal therapy biopsies are positive yet no consensus nor guidelines exist in published literature for the interpretation of surveillance mpMRI post ablation [15]. Prostate Imaging for Recurrence Reporting (PI-RR), a standardized algorithm by a panel of international experts for assessing local recurrence at mpMRI after whole gland therapy, was recently published [16]. This is the first published algorithm proposed for mpMRI assessment of recurrent prostate cancer following whole gland radiation therapy or radical prostatectomy and is not designed for assessment after focal treatment. This algorithm, therefore, has limited value in our cohort as significant “normal” untreated prostate tissue remained after focal therapy.
Application of PIRADs v2.1, developed for assessment of lesions detected on mpMRI in treatment naïve patients, is controversial in post FT mpMRI. In a recent study assessing oncologic and safety outcomes of cryotherapy for partial gland ablation of prostate cancer, PI-RADS criteria was used in interpretation of post treatment mpMRI due to lack of consensus guidelines and literature gap in post focal treatment mpMRI [17]. As FT preserves significant untreated prostate tissue, we applied PIRADS v2.1 in our interpretation of post FT mpMRI. Recently Giganti et al. proposed a novel scoring system for prostate imaging after focal ablation therapy and we endeavor to apply the scoring system in our future studies [18].
Our results demonstrated high sensitivity of post FT mpMRI for cancer detection with sensitivity of 100% and 92% for per participant and per lesion analyses respectively. Specificity of mpMRI in our post FT cohort was low at 14.82%. We adopted a conservative approach in our initial experience interpreting post focal cryotherapy mpMRI and equivocal lesions were assigned P3 to ensure identification of all possible cancers within the remnant prostate for accurate assessment of oncological efficacy. This approach explains the high sensitivity and low specificity. Not surprisingly, only 20.83% of P3 lesions were biopsy proven positive cancers (all were non-csPCa) whilst the percentage of biopsy positive cancers was higher (58.06%) in the P4/5 group. We believed this comprehensive post-treatment mpMRI interpretation and biopsy protocol allowed us to ascertain the short-term treatment efficacy of FT as accurately as practically possible. A recent review of equivocal PIRADS 3 lesions by Schoots et al. reported a wide variation in prevalence of csPCa, depending on previous biopsy status; higher prevalence in men with 1st biopsies (21%) compared to men with previous negative biopsies and active surveillance biopsies (16–17%) [19]. Similarly, in our post treatment and post biopsy cohort, no csPCa was detected in all P3 lesions.
In a recent retrospective study of 75 patients who underwent surveillance mpMRI and MRI guided biopsy 1 year post focal cryotherapy by Baskin et al., the specificity of MRI to rule out residual disease was poor and 91.7% of patients with clinically significant prostate cancer (GG≥2 disease) had negative or low risk mpMRI findings (PI-RADS 1–3) [20]. However, radiologist experience in this study was unclear, mpMRI findings were extracted from medical records and were not re-reviewed by radiologists experienced in mpMRI interpretation, possibly accounting for the poor performance of mpMRI in the cohort. Moreover, detailed per lesion and per patient analysis of mpMRI finding was not performed. In contrast, PIRADS 3 mpMRI was designated positive in our study, resulting in a high proportion of positive mpMRI and all patients with residual csPCa were identified on surveillance mpMRI.
While a negative post treatment mpMRI is the best possible outcome post FT, recent published studies by Baskin et al. and Aker at al found significant csPCa in patients with negative post treatment mpMRI [17], [20]. Despite a single experienced genitourinary radiologist interpreting all post treatment mpMRI, almost 1 in 4 and 1 in 5 patients with negative mpMRI has csPCa at 6 months and 18 months post cryoablation respectively [17].
An equivocal lesion was assigned P3 in an attempt to avoid “missing” csPCa post focal treatment as we attempted to accurately establish the short term oncological outcome; this approach accounts for high prevalence of positive mpMRI. No established criteria exists for surveillance of majority of our patients with positive mpMRI post FT. We found statistically significant correlation between P3 lesions and non-csPCa as well as statistically significant correlation between mean ADC values and tumor grades. Our data suggests that active clinical surveillance with serum PSA and clinical assessment, may be appropriate in post FT participants with low PIRADS score lesions, with a view to repeat mpMRI when clinically necessary, potentially avoiding an invasive re-biopsy.
The positive correlation of mean tumor ADC values with csPCa renders ADC value a potentially valuable triage tool. In our study, ADC values were significantly lower in csPCa. Equivocal lesions with decreasing trend of ADC values on surveillance mpMRI may potentially be upgraded for targeted biopsy. P4/5 lesions with low ADC values may imply higher risk of residual csPCa, necessitating re-biopsy. Our findings in this initial experience with early post ablation mpMRI data in this small cohort are potentially invaluable in future development of active surveillance algorithm post FT.
The main strength of our study is the prospective nature of our data. A dedicated experienced uroradiologist prospectively interpreted all post-ablation mpMRI prior to repeat biopsy, eliminating inter-reader variability. We established the ground truth by targeting and correlating all P ≥ 3 lesions with histopathology obtained via transperineal MRI-TRUS targeted biopsy. MRI-ultrasound fusion registration has an estimated target registration error of 2.4–2.5 mm. As such, positive biopsy cores within 2.5 mm margin around the MRI detected lesion was deemed concordant [21]. Additionally, a saturation biopsy (>24 cores, adjusted to prostate volume) was performed in order to overcome any “miss” from the fusion biopsy and to assess the “normal” prostate on mpMRI. Third, all MRI-TRUS biopsies were performed by an experienced urologist and all biopsy cores were interpreted by a senior uropathologist with 9 years experience, eliminating inter-operator and inter-reader variability. The accuracy of targeted biopsy can be influenced by several factors including operator experience; this bias is significantly diminished in our cohort. MRI-TRUS targeted biopsy is the best reference standard achievable in this patient cohort who opted for focal ablation of prostate cancer. There is no perceived selection bias as all enrolled men underwent surveillance mpMRI followed by mandatory biopsy.
Our small sample size is a major limitation of our study as the study design was structured for the primary outcome of the trial to determine the functional quality-of-life related primary end point. We included all mpMRI positive lesions in our analysis, increasing our sample size for statistical analysis. A single experienced radiologist interpreting all post treatment mpMRI precludes assessment of inter-reader variability. Similarly, a single experienced radiologist reading all mpMRI and a single experienced urologist performing all fusion biopsies may limit the extrapolation of these findings to other institutions. Sampling errors and registration errors in MRI-TRUS biopsy are well described, affecting accuracy of targeted biopsy in small lesions [22]. Scarring, fibrosis, adhesions and gland distortion post focal therapy present new challenges to the urologist performing the biopsy. Sampling error in re-biopsy of post ablation cohort may therefore, potentially increase. Our findings will require further validation with longer term follow up in a larger cohort.
In conclusion, mpMRI is highly sensitive in detecting residual prostate cancer post focal cryotherapy in our initial experience. ADC values and PIRADS scores may be of value in differentiating csPCa from non-csPCa. In active surveillance post ablation, mpMRI may have important clinical applications, stratifying men with high risk lesions requiring re-biopsy from men with low risk lesions who may benefit from non-invasive imaging and clinical surveillance. This reduces unnecessary re-biopsy, reducing morbidity and health care costs, further improving quality of life in men with localised prostate cancer. Our results are potentially invaluable in future consensus development for post FT mpMRI and clinical surveillance.
Funding information
Dr KJ Tay received funding support from National Medical Research Council, Singapore.
Grant number - NCT04138914.
Ethical statement
This study received IRB approval (SingHealth 2018/2482).
CRediT authorship contribution statement
Jyothirmayi Velaga: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis. Kae Jack Tay: Writing – review & editing, Supervision, Methodology, Funding acquisition, Conceptualization. Guanqi Hang: Writing – review & editing, Methodology, Investigation, Formal analysis, Data curation. Yu Guang Tan: Writing – review & editing, Project administration, Funding acquisition. John SP Yuen: Writing – review & editing. Rajan T. Gupta: Investigation. Thomas J. Polascik: Investigation. Nye Thane Ngo: Writing – review & editing, Investigation. Yan Mee Law: Writing – review & editing, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Chen R.C., Rumble R.B., Loblaw D.A., et al. Active surveillance for the management of localized prostate cancer (cancer care Ontario Guideline): American society of clinical oncology clinical practice guideline endorsement. J. Clin. Oncol. 2016;34(18):2182–2190. doi: 10.1200/JCO.2015.65.7759. [DOI] [PubMed] [Google Scholar]
- 2.Ficarra V., Novara G., Rosen R.C., et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur. Urol. 2012;62(3):405–417. doi: 10.1016/j.eururo.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 3.Patient-Reported Outcomes Through 5 Years for Active Surveillance, Surgery, Brachytherapy, or External Beam Radiation With or Without Androgen Deprivation Therapy for Localized Prostate Cancer | Oncology | JAMA | JAMA Network. 〈https://jamanetwork.com/journals/jama/fullarticle/2758599〉. Accessed November 20, 2022. [DOI] [PMC free article] [PubMed]
- 4.Ahmed H.U., Freeman A., Kirkham A., et al. Focal therapy for localized prostate cancer: a phase I/II trial. J. Urol. 2011;185(4):1246–1254. doi: 10.1016/j.juro.2010.11.079. [DOI] [PubMed] [Google Scholar]
- 5.Gage A.A., Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37(3):171–186. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 6.Bozzini G., Colin P., Nevoux P., Villers A., Mordon S., Betrouni N. Focal therapy of prostate cancer: energies and procedures. Urol. Oncol. 2013;31(2):155–167. doi: 10.1016/j.urolonc.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Gaur S., Turkbey B. Vol. 56. Elsevier; 2018. Prostate MR Imaging for Posttreatment Evaluation and Recurrence; pp. 263–275. (Radiologic Clinics). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant K., Lindenberg M.L., Shebel H., et al. Functional and molecular imaging of localized and recurrent prostate cancer. Eur. J. Nucl. Med Mol. Imaging. 2013;40(Suppl 1):S48–S59. doi: 10.1007/s00259-013-2419-6. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokuda B., Yamada K., Takahata A., et al. Time-course changes in multiparametric magnetic resonance imaging following focal cryotherapy for localized prostate cancer: Initial experience. Eur. J. Radiol. 2023;160 doi: 10.1016/j.ejrad.2023.110714. [DOI] [PubMed] [Google Scholar]
- 10.Barret E., Harvey-Bryan K.-A., Sanchez-Salas R., Rozet F., Galiano M., Cathelineau X. How to diagnose and treat focal therapy failure and recurrence. Curr. Opin. Urol. 2014;24(3):241–246. doi: 10.1097/MOU.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 11.Tay K.J., Scheltema M.J., Ahmed H.U., et al. Patient selection for prostate focal therapy in the era of active surveillance: an International Delphi Consensus Project. Prostate Cancer Prostatic Dis. 2017;20(3):294–299. doi: 10.1038/pcan.2017.8. [DOI] [PubMed] [Google Scholar]
- 12.Scheltema M.J., Chang J.I., van den Bos W., et al. Preliminary diagnostic accuracy of multiparametric magnetic resonance imaging to detect residual prostate cancer following focal therapy with irreversible electroporation. Eur. Urol. Focus. 2019;5(4):585–591. doi: 10.1016/j.euf.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Tay K.J., Amin M.B., Ghai S., et al. Surveillance after prostate focal therapy. World J. Urol. 2019;37(3):397–407. doi: 10.1007/s00345-018-2363-y. [DOI] [PubMed] [Google Scholar]
- 14.Lebastchi A.H., George A.K., Polascik T.J., et al. Standardized nomenclature and surveillance methodologies after focal therapy and partial gland ablation for localized prostate cancer: an international multidisciplinary consensus. Eur. Urol. 2020;78(3):371–378. doi: 10.1016/j.eururo.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah T.T., Kasivisvanathan V., Jameson C., Freeman A., Emberton M., Ahmed H.U. Histological outcomes after focal high-intensity focused ultrasound and cryotherapy. World J. Urol. 2015;33(7):955–964. doi: 10.1007/s00345-015-1561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panebianco V., Villeirs G., Weinreb J.C., et al. Prostate magnetic resonance imaging for local recurrence reporting (PI-RR): international consensus -based guidelines on multiparametric magnetic resonance imaging for prostate cancer recurrence after radiation therapy and radical prostatectomy. Eur. Urol. Oncol. 2021;4(6):868–876. doi: 10.1016/j.euo.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Aker M.N., Brisbane W.G., Kwan L., et al. Cryotherapy for partial gland ablation of prostate cancer: Oncologic and safety outcomes. Cancer Med. 2023 doi: 10.1002/cam4.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giganti F., Dickinson L., Orczyk C., et al. Prostate imaging after focal ablation (PI-FAB): a proposal for a scoring system for multiparametric MRI of the prostate after focal therapy. Eur. Urol. Oncol. 2023;(23):00083–00084. doi: 10.1016/j.euo.2023.04.007. S2588-9311. [DOI] [PubMed] [Google Scholar]
- 19.Schoots I.G. MRI in early prostate cancer detection: how to manage indeterminate or equivocal PI-RADS 3 lesions? Transl. Androl. Urol. 2018;7(1):70–82. doi: 10.21037/tau.2017.12.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baskin A., Charondo L.B., Balakrishnan A., et al. Medium term outcomes of focal cryoablation for intermediate and high risk prostate cancer: MRI and PSA are not predictive of residual or recurrent disease. Urol. Oncol. 2022;40(10):451. doi: 10.1016/j.urolonc.2022.06.010. e15-451.e20. [DOI] [PubMed] [Google Scholar]
- 21.Hu Y., Ahmed H.U., Taylor Z., et al. MR to ultrasound registration for image-guided prostate interventions. Med. Image Anal. 2012;16(3):687–703. doi: 10.1016/j.media.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Meng X., Rosenkrantz A.B., Huang R., et al. The institutional learning curve of magnetic resonance imaging-ultrasound fusion targeted prostate biopsy: temporal improvements in cancer detection in 4 years. J. Urol. WoltersKluwer. 2018;200(5):1022–1029. doi: 10.1016/j.juro.2018.06.012. [DOI] [PubMed] [Google Scholar]