Abstract
Objective
To evaluate the efficacy and safety of electroacupuncture (EA) on ulcerative colitis (UC) and explore the influence of EA parameters and acupoint compatibility to optimize the clinical treatment plan.
Methods
After searching eight databases, data were extracted and analyzed to determine the quality and bias of the study's methodological design, and randomized controlled trial (RCT) datas were meta-analyzed.
Results
Twelve studies that meet the criteria were included. The results of meta-analysis indicated that, compared with the control group, experimental group had better clinical efficacy [RR = 1.27, 95%CI = (1.19, 1.36), P < 0.01], Other indicators such as cure rate [RR = 1.73, 95%CI = (1.43, 2.09), P < 0.01], effective rate of mucosal lesions under enteroscopy [RR = 1.24, 95%CI = (1.11, 1.38), P < 0.01], serum inflammatory factor TNF-α [MD = −41.11, 95%CI = (−46.01, 36.22), P < 0.01] were significantly better than those in the control group. Sixteen acupoints on the Ren, Bladder, Stomach, Spleen, and Liver meridians were used 74 times. RN4-ST25 is the most compatible acupoints.
Conclusion
The clinical efficacy of EA in treating UC is superior than the control group's, and it has curative effects in terms of cure rate, efficacy of mucosal lesions under colonoscopy, serum inflammatory factors, and Traditional Chinese Medicine (TCM) syndrome scores. Combining acupoints of the Bladder, Stomach, and Ren meridians and using dense wave for 30 min each time for more than 6 weeks may be optimal for UC patients.
Keywords: Electroacupuncture(EA), Ulcerative colitis(UC), Meta-analysis, Acupoints, Meridians
1. Introduction
Ulcerative colitis (UC) is a chronic, nonspecific inflammatory disease of the colon and rectum, notably between the colonic mucosa and submucosa [1]. It is characterized by diarrhea, mucopurulent blood, blood in the stool, and abdominal pain, with extra-intestinal manifestations affecting the mucous membranes, joints, eyes, lungs, and neurological system [1,2]. The peak age of UC onset is between 30 and 40 years, and the incidence of UC in men and women is approximately equal [[1], [2], [3]]. The pathophysiology of UC remains unknown; however, studies have shown that it is closely associated with genetic susceptibility, environmental factors, and immunological disorders [[2], [3], [4]]. Currently, the main clinical treatments for UC are medication control and surgery; however, both methods have certain limitations [5,6]. Medication is commonly used to control symptoms and slow the disease progression; however, it may have different effects owing to individual differences, while side effects and drug resistance are also limiting factors. Surgical treatment is usually the last resort; however, surgical risks and postoperative changes may also affect patients to varying degrees. Therefore, safer and more effective therapeutic options are urgently required. Recently, traditional Chinese acupuncture has gradually begun to be used in the treatment of UC because of its advantages of low cost and few side effects, as well as its anti-inflammatory and immunomodulatory functions at specific acupoints [7,8]. Electroacupuncture (EA) is an emerging non-invasive therapy that combines acupuncture with electrical stimulation. Compared with traditional acupuncture, EA can further enhance the therapeutic effect through electrical current stimulation. EA can also adjust the parameters of electrical current intensity, frequency, and waveform according to the patient's individual differences and conditions to achieve a personalized treatment plan. When combined with drugs, EA can improve the therapeutic efficacy while reducing the drug dosage and alleviating the side effects. Studies have shown that EA has good therapeutic efficacy for pain management, neurological disorders, gastrointestinal disorders, and other diseases [9]. Multiple studies have demonstrated that EA, alone or in conjunction with medicines, has a definite effect on UC [10,11]. However, most of these studies were performed as experiments on animals. There are few clinical reports in this area, and there are no evidence-based medical studies on EA intervention in UC. To offer an evidence-based foundation for the future promotion and implementation of EA in the treatment of UC, this study performed a meta-analysis of the clinical literature on EA for the treatment of UC and systematically evaluated its efficacy and safety.
2. Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed in the review process and analyses [12]. The study protocol was registered in PROSPERO (CRD42022362389).
2.1. Sources of studies and search methods
Computer searches were conducted using the China Knowledge Network Database (CNKI), Wanfang Database, China Biomedical Database (CBM), Vipers Journal Database (VIP), PubMed, Embase, Web of Science (WOS), and Cochrane Library databases. The retrieval period was from the establishment of the database to January 3, 2023. The most common method for searching for Chinese and English literary works is a combination of subject terms and free words. The Chinese search terms used “ulcerative colitis” and “electro-acupuncture” as the subject words; “ulceration,” “colitis,” and “ulcerative” were used as free words. The English search terms used “electroacupuncture,” “colitis, ulcerative,” and “ulcerative colitis” as subject headings and “colitis,” “inflammatory bowel disease,” “electric stimulation disease,” “electric stimulation therapy,” and “transcutaneous electric nerve stimulation” as free words to search for relevant literature.
2.2. Screening of selected studies
2.2.1. Inclusion criteria
(1) Research subjects: According to the criteria for diagnosing and treating UC or inflammatory bowel disease (IBD), patients with a confirmed diagnosis of UC can be of any race, nationality, age, sex, and Traditional Chinese Medicine (TCM) syndrome type, duration, or severity. (2) Research Type: Regardless of whether the blind method is stated in the literature, the study must be a published clinical randomized controlled trial (RCT) with complete experimental data. (3) Intervention: According to the literature, the experimental group's primary treatment strategies were EA or EA combined with additional therapies, whereas the control group's interventions included Western medicine. However, the treatment courses for the experimental and control groups were the same as those for the other fundamental intervention strategies. There were no constraints on the frequency of treatment methods or drug dosage between the two groups. (4) Outcomes: Clinical efficacy was the primary indicator, while the clinical cure rate, efficacy of mucosal lesions under colonoscopy, serum inflammatory factors, TCM syndrome scores, and incidence of adverse reactions were secondary outcome indicators.
2.2.2. Exclusion criteria
(1) Literature that is unpublished, repeatedly published, and difficult to access in full text or complete data. (2) Case studies, reviews, conference papers, retrospective research, and experiments involving animals and cells. (3) Literature on diseases with unclear diagnoses. (4) Non-RCTs or studies with multiple control groups. (5) Study participants and intervention strategies that did not meet the inclusion criteria. (6) Studies with different treatment courses for the experimental and control groups. (7) Literature with unclear outcome evaluation indicators.
2.2.3. Data extraction
The corresponding material was imported into the Endnote X9 software to eliminate duplicates. By examining titles and abstracts, the two researchers eliminated duplicate studies that were not discovered by the software, studies submitted in both Chinese and English for the same article, and studies that were clearly inconsistent with the research content. We downloaded the full texts of the remaining studies, read them carefully, and selected studies that were suitable for inclusion based on the previously defined inclusion and exclusion criteria. Finally, information on the first author, publication date, sample situation (number of cases, age, sex, and course of the disease), intervention situation (measures, intervention time, frequency, and course of treatment), and outcome indicators (total effective rate, cure rate, and adverse reactions) were extracted from the literature that met the inclusion criteria. During this procedure, any conflicts were handled through negotiation between the two investigators; if no agreement could be reached, a third investigator assisted in making the final determination.
2.3. Quality assessment
The Cochrane Risk of Bias Assessment Tool [13] was used to assess the methodological quality of the included studies. The modified Jadad scale [14] was then used to assign a quality score ranging from 1 to 7, with 4–7 points indicating high-quality research and 1–3 points indicating low-quality research. The evaluation method was initially reviewed and evaluated separately by two researchers and a third person assisted in resolving any discrepancies to establish a consensus.
2.4. EA parameters
The meridians of each acupoint were identified by counting the number of times the acupoints were mentioned in each study. The compatibility and frequency of use between the two acupoints in the prescription were summarized, and Gephi software was used to build a network interaction diagram to observe and analyze the degree of connection between the acupoints, thereby revealing the inherent rules of acupoint s matching.
2.5. Statistical methods
RevMan 5.3 and StataSE 15 software were used to conduct a meta-analysis of the included studies. First, we evaluated the heterogeneity of the included studies using the chi-square test and I2 statistic. Since there was no heterogeneity when P > 0.10 and I2 < 50%, the fixed effects model was used for meta-analysis. Heterogeneity was suggested with P ≤ 0.10 and I2 ≥ 50%. At this point, it is necessary to investigate the source of heterogeneity using a subgroup analysis or sensitivity analysis to eliminate the source of heterogeneity and perform a re-analysis or to directly utilize a random-effects model for analysis, depending on the actual circumstances. The standard mean difference (SMD) is typically employed as the statistic of choice in meta-analyses of measurement data. Relative risk (RR) is used for dichotomous variables to represent the magnitude of the effect. Each effect was provided with a 95% confidence interval (CI), and P < 0.05 showed statistical significance.
3. Results
3.1. Retrieved studies
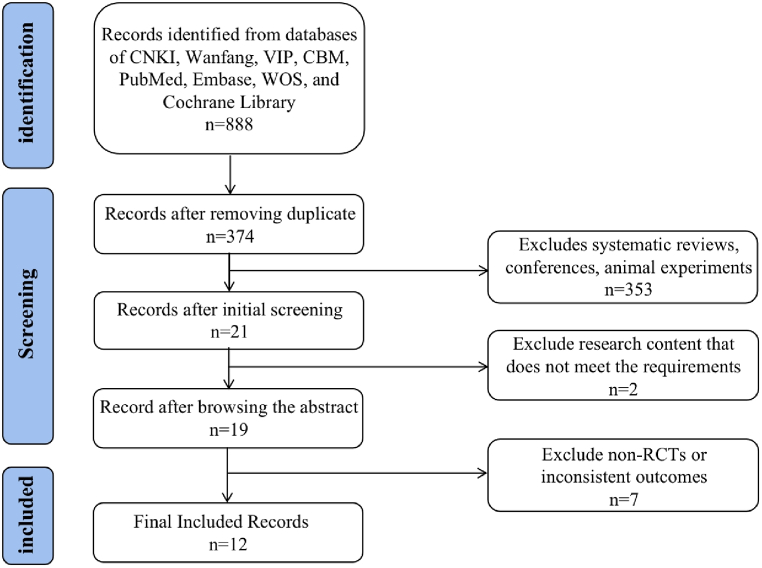
According to the search strategy, a total of 888 references were identified, of which 514 were deduplicated, and after reviewing the titles and abstracts, 355 references irrelevant to UC and EA were discarded. The remaining studies were read in their entirety, and seven articles with non-RCTs or inconsistent outcome indicators were eliminated. Finally, 12 studies [[15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26]] were selected, comprising 11 academic journals and one master's thesis (Fig. 1).
Fig. 1.
Flowchart of study selection.
3.2. Characteristics of the studies
There were a total of 976 samples in the 12 included studies, with 488 in the treatment group and 488 in the control group. The sample size varied from 30 to 220, the individuals' ages ranged from 21 to 73 years, and the disease duration ranged from >3 months to 11 years. Most study participants in the experimental groups received EA or EA-drug combination therapy. Five studies utilized EA + Western medicine to treat UC; two studies used EA + TCM therapy; four studies used EA + Integrated Chinese and Western medicine therapy; one study used EA alone. The control group was either administered sulfasalazine (SASP) or mesalazine (MS). Overall, 11 of the 12 studies examined the overall clinical treatment efficacy, whereas the remaining studies simply analyzed the clinical efficacy of certain UC symptoms. In addition, seven studies reported changes in colonoscopy, three studies presented changes in TCM syndrome scores, three studies detailed changes in serum inflammatory factors, and five studies reported adverse reactions; however, none of the studies reported follow-up data after treatment (Table 1).
Table 1.
Characteristics of included studies.
| Author | Year | Experiment method | Total samples(EG/CG) | Gender (men/women) | Age(years) | Course of disease |
Interventions (EG/CG) |
Total efficiency | Total clinical efficacy(EG/CG) | Course of treatment | Outcomes | AR(EG/CG) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shi YQ | 2006 | RCT,Single blind | 60 (30/30) | EG(18/12) CG(16/14) |
EG(42.31 ± 6.5) CG(43.64 ± 5.7) |
EG(2.7 ± 1.3)y CG(2.5 ± 1.5)y |
EA/SASP | EG(28/2); CG(25/5) | 93.3/83.3 | 1 m | ①⑦ | NR |
| Zhou GY | 2008 | RCT,non-blind | 220 (110/110) | EG(67/43) CG(64/46) |
EG(48.60 ± 7.48) CG(50.24 ± 6.95) |
EG(4.75 ± 1.69)y CG(4.89 ± 1.76)y |
SASP + Moxibustion + EA/SASP | EG(93/17); CG(75/35) | 84.5/68.2 | 40 d | ①②④ | 9/20 |
| Jia YB | 2010 | RCT,non-blind | 90 (45/45) | EG(21/24) CG(23/22) |
EG(37.8 ± 8.5) CG(37.1 ± 8.7) |
EG(7.2 ± 3.3)y CG(6.2 ± 3.4)y |
EA + Acupoint application/MS | EG(41/4); CG(33/12) | 91.1/73.3 | 8 w | ①② | NR |
| Ge F | 2012 | RCT,non-blind | 60 (30/30) | (Total) 27/22 |
(Total) 31.7 ± 4.5 |
(Total) (3.8 ± 2.1)y | SASP + EA/SASP | NR | NR | 8 w | ①④ | 8/7 |
| Teng Y | 2014 | RCT,non-blind | 80 (40/40) | EG(22/18) CG(21/19) |
EG(42.3) CG(43.7) |
EG(0.4–10y)y CG(0.5–11)y |
SASP + TCM + EA/SASP | EG(38/2); CG(30/10) | 95/75 | 2 m | ①②③④ | 0/0 |
| Ge F | 2014 | RCT,non-blind | 62 (31/31) | EG(16/15) CG(17/14) |
EG(35.6 ± 7.5) CG(38.4 ± 7.8) |
EG(3.7 ± 2.8)y CG(4.0 ± 2.5)y |
SASP + EA/SASP | EG(29/2); CG(22/9) | 93.5/71 | 2 m | ①②④ | 9/8 |
| Ge F | 2015 | RCT,non-blind | 50 (25/25) | (Total) 27/22 |
(Total) 38.5 ± 6.5 |
(Total) (4.1 ± 2.7)y |
SASP + EA/SASP | EG(24/1); CG(17/8) | 96/68 | 2 m | ①④ | 5/4 |
| Shan HY | 2018 | RCT,non-blind | 80 (40/40) | EG(20/20) CG(19/21) |
EG(65.52 ± 8.15) CG(64.18 ± 7.83) |
EG(29.07 ± 9.31)m CG(28.44 ± 9.66)m |
MS + EA + Five-tone therapy/MS | EG(37/3); CG(29/11) | 92.5/72.5 | 8 w | ①②⑤ | NR |
| Teng Y | 2018 | RCT,non-blind | 100 (50/50) | EG(26/24) CG(25/25) |
EG(43.3) CG(43.8) |
EG(0.3–11)y CG(0.4–12)y |
TCM + EA/SASP | EG(38/2); CG(31/9) | 98/84 | 2 m | ①② | NR |
| Chen ZX | 2019 | RCT,non-blind | 30 (15/15) | EG(11/4) CG(9/6) |
EG(37.40 ± 13.80) CG(39.13 ± 10.89) |
EG(3.69 ± 3.38)y CG(3.80 ± 2.19)y |
MS + EA/MS | EG(13/2); CG(6/9) | 86.67/40 | 2 w | ①⑥⑧ | 0/0 |
| Wang HL | 2020 | RCT,non-blind | 64 (32/32) | EG(24/8) CG(22/10) |
EG(27.24 ± 2.23) CG(26.12 ± 5.09) |
EG(5.77 ± 1.99)y CG(5.34 ± 2.12)y |
MS + EA + TCM/MS | EG(29/3); CG(25/7) | 90.6/78.1 | 8 w | ①②⑥⑦ | NR |
| Liu HR | 2021 | RCT,non-blind | 80 (40/40) | EG(22/18) CG(23/17) |
EG(42.74 ± 7.30) CG(42.69 ± 6.72) |
EG(3.06 ± 0.64)y CG(2.94 ± 0.52)y |
MS + EA/MS | EG(37/3); CG(28/12) | 92.5/70 | 2 w | ①⑥⑦ | NR |
Note: ① Clinical effective rate ② Comparison of efficacy under colonoscopy ③ Pathological histology ④ Adverse reactions ⑤ Self-rating anxiety and depression scale ⑥ TCM syndrome scores ⑦ Changes of serum inflammatory factors ⑧ Modified mayo score.
3.3. EA parameters
Acupoint selection and surgical characteristics varied. All studies employed continuous waves, with nine adopting sparse waves and three adopting dense waves. The duration of a single treatment in the included studies ranged from 10 to 60 min, with the majority of treatments lasting 20 or 30 min; this duration was not mentioned in two studies. In most studies, EA was conducted once a day, 5–7 days per week, and the duration of treatment ranged from 2 weeks to 2 months (Table 2).
Table 2.
EA parameters of included studies.
| Author | Years | Acupuncture points | Waveform | Time (min) | Related parameters |
|---|---|---|---|---|---|
| Shi YQ | 2006 | RN4, RN6, ST25, ST36, SP9, ST37 | Sparse wave | 30 | 2Hz,1 mA |
| Zhou GY | 2008 | ST25, RN12, RN6, ST36, SP9, ST37 | Sparse wave | 30 | 2Hz,3–5 mA |
| Jia YB | 2010 | (1)RN12, ST25, RN4, RN6; (2)ST37, BL20, BL25, ST36 (two groups are used alternately) |
Dense wave | 30 | 60 Hz |
| Ge F | 2012 | (1) BL18, BL20, BL25, BL23, BL32; (2)ST25,RN6, RN4, ST37, SP6, LR3 (the two groups are used alternately) |
Sparse wave | 30 | Low frequency |
| Teng Y | 2014 | RN12,ST25, RN4, ST36, SP6 | Dense wave | NR | 50-100/min |
| Ge F | 2014 | (1) BL18, BL20, BL25, BL23, BL32; (2)ST25,RN6, RN4, ST37, SP6, LR3 (the two groups are used alternately) |
Sparse wave | 30 | Low frequency |
| Ge F | 2015 | (1) BL18, BL20, BL25, BL23, BL32; (2)ST25,RN6, RN4, ST37, SP6, LR3 (the two groups are used alternately) |
Sparse wave | 30 | Low frequency |
| Shan HY | 2018 | Shendao eight array acupoints | Sparse wave | 20 | The patient's maximum tolerance |
| Teng Y | 2018 | RN12, ST25, RN4, ST36, SP6 | Dense wave | NR | 50-100/min |
| Chen ZX | 2019 | BL33, BL34 | Sparse wave | 60 | 5Hz-0.5 ms-10s(on)-90s(off) |
| Wang HL | 2020 | RN12, ST25, ST36, SP9, BL20, LR3 | Sparse wave | 30 | 1–1.5Hz |
| Liu HR | 2021 | BL33, BL34 | Sparse wave | 60 | 5Hz-0.5 ms-10s(on)-90s(off) |
3.4. Risk of bias
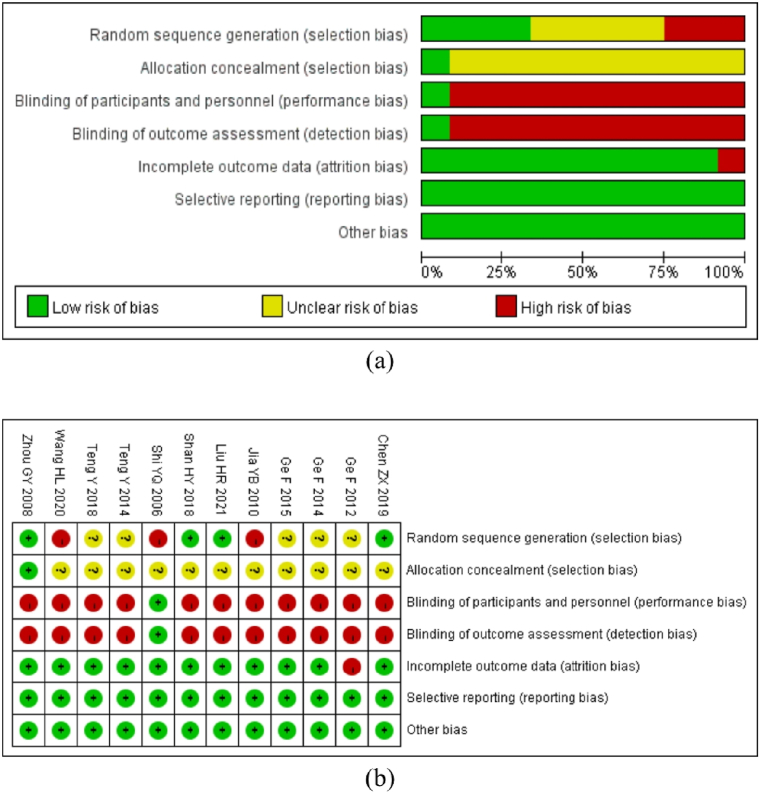
All the studies mentioned randomization (Fig. 2a and b); however, only four studies used a random number method to assign participants (low risk), three studies were randomized according to the order of visits (high risk), and the remaining five studies only mentioned the word “random” (unclear risk) without describing the specific grouping method. Due to the clinical treatment characteristics of EA, it is difficult to execute the blind method of acupuncture operators; therefore, the single blind technique is used in just one study. Only one study mentioned an allocation concealment strategy. One literature outcome was deemed as “high risk” due to its lack of total efficacy. There was no other potential risk of bias or selective reporting of the results, and all studies disclosed all data included in the results. The studies were evaluated using a modified version of the Jadad rating system (Table 3). The results revealed that two of the studies were of high quality (one study received five points and one study received four points), whereas the remaining ten studies were of low quality (five studies received two points, two studies received one point, and three studies received three points).
Fig. 2.
Risk of bias assessments. Note: (a) overall risk of bias of included studies; (b) risk of bias for each included study. “+“: low risk of bias; “-“: high risk of bias; “?“: unclear risk of bias.
Table 3.
Modified Jadad rating scale.
| Author, Year | Random sequences | Allocation hidden | Blinding method | Withdrawal | Jadad score |
|---|---|---|---|---|---|
| Shi YQ 2006 | 0 | 1 | 2 | 0 | 3 |
| Zhou GY 2008 | 2 | 2 | 0 | 1 | 5 |
| Jia YB 2010 | 0 | 1 | 0 | 0 | 1 |
| Ge F 2012 | 1 | 1 | 0 | 0 | 2 |
| Teng Y 2014 | 1 | 1 | 0 | 0 | 2 |
| Ge F 2014 | 1 | 1 | 0 | 0 | 2 |
| Ge F 2015 | 1 | 1 | 0 | 0 | 2 |
| Shan HY 2018 | 1 | 1 | 0 | 0 | 2 |
| Teng Y 2018 | 2 | 1 | 0 | 0 | 3 |
| Chen ZX 2019 | 2 | 1 | 0 | 1 | 4 |
| Wang HL 2020 | 0 | 1 | 0 | 0 | 1 |
| Liu HR 2021 | 2 | 1 | 0 | 0 | 3 |
3.5. Meta-analysis results
3.5.1. Clinical efficiency
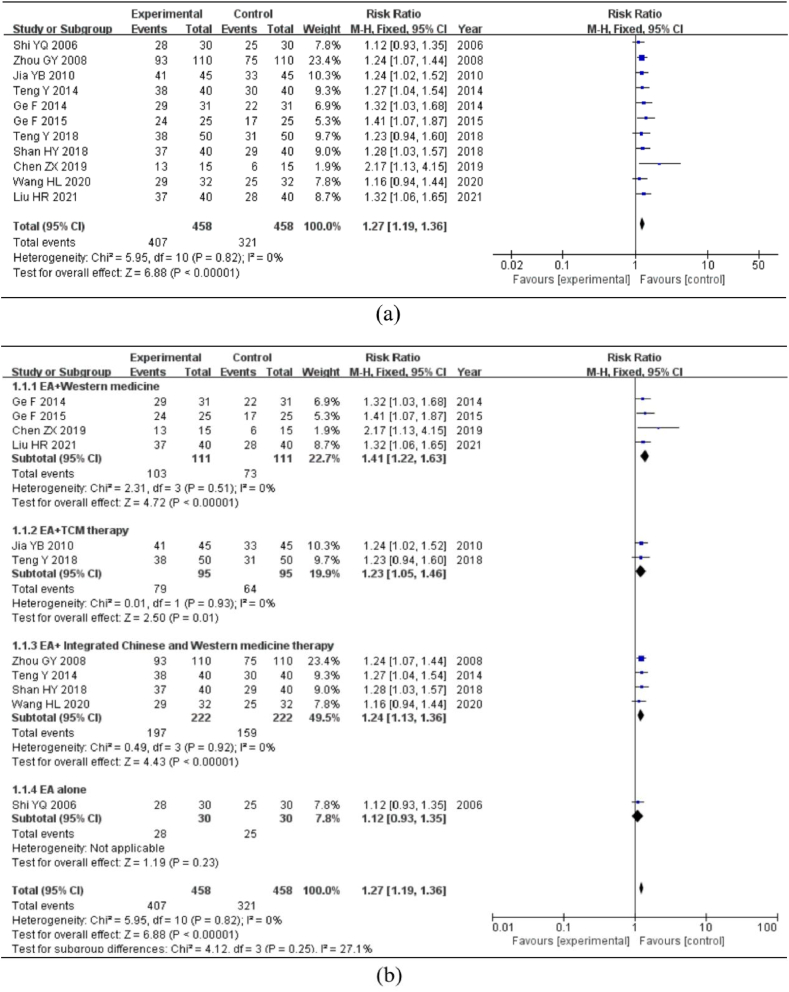
Overall, 11 of the 12 included studies discussed the total clinical efficacy in 836 patients (418 patients in the experimental group and 418 patients in the control group). P > 0.1 and I2 = 0% indicated that there was no heterogeneity among the included studies; consequently, the fixed effect model was used for the meta-analysis. The analysis showed that the total clinical effectiveness rate in the experimental group was significantly higher than that in the control group [RR = 1.27, 95%CI = (1.19, 1.36), P < 0.01] (Fig. 3a).
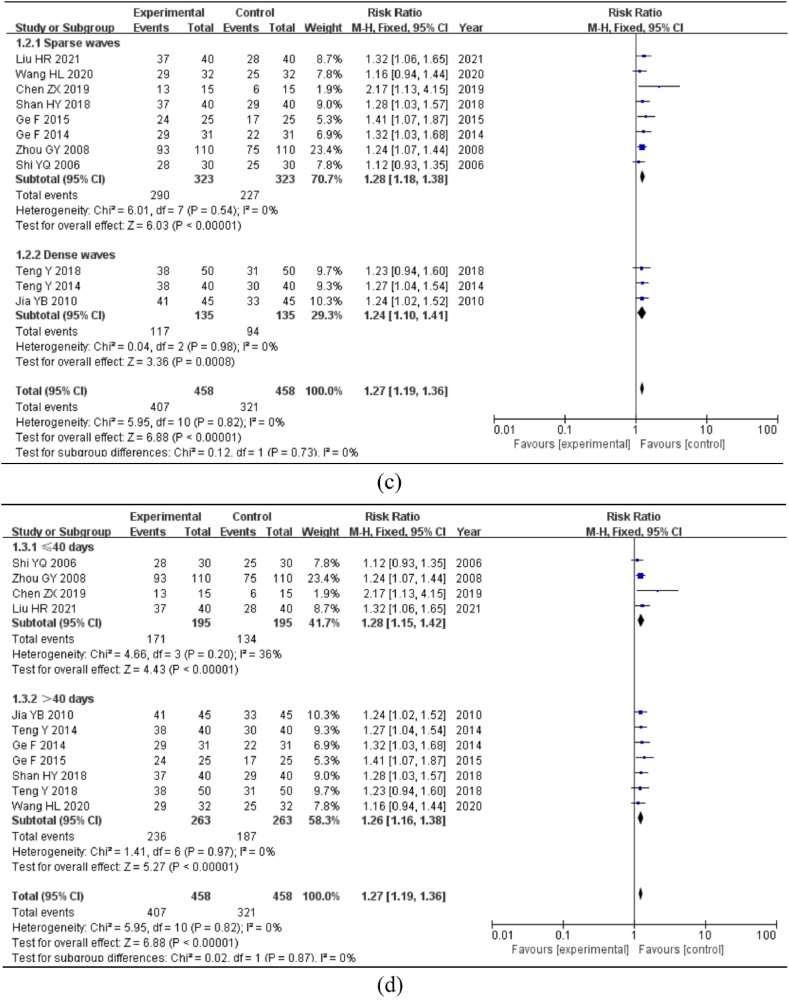
Fig. 3.
Subgroup analysis of total clinical efficacy. Note:(a) Meta-analysis of total clinical efficacy. (b) Subgroup analysis of the effects of different interventions. (c) Subgroup analysis of the effects of different EA waveform. (d) Subgroup analysis of the effects of different courses of treatment.
The subgroup analysis was separated into EA + Western medicine, EA + TCM therapy, EA + Integrated Chinese and Western medicine therapy, and EA alone, based on various intervention approaches. There was no heterogeneity across all groups (P > 0.1, I2 = 0%); hence, a fixed-effects model was used for analysis. The intervention method in four studies was EA + Western medicine [RR = 1.41, 95%CI = (1.22, 1.63), P < 0.01]; the intervention method in two studies was EA + TCM therapy [RR = 1.23, 95%CI = (1.05, 1.46), P < 0.01]; the intervention method in four studies was EA + Integrated Chinese and Western medicine therapy [RR = 1.24, 95%CI = (1.13, 1.36), P < 0.01]; the intervention method in one study was EA alone [RR = 1.12, 95%CI = (0.93, 1.35), P = 0.23]. The analysis revealed that EA alone had no significant effect on the total clinical efficacy. Compared with the control group, the other three experimental groups showed increased total clinical efficacy at the conclusion of therapy to varying degrees (Fig. 3b).
For the subgroup analysis, the EA treatment waveform was separated into a sparse wave group and a dense wave group. The heterogeneity test revealed no heterogeneity between the studies (P > 0.1, I2 = 0%); hence, the fixed effects model was used for analysis. In eight studies, the EA waveform was sparse [RR = 1.28, 95%CI = (1.18, 1.38), P < 0.01]; however, in three studies, the EA waveform was dense [RR = 1.24, 95%CI = (1.10, 1.41), P < 0.01]. The statistically significant differences indicated that the total clinical efficacy effect of the experimental group was superior to that of the control group, regardless of whether the treatment waveform was sparse or dense (Fig. 3c).
Using the 40-day treatment duration as the node, the group was separated into two subgroups for analysis: >40 days and ≤40 days. The heterogeneity test revealed no heterogeneity between the studies (P > 0.1, I2 < 50%); hence, the fixed-effects model was employed for analysis. The duration of four of the studies was 40 days [RR = 1.28, 95%CI = (1.15, 1.42), P < 0.01], whereas seven studies were >40 days [RR = 1.26, 95%CI = (1.16, 1.38), P < 0.01]. The difference was statistically significant, indicating that the duration of EA therapy ranged between two weeks and two months and that the total clinical efficacy of the experimental group was superior to that of the control group (Fig. 3d).
3.5.2. Cure rate
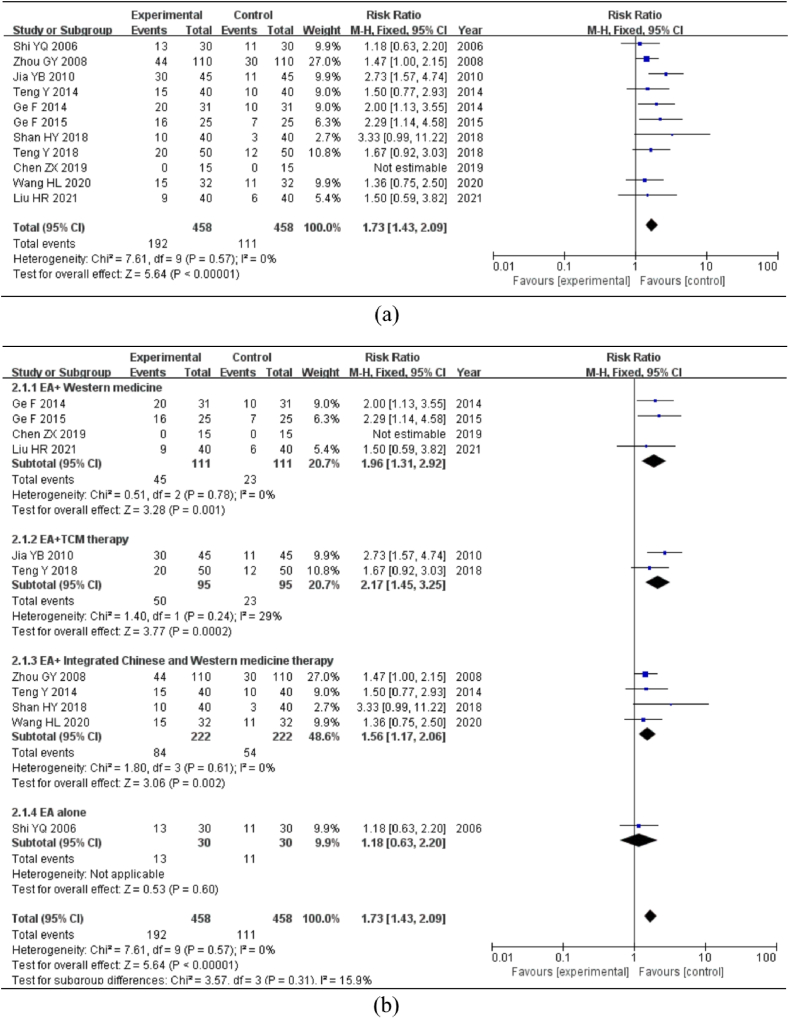
Eleven studies included the cure rate indicator. According to the heterogeneity test, P > 0.1 and I2 = 0%, there was no heterogeneity among the included studies. A meta-analysis was conducted using a fixed-effects model. The results indicated [RR = 1.73, 95%CI = (1.43, 2.09), P < 0.01]; the difference was statistically significant, showing that the experimental group had a considerably higher cure rate than the control group (Fig. 4a).
Fig. 4.
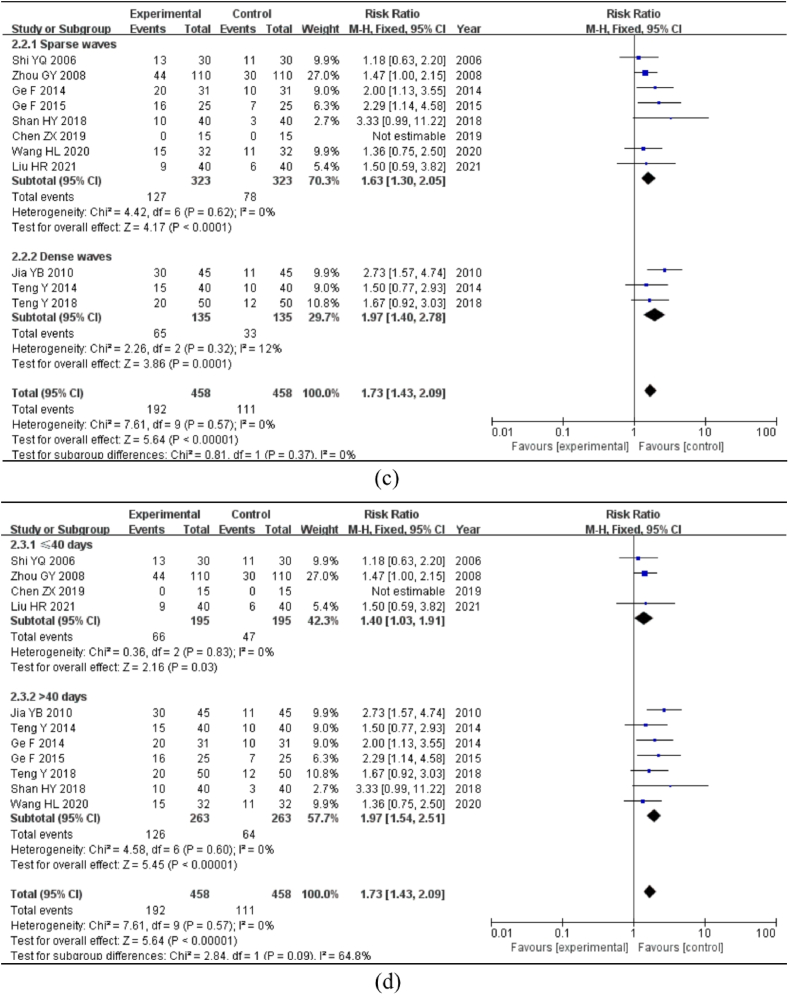
Meta-analysis and subgroup analysis of cure rate. Note: (a) Meta-analysis of cure rate. (b) Subgroup analysis of the cure rate of different interventions. (c) Subgroup analysis of the cure rate of different EA waveform. (d) Subgroup analysis of the cure rate of different courses of treatment.
The subgroup analysis was separated into EA + Western medicine, EA + TCM therapy, EA + Integrated Chinese and Western medicine therapy, and EA alone, based on various intervention approaches. The heterogeneity test revealed no heterogeneity between the studies (P > 0.1, I2 < 50%); hence, the fixed-effects model was employed for analysis. Four studies used EA + Western medicine [RR = 1.96, 95%CI = (1.31, 2.92), P < 0.01]; two studies used EA + TCM therapy [RR = 2.17, 95%CI = (1.45, 3.25), P < 0.01]; four studies used EA + Integrated Chinese and Western medicine therapy [RR = 1.56, 95%CI = (1.17, 2.06), P < 0.01]; one study used EA alone [RR = 1.18, 95%CI = (0.63, 2.20), P = 0.6]. A comprehensive analysis revealed that the EA-alone group was not statistically significant; however, the cure rates of the other three experimental groups were higher than those of the control group (Fig. 4b).
For the subgroup analysis, the EA waveform was separated into sparse and dense wave groups. The heterogeneity test revealed no heterogeneity between the studies (P > 0.1, I2 < 50%); hence, the fixed-effects model was employed for analysis. In eight studies, the EA waveform was sparse [RR = 1.63, 95%CI = (1.30, 2.05), P < 0.01]; however, in three studies, the EA waveform was dense [RR = 1.97, 95%CI = (1.40, 2.78), P < 0.01]. All differences were statistically significant, indicating that the experimental group had a higher cure rate than the control group and that the dense-wave group had a higher cure rate (Fig. 4c).
For the analysis, the treatment period was divided into two subgroups: >40 days and ≤40 days. The heterogeneity test revealed no heterogeneity between the studies (P > 0.1, I2 < 50%); hence, the fixed-effects model was employed for analysis. The duration of four of the studies were ≤40 days [RR = 1.40, 95% CI = (1.03, 1.91), P < 0.01], while seven studies were >40 days [RR = 1.97, 95% CI = (1.54, 2.51), P < 0.01]. The differences were statistically significant, indicating that the cure rate of the experimental group was superior to that of the control group regardless of the treatment length >40 days or ≤40 days, and that the cure rate >40 days had a pronounced effect (Fig. 4d).
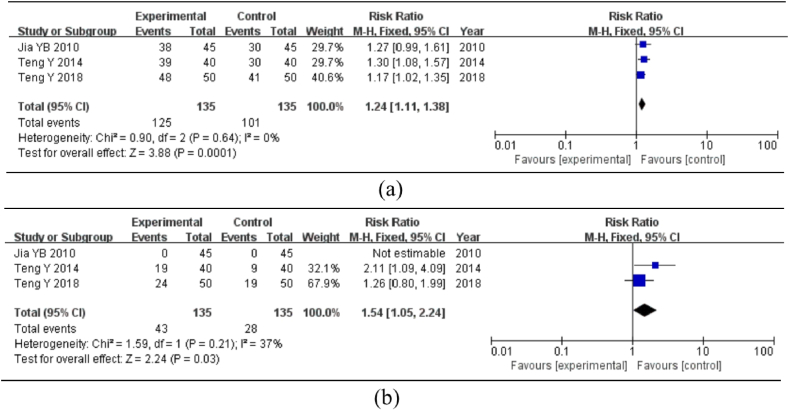
3.5.3. Mucosal lesions colonoscopy
Three studies described the indicators of the efficacy of mucosal lesions under colonoscopy (cure: the degree of recovery of mucosal lesions in colonoscopy re-examination was grade 2 or above; effective: the degree of recovery of mucosal lesions in colonoscopy re-examination was grade 1 or above). I2 = 0%, P > 0.10, suggesting no heterogeneity. Hence, the fixed-effects model was employed to calculate the effective rate. The meta-analysis revealed that the difference was statistically significant [RR = 1.24, 95%CI = (1.11, 1.38), P < 0.01], showing that the experimental group had a significantly higher rate of success in treating intestinal mucosal lesions than the control group (Fig. 5a). The cure rate was depicted as follows: I2 = 37%, P > 0.1, showing no substantial heterogeneity; hence, the fixed-effects model was employed. The results of the meta-analysis revealed that the difference was not statistically significant [RR = 1.54, 95%CI = (1.05, 2.24), P = 0.03], indicating that the experimental group had no clear benefit over the control group in terms of the cure rate of intestinal mucosal lesions (Fig. 5b).
Fig. 5.
Meta-analysis of mucosal lesions under colonoscopy. Note: (a) Effective rate of mucosal lesions under colonoscopy. (b) Cure rate of mucosal lesions under colonoscopy.
3.5.4. TNF-α
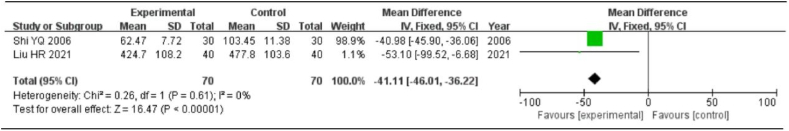
Two studies discussed the variations of the serum inflammatory factor, TNF-α. The heterogeneity test revealed no heterogeneity between the studies (P > 0.1, I2 < 50%); hence, the fixed-effects model was employed for analysis. The results of the meta-analysis showed that the difference was not statistically significant [MD = −41.11, 95%CI = (−46.01, 36.22), P < 0.01], indicating that the experimental group could significantly reduce the index of TNF-α compared to the control group (Fig. 6).
Fig. 6.
Meta-analysis of serum inflammatory factor TNF-α.
3.5.5. TCM syndrome scores
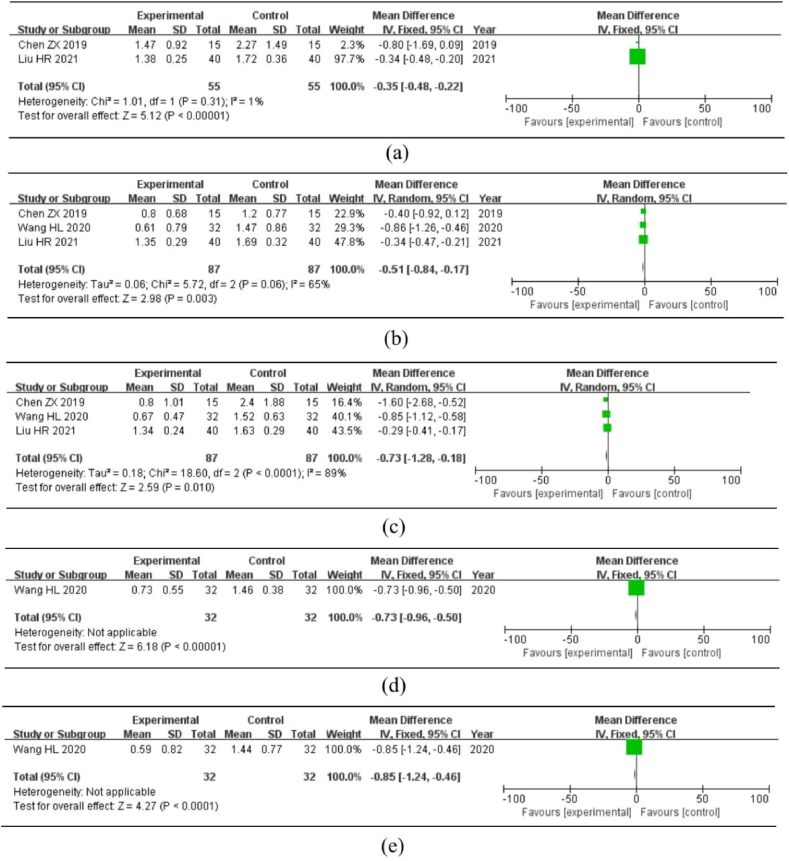
Three studies described scores for various TCM syndromes, including chills, cold limbs and body, abdominal pain, tenesmus, diarrhea, and blood in the stool. Cold limbs and body were utilized as research indicators in two studies, and the fixed-effect model meta-analysis was chosen because there was no clear heterogeneity in the studies (P > 0.1, I2 < 50%). The treatment effect of the experimental group was superior to that of the control group, and the results were statistically significant [MD = −0.35, 95%CI = (−0.48, −0.22), P < 0.01] (Fig. 7a). Diarrhea and stomach pain were used as research indicators in three different studies. Following the heterogeneity test, I2 > 50% and P < 0.1, indicated considerable heterogeneity in the study. A random-effects model was employed for meta-analysis because the number of included studies was too small to conduct subgroup and sensitivity analyses. Moreover, the data revealed abdominal pain [MD = −0.51, 95%CI = (−0.84, −0.17), P < 0.01] (Fig. 7b) and diarrhea [MD = −0.73, 95%CI = (−1.28, −0.18), P = 0.01] (Fig. 7c). Therefore, the experimental group was able to considerably minimize the abdominal pain and diarrhea symptoms. Only one study evaluated the indicators of bloody stool and tenesmus: bloody stool [MD = −0.73, 95% CI = (−0.96, −0.50), P < 0.01] (Fig. 7d) and tenesmus [MD = −0.85, 95%CI = (−1.24, −0.46), P < 0.01] (Fig. 7e), indicating that compared to the control group, the experimental group had a more significant effect on the reduction of bloody stool and tenesmus syndrome scores.
Fig. 7.
Meta-analysis of different TCM syndrome scores. Note: (a) Cold limbs and body. (b) Abdominal pain. (c) Diarrhea. (d) Bloody stool. (e) Tenesmus.
3.5.6. Adverse reactions
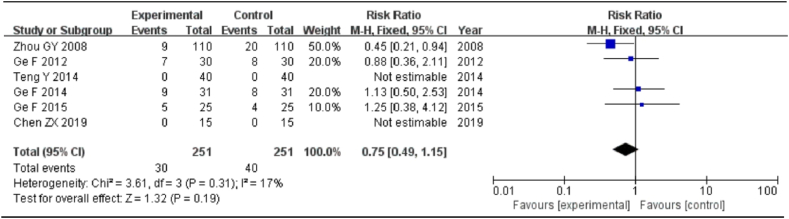
Adverse reactions were mentioned in six studies (30 cases in the experimental group and 40 cases in the control group). Adverse reactions in the experimental group included headache in six cases, nausea in four cases, abdominal discomfort (including abdominal pain and diarrhea) in seven cases, leukopenia in seven cases, and localized skin rash in six cases. Adverse reactions in the control group included headache in four cases, nausea in ten cases, abdominal discomfort (including abdominal pain and diarrhea) in 13 cases, leucopenia in 11 cases, and local skin rash in two cases. The following results were obtained: I2 = 17% and P = 0.31, indicating that the heterogeneity was small; hence, the fixed-effects model was utilized. The meta-analysis revealed that the difference was not statistically significant [RR = 0.75, 95%CI = (0.49, 1.15), P = 0.19], indicating that the experimental group was not affected by the occurrence of adverse reactions compared with the control group (Fig. 8).
Fig. 8.
Meta-analysis of adverse reactions.
3.5.7. Bias analysis
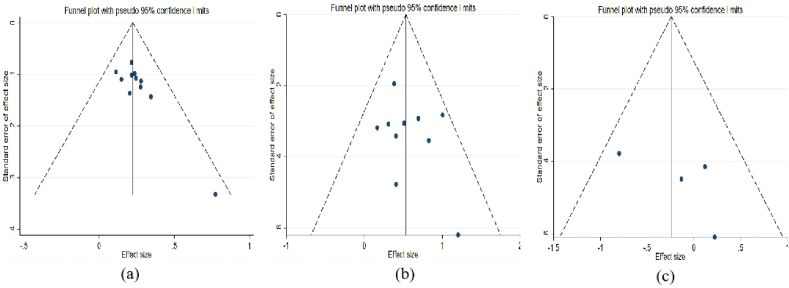
On the basis of stataSE 15, funnel plots for the total clinical efficacy studies were created. It is evident that the graph's distribution of scattered points is skewed, and there may be a publication bias. Begg's method and Egger's method were used to examine the included studies for bias. Begg's test revealed P = 0.029 < 0.05, whereas Egger's test revealed P = 0.010 < 0.05, indicating publication bias in these 11 studies (Fig. 9a). The scatter distribution of the cure rate in the graph is essentially symmetrical and within the 95% confidence interval. Begg's test results were P = 0.721 > 0.05, and Egger's test results were P = 0.371 > 0.05, indicating that there was no clear publication bias(Fig. 9b). Regarding adverse reactions, the distribution of scattered points was essentially symmetrical in the graph. Begg's test revealed P = 0.734 > 0.05, and Egger's test revealed P = 0.348 > 0.05, demonstrating no significant publication bias (Fig. 9c). Due to an insufficient number of studies on the remaining indicators, no deviation analysis was conducted.
Fig. 9.
Bias analysis. Note: (a) Bias analysis of total clinical efficacy. (b) Bias analysis of cure rate. (c) Bias analysis of adverse reactions.
3.6. Acupoint compatibility law
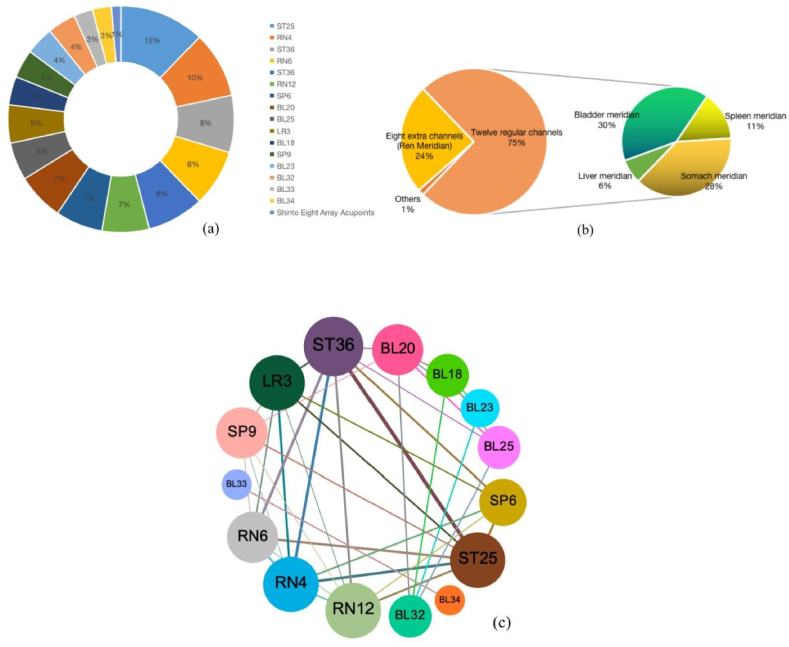
In the included twelve studies, 16 specific acupoints were used (the Shinto Eight Array acupoints were regarded as a group of acupoints). The frequencies of the selected acupoints were as follows: ST25 (nine times), RN4 (seven times), ST36 (six times), RN6 (six times), ST37 (six times), RN12 (five times), SP36 (five times), BL20 (five times), BL25 (four times), LR3 (four times), BL18 (three times), SP9 (3 times), BL23 (3 times), BL32 (3 times), BL33 (2 times), BL34 (2 times), and Shinto Eight Formation Points (1 time) (Fig. 10a). Most of these tumors are located in the lower abdomen, lumbosacral area, and below the knee joint. Most of these acupoints originated from the 12 main meridians and the Ren meridian, with the 12 main meridians primarily concentrated in the bladder, stomach, spleen, and liver meridians (Fig. 10b).
Fig. 10.
Analysis rule for selecting acupoints. Note:(a) Distribution diagram of acupoint use. (b) Distribution diagram of acupoint meridian tropism. (c) Network diagram of acupoint compatibility.
Gephi software was used to create a network diagram to visually evaluate the degree of connection between the acupoints, analyze the compatibility between various acupoints, and uncover the underlying laws of acupoint matching (Fig. 10c). The circles and lines in the figure represent the connections between the acupoints. The larger the circle and the thicker the line, the closer the connection between the two acupoints and the higher the frequency of co-occurrence. According to the graph, the RN4-ST25 combination was utilized most frequently, and the connection between them was the thickest. We speculate that a combination of acupoints with higher frequency may have greater therapeutic effectiveness.
4. Discussion
UC is a chronic relapsing inflammatory disease of the colon and rectum. The primary symptoms include diarrhea with blood, stomach pain, and discomfort [1,2]. According to the TCM theory of etiology and pathophysiology, the early stages of UC are dominated by damp heat and cold. Intestinal qi stagnation and blood stasis emerge in the middle stages of the disease, exacerbating UC symptoms. In the later stages of the disease, both the spleen and kidneys are damaged, resulting in splenic and kidney deficiencies and a prolonged, unhealed condition [27]. Currently, there is no cure for UC and surgery may be associated with recurrent symptoms [5,6]. In recent years, the therapeutic mechanisms of acupuncture for UC have been widely studied. The brain-gut axis is the structural basis for acupuncture to mobilize multiple targets and systems to regulate gastrointestinal function. Studies have shown that acupuncture can activate the vagus and sympathetic nerves to bidirectionally regulate gastrointestinal motility, activate the cholinergic anti-inflammatory pathway to act on immune cells and inhibit intestinal inflammation, regulate the HPA axis and inhibit visceral hypersensitivity, correct the imbalance of the intestinal flora, improve the intestinal microenvironment, and inhibit the nociceptive sensitization of UC via multiple pathways [28]. These mechanisms suggest that acupuncture can improve the pathological processes and symptoms associated with UC by modulating multiple targets in the brain-gut axis communication loop. However, the clinical efficacy and optimal therapeutic parameters of EA for UC have not been fully elucidated; therefore, this study analyzed the clinical efficacy of EA in the treatment of UC and explored its optimal therapeutic parameters to provide a reference for clinical promotion.
This analysis includes a total of twelve studies after screening. In the earlier years, the recommended first-line treatment for UC was SASP, which has since been discontinued because of adverse consequences. Currently, 5-aminosalicylic acid is the recommended first-line treatment, and MS is a representative drug that can be delivered as a suppository, foam, enema, or oral preparation [5,29]. Consequently, these two medicines were used as positive controls in the included studies based on various years. Based on this meta-analysis, the total clinical efficacy of the experimental group in the treatment of UC was greater than that of the control group. The clinical efficacies of EA + Western medicine, EA + TCM therapy, and EA + Integrated Chinese and Western medicine therapies were superior to that of the control group, with EA + Western medicine showing the highest efficacy rate. These three groups were also superior to the control group in terms of the cure rate. EA + TCM therapy had the highest cure rate, which may be attributable to the fundamental concepts of the clinical syndrome and TCM treatment. Chinese medicine therapy is adept at identifying the essence of a disease by focusing on the relationship between the whole and its parts. It can balance yin and yang by bolstering righteousness and eradicating evil spirits in response to changes in deficiency and reality generated by the conflict between good and evil, thus considerably enhancing treatment rates.
Colonoscopy is crucial in the diagnosis and treatment of UC because it can evaluate the occurrence of colorectal tumors and disease severity [30]. The results of this meta-analysis revealed that compared to the control group, the experimental group was able to enhance the effective rate of mucosal lesions under colonoscopy, but had no discernible influence on the cure rate. TNF-α is particularly important for the induction and maintenance of intestinal inflammation in UC patients. Studies have shown that TNF-α is expressed in the mucosa of the human gastrointestinal tract and that its expression is strongly enhanced during UC inflammation [31]. In UC patients, the expression level of TNF-α is significantly increased; additionally, it can contribute to the exacerbation and persistence of the disease by promoting inflammatory responses, triggering tissue damage, facilitating the activation of immune cells, and destroying the intestinal barrier. Tian et al. demonstrated that EA stimulation of ST36 cells could reduce the serum TNF-α concentration and colonic TNF-α mRNA expression levels and attenuate colonic inflammatory injury [32]. According to the results of this meta-analysis, the experimental group had a significant impact on the serum TNF-levels in individuals with UC. The TCM syndrome score is mostly used for evaluating the curative effects and has become an increasingly popular method for evaluating TCM clinical curative effect evaluation [33]. Compared to the control group, the experimental group showed a greater reduction in the effect on syndrome scores, such as chills, abdominal pain, tenesmus, diarrhea, and blood in the stool. Although the TCM syndrome score currently lacks a unified standard at the present time, it has guiding value as an outcome measure for evaluating the clinical efficacy of TCM in China. In terms of adverse reactions, the experimental group had no significant effect on the incidence of adverse reactions compared with the control group, which may be due to the smaller number of included studies.
The selection and compatibility of acupoints have the greatest effect on the effectiveness of acupuncture [34]. Studies have confirmed that acupuncture at different parts of the acupoints can activate different autonomic nerve pathways to regulate gastrointestinal motility in both directions. Low-intensity EA in the hind limb area drives the vagus nerve-adrenal axis, resulting in systemic anti-inflammatory effects that depend on NPY + adrenal chromaffin cells, while high-intensity EA on the abdomen activates NPY + splenic noradrenergic neurons through the spinal cord sympathetic axis to fight inflammation. Therefore, further exploration of the selection and compatibility of acupoints will help propose a better clinical treatment plan for the complex symptoms of UC [35]. Most of these acupoints are observed to be located in the lower abdomen, lumbosacral area, or below the knee joint. The compatibility and use of acupoints exhibit the following characteristics: (1) the combination of upper and lower acupoints, such as abdominal and lower limb acupoints. (2) The compatibility and combination of Shu and Mu points, such as ST25 and BL25, are used alternately. (3) Surface and inner meridian acupoint matching methods, such as ST36 and SP6, and (4) additional combinations, such as the application of the Shinto Eight Array acupoints in conjunction with the Five Elements and Eight Diagrams. We found that ST25 was the most frequently used. ST25 can regulate gastrointestinal function. Shi et al. demonstrated that acupuncture stimulation of ST25 activated mast cell degranulation and downregulated the apoptosis of colonic epithelial cells in a rat model of colitis [36]. Hou et al. demonstrated that the EA of ST25 inhibited inflammatory responses, improved blood circulation in the intestinal mucosa, and accelerated ulcer healing [37]. ST-36 is second only to ST25 in terms of the frequency of use and is an important acupoint for gastrointestinal health care. Song et al. showed that EA ST-36 ameliorated DSS-induced acute colitis by inhibiting NLRP3/IL-1β and promoting Nrf2/HO-1-induced macrophage polarization [38]. Chen et al. found that reduced rectal compliance and visceral hypersensitivity were present in the post-inflammatory state of rats with DSS-induced colitis. EA at ST36 with 100 Hz, 0.1 s-on, 0.4 s-off, 0.5 ms, and 0.5 mA reduces visceral hypersensitivity, possibly via the mast cell-triggered NGF/TrkA/TRPV1 peripheral afferent pathway [39]. An effective combination of different acupoints can integrate and amplify their efficacy, generate synergistic effects, enhance clinical efficacy, and broaden the treatment spectrum [40]. By drawing an acupoint compatibility network diagram, we found that RN4-ST25 was used most frequently and that the combination of the two acupoints exerted the effects of clearing heat and dampness, regulating Qi, and invigorating the spleen. In addition, the use of RN4-RN6 can strengthen the kidney and nourish Qi, and BL33-BL34 can warm the kidney and remove dampness. These findings provide meaningful clinical evidence of the efficacy of UC treatment.
In addition to the selection and compatibility of acupoints, the selection of the treatment period and EA parameters, such as waveform, frequency, and intensity are also significant factors that affect efficacy [41]. We discovered that, for the treatment of UC, the majority of studies selected sparse or dense waves. The fast frequency, called “dense wave” (50–100 Hz), which is generally 50–100/s, has a powerful inhibitory impact that can relax and alleviate the clinical symptoms of stomach pain. The low frequency (2–5 Hz) is referred to as a sparse wave (2–5/s). It has a clear excitatory effect, may stimulate the circulation of Qi and blood, and aids in the healing of intestinal mucosal tissue [42]. We found that both sparse and dense waves were beneficial for enhancing the clinical efficacy of UC symptoms, with minimal distinction between the two. Dense waves are clearly superior to sparse waves in terms of the curing rate. In addition, the length of needle retention has a significant impact on acupuncture efficacy. In this review, we discovered that the majority of studies selected 30 min of treatment at a time; therefore, we hypothesized that the effect of 30 min of treatment may be fully exerted, and the clinical result is superior. Regardless of whether the treatment duration was >40 or ≤40 days, the clinical effectiveness rate of the experimental group was higher than that of the control group, and the difference between the two groups was small. A treatment course >40 days has apparent advantages in terms of the cure rate. Therefore, we hypothesized that EA utilizing dense waves with a duration of 30 min of electrification and a course of treatment spanning over 6 weeks may be the most effective treatment for UC patients.
This study has some limitations. First, the sample size and number of included RCTs were small and both were single-center studies, which may have led to deviations in the results. Moreover, all included studies were completed in China, suggesting a significant publication bias. Second, the overall quality of the studies included in the meta-analysis was poor. Most studies lack clarity regarding the generation and distribution of random sequences, whereas some studies lack blind techniques. Third, since UC is a progressive condition and none of the included studies contained follow-up data, it was impossible to determine the long-term impact of EA on UC. Fourth, the acupuncture parameters presented in the study were not exhaustive, which may be due to subjective syndrome differentiation among doctors, resulting in clinical differences in acupuncture parameters. Therefore, we only conducted a preliminary analysis of the available parameters. Finally, most studies integrated EA with pharmaceuticals as a treatment, and there are few studies on EA alone. Therefore, we cannot draw a clear conclusion on the superiority of EA based on the experimental evidence included in this study; however, it still has guiding significance for clinical practice.
5. Conclusions
In conclusion, the clinical efficacy of the EA treatment group in the treatment of UC was better than that of the control group and had certain curative effects in terms of the cure rate, the efficacy of mucosal lesions under colonoscopy, serum inflammatory factors, and TCM syndrome scores. At the same time, we speculate that the combination of acupoints on the bladder meridian, stomach meridian, and Ren meridian, along with dense waves lasting 30 min each time for more than 6 weeks, may be the best solution for UC patients. Owing to some of the limitations of this study, large-sample, multicenter, high-quality RCT studies are needed to support verification in the future. In addition, it is necessary to continuously quantify the parameters of EA, help formulate the best treatment method, and strengthen the long-term follow-up of patient prognoses to provide safer and more reliable data for clinical use.
Authors' contributions
Jianheng Hao: Conceived and designed the experiments; Performed the experiments; Wrote the paper. Yuemeng Zhao; Yuxia Cao: Analyzed and interpreted the data; Performed the experiments. Haijun Wang: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data. Laixi Ji: Conceived and designed the experiments; Wrote the paper.
Funding
This work was supported by the National Natural Science Foundation of China (NO. 82074549).
Ethical approval
This article does not contain any studies with human participants or animals.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e20789.
Contributor Information
Haijun Wang, Email: whjdavid@163.com.
Laixi Ji, Email: jlx@sxtcm.edu.cn.
Abbreviations
- UC
ulcerative colitis
- EA
electroacupuncture
- y
year
- m
month
- w
week
- d
day
- AR
adverse reaction
- EG
experiment group
- CG
control group
- SASP
sulfasalazine
- MS
mesalamine
- TCM
traditional Chinese medicine
- NR
not reported
- TNF
tumor necrosis factor
- ST25
tian shu
- ST36
zu san li
- ST37
shang ju xu
- RN4
guan yuan
- RN6
qi hai
- RN12
zhong wan
- SP9
yin ling quan
- SP36
san yin jiao
- BL18
gan shu
- BL20
pi shu
- BL23
shen shu
- BL25
da chang shu
- BL32
ci liao
- BL33
zhong liao
- BL34
xia liao
- LR3
tai chong
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Ungaro R., Mehandru S., Allen P.B., et al. Ulcerative colitis. Lancet. 2017;389:1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segal J.P., LeBlanc J.F., Hart A.L. Ulcerative colitis: an update. Clin. Med. 2021;21:135–139. doi: 10.7861/clinmed.2021-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi T., Siegmund B., Le Berre C., et al. Ulcerative colitis. Nat. Rev. Dis. Prim. 2020;6:74. doi: 10.1038/s41572-020-0205-x. [DOI] [PubMed] [Google Scholar]
- 4.Cui G., Yuan A. A systematic review of epidemiology and risk factors associated with Chinese inflammatory bowel disease. Front. Med. 2018;5:183. doi: 10.3389/fmed.2018.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kayal M., Shah S. Ulcerative colitis: current and emerging treatment strategies. J. Clin. Med. 2019;9:94. doi: 10.3390/jcm9010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko C.W., Singh S., Feuerstein J.D., et al. AGA clinical practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterol. 2019;156:748–764. doi: 10.1053/j.gastro.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein D.J. Massage acupuncture, moxibustion, and other forms of complementary and alternative medicine in inflammatory bowel disease. Gastroenterol. Clin. N. Am. 2017;46:875–880. doi: 10.1016/j.gtc.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Song G., Fiocchi C., Achkar J.P. Acupuncture in inflammatory bowel disease. Inflamm. Bowel Dis. 2019;25:1129–1139. doi: 10.1093/ibd/izy371. [DOI] [PubMed] [Google Scholar]
- 9.Ulett G.A., Han S., Han J. Electroacupuncture: mechanisms and clinical application. Biol. Psychiatr. 1998;44:129–138. doi: 10.1016/s0006-3223(97)00394-6. [DOI] [PubMed] [Google Scholar]
- 10.Liu G.H., Liu H.M., Chen Y.S., et al. Effect of electroacupuncture in mice with dextran sulfate sodium-induced colitis and the influence of gut microbiota. Evid. Based. Complement. Alternat. Med. 2020;2020 doi: 10.1155/2020/2087903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horta D., Lira A., Sanchez-Lloansi M., et al. A prospective pilot randomized study: electroacupuncture vs. sham procedure for the treatment of fatigue in patients with quiescent inflammatory bowel disease. Inflamm. Bowel Dis. 2019;26:484–492. doi: 10.1093/ibd/izz091. [DOI] [PubMed] [Google Scholar]
- 12.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg. 2021;88 doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 13.Joanne E., McKenzie Georgia, et al. Meta-analysis and the Cochrane collaboration: 20 years of the Cochrane statistical methods group. Syst. Rev. 2013;2:80. doi: 10.1186/2046-4053-2-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark H.D., Wells G.A., Huët C., et al. Assessing the quality of randomized trials: reliability of the Jadad scale. Contr. Clin. Trials. 1999;20:448–452. doi: 10.1016/s0197-2456(99)00026-4. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y.Q., Liu S.P., Liu J.G. Effects of acupuncture and moxibustion on cytokines in patients with ulcerative colitis. Hubei J. Tradit. Chin. Med. 2006;2:11–12. [Google Scholar]
- 16.Zhou G.Y., Jin J.H. Clinical observation on ulcerative colitis treated by electroacupuncture and moxibustion combined with drugs. Zhonghua J. Tradit. Chin. Med. 2008;9:2069–2071. [Google Scholar]
- 17.Jia Y.B., Feng X.X., Liu X.F. Electroacupuncture combined with acupoint sticking in the treatment of chronic ulcerative colitis. Chin. Acupunct. Moxibustion. 2010;30:717–719. [PubMed] [Google Scholar]
- 18.Ge F., Ma X.P., Xiao M.B. Observation on the effect of electroacupuncture combined with sulfasalazine in the treatment of ulcerative colitis. Traffic. Med. 2012;26:173–174. [Google Scholar]
- 19.Teng Y., Dong W.G., Tang S.Q., Baijiang cao. Mixture combined with dense wave electroacupuncture in the treatment of 40 cases of ulcerative colitis. Henan. Chin. Med. 2014;3:2362–2365. [Google Scholar]
- 20.Ge F., Zhu S.L., Xiao M.B., et al. Study on the mechanism of electroacupuncture combined with drugs in the treatment of UC based on the brain-gut axis: clinical data of 31 cases. Jiangsu. Chin. Med. 2014;46:65–67. [Google Scholar]
- 21.Ge F., Ji Y., Ma X.P., et al. Observation on the clinical effect of acupuncture and medicine in the treatment of ulcerative colitis. Chin. Contemp. Med. 2015;22:164–165+168. [Google Scholar]
- 22.Shan H.Y., Zhang X.Y., Gu H.Y., et al. Clinical observation of 40 cases of ulcerative colitis with anxiety and depression treated by electroacupuncture combined with five-tone therapy. Chin. J. Integr. Med. Dig. Med. 2018;26:775–777+782. [Google Scholar]
- 23.Teng Y., Tang S.Q. Efficacy observation of Sibai Huanglian Healing Powder combined with dense wave electroacupuncture in the treatment of 50 cases of ulcerative colitis. Hunan J. Tradit. Chin. Med. 2018;34:62–64. [Google Scholar]
- 24.Chen Z.X. Nanjing University of Traditional Chinese Medicine; 2019. Therapeutic Effect and Mechanism of Electroacupuncture at Bliao Point on Spleen-Kidney Yang Deficiency Type Ulcerative Colitis. [Google Scholar]
- 25.Wang H.L., Chen M., Hou B., et al. Clinical study of Tongxie Yaofang combined with electroacupuncture in the treatment of ulcerative colitis with liver-spleen disharmony Sichuan. Chin. Med. 2020;38:124–127. [Google Scholar]
- 26.Liu H.R., Li J., Lu M., et al. Effect of deep electroacupuncture at Bliao point on Th17/Treg cell immune balance and serum inflammatory factors in patients with ulcerative colitis due to spleen and kidney yang deficiency. Mod. Biol. Adv. Med. 2021;21:4293–4297. [Google Scholar]
- 27.Zhang L., Wu Y.B., Dai Y.K., et al. Efficacy and safety of Qingre-Chushi therapies in active ulcerative colitis: a network meta-analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0257599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song G., Fiocchi C., Achkar J.P. Acupuncture in inflammatory bowel disease. Inflamm. Bowel Dis. 2019;25:1129–1139. doi: 10.1093/ibd/izy371. [DOI] [PubMed] [Google Scholar]
- 29.Murray A., Nguyen T.M., Parker C.E., et al. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2020;8:CD000544. doi: 10.1002/14651858.CD000544.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Travis S.P.L., Schnell D., Krzeski P., et al. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterol. 2013;145:987–995. doi: 10.1053/j.gastro.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 31.Billiet T., Rutgeerts P., Ferrante M., et al. Targeting TNF-α for the treatment of inflammatory bowel disease. Expet Opin. Biol. Ther. 2014;14:75–101. doi: 10.1517/14712598.2014.858695. [DOI] [PubMed] [Google Scholar]
- 32.Tian L., Huang Y.X., Tian M., et al. Downregulation of electroacupuncture at ST36 on TNF-α in rats with ulcerative colitis. World J. Gastroenterol.: WJG. 2003;9:1028. doi: 10.3748/wjg.v9.i5.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo H., Liao X., Wang Q. Application of TCM syndrome score in efficacy evaluation: a comparative study based on 240 randomized controlled trials. Chin. J. Integr. Tradit. West. Med. 2015;35:1261–1266. [PubMed] [Google Scholar]
- 34.Huang Z.Q., Pei J., Wang W.M. A brief review on the research progress of acupuncture and acupoint compatibility. Chin. Acupunct. Moxibustion. 2015;4:19–23. [Google Scholar]
- 35.Liu S., Wang Z.F., Su Y.S., et al. Somatotopic organization and intensity dependence in driving distinct NPY-expressing sympathetic pathways by electroacupuncture. Neuron. 2020;108:436–450. doi: 10.1016/j.neuron.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Y., Qi L., Wang J., et al. Moxibustion activates mast cell degranulation at the ST25 in rats with colitis. World J. Gastroenterol.: WJG. 2011;17:3733. doi: 10.3748/wjg.v17.i32.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou T.S., Han X.X., Yang Y., et al. Protective effect of electroacupuncture on intestinal microecology in rats with ulcerative colitis. Acupunct. Res. 2014;39:27–34. [PubMed] [Google Scholar]
- 38.Song S., An J., Li Y., et al. Electroacupuncture at ST-36 ameliorates DSS-induced acute colitis via regulating macrophage polarization induced by suppressing NLRP3/IL-1β and promoting Nrf2/HO-1. Mol. Immunol. 2019;106:143–152. doi: 10.1016/j.molimm.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y., Cheng J., Zhang Y., et al. Electroacupuncture at ST36 relieves visceral hypersensitivity via the NGF/TrkA/TRPV1 peripheral afferent pathway in a rodent model of post-inflammation rectal hypersensitivity. J. Inflamm. Res. 2021;14:325. doi: 10.2147/JIR.S285146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L.L., Liu T., Wang H.L., et al. To explore the mechanism of acupuncture in the treatment of digestive system diseases based on the synergistic effect of acupoint compatibility. Clin. J. Acupunct. Moxibust. 2022;38:98–101. [Google Scholar]
- 41.Chen S., Shen Y. An overview of the research on electroacupuncture parameter elements. Chin. Acupunct. Moxibustion. 2022;11:107–110. [Google Scholar]
- 42.Li X.L., Jia W.R., Wu X.L., et al. The difference in therapeutic effect of hand acupuncture and electroacupuncture with different stimulation parameters in mice with ulcerative colitis. Univ. Chin. Med. 2022;15:949–957. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.