Abstract
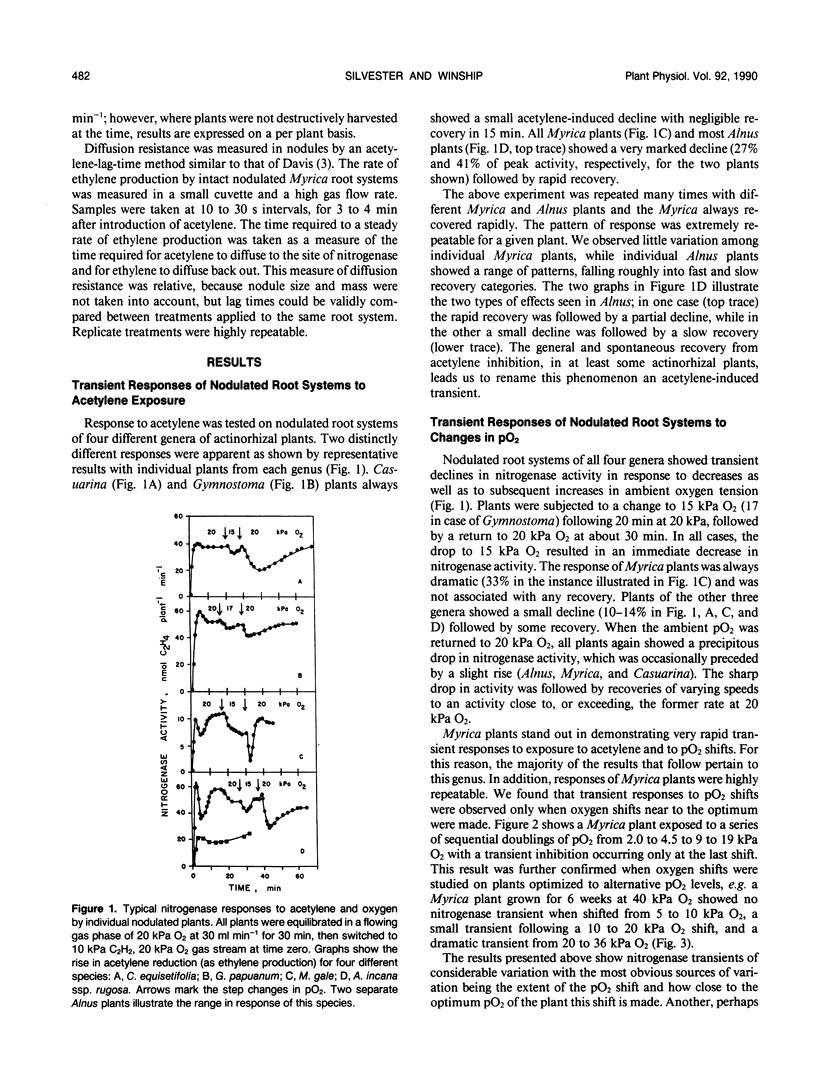
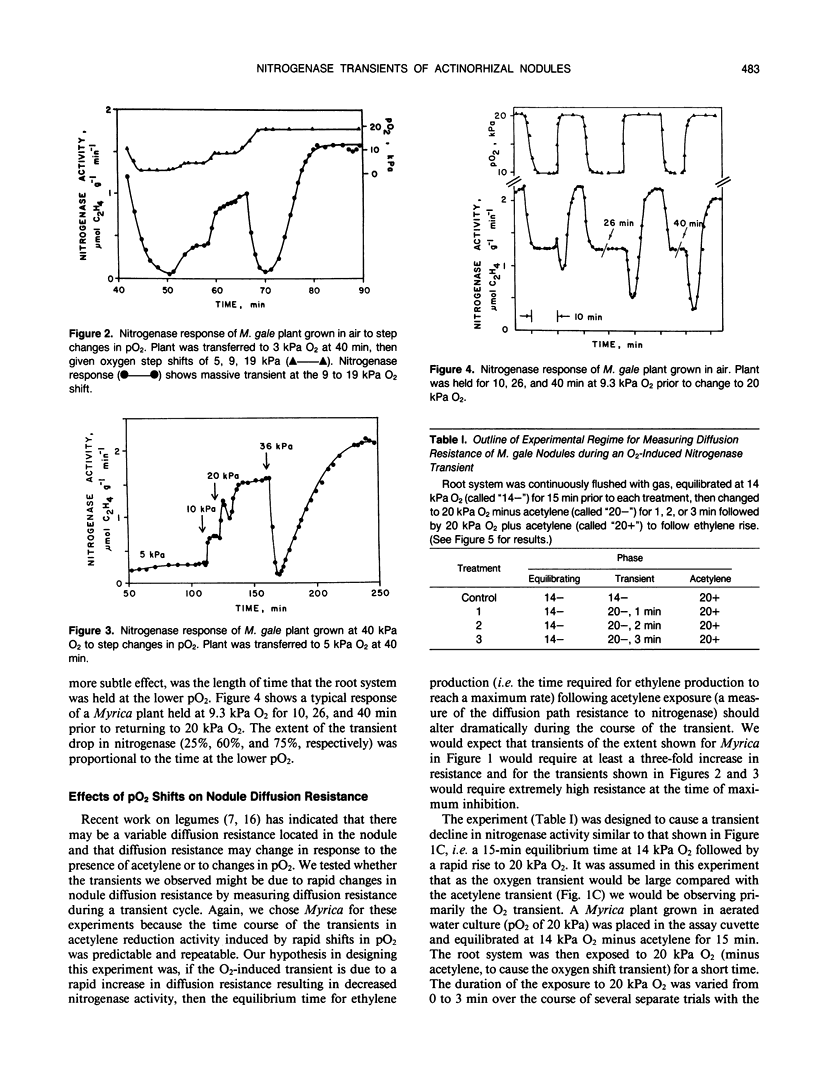
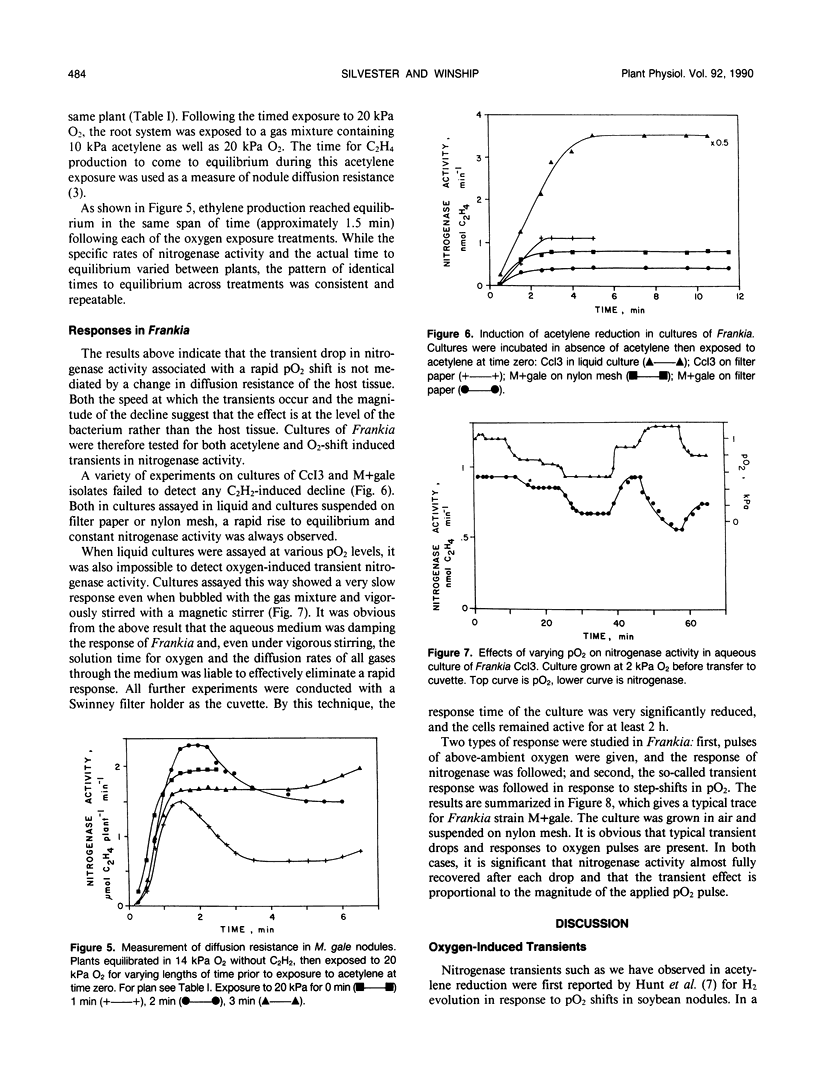
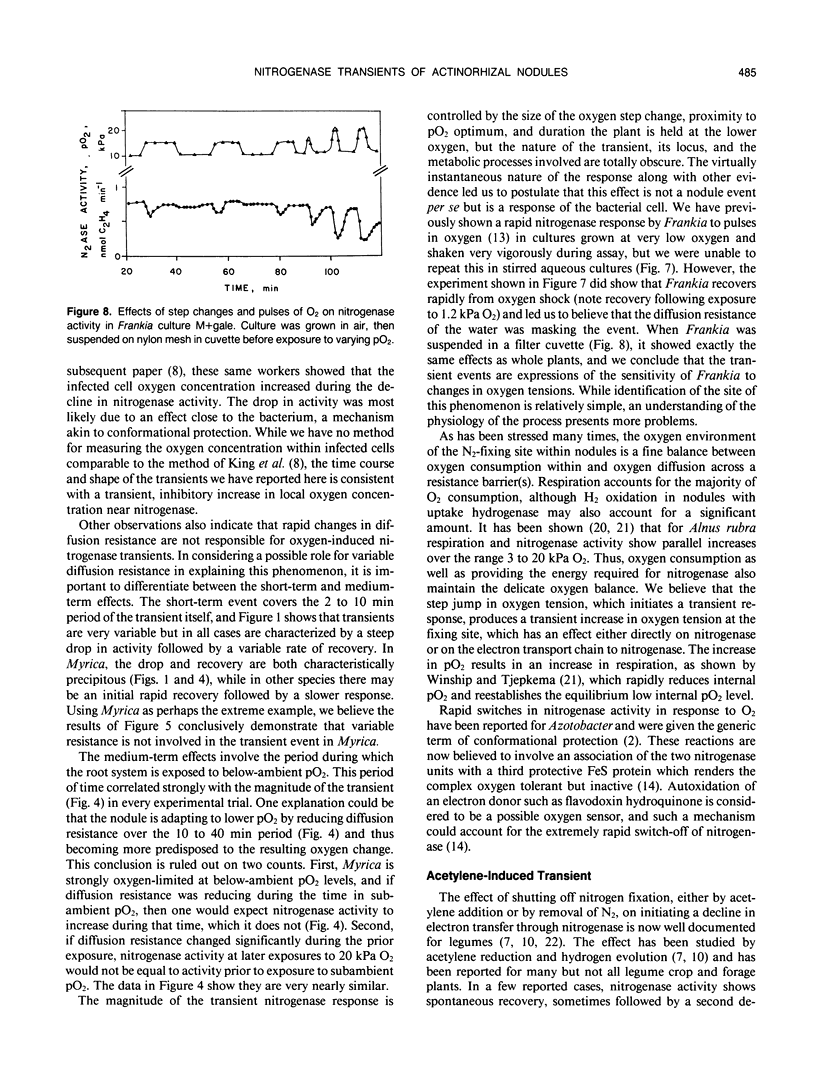
Nitrogenase activity in root nodules of four species of actinorhizal plants showed varying declines in response to exposure to acetylene (10% v/v). Gymnostoma papuanum (S. Moore) L. Johnson. and Casuarina equisetifolia L. nodules showed a small decline (5-15%) with little or no recovery over 15 minutes. Myrica gale L. nodules showed a sharp decline followed by a rapid return to peak activity. Alnus incana ssp. rugosa (Du Roi) Clausen. nodules usually showed varying degrees of decline followed by a slower return to peak or near-peak activity. We call these effects acetylene-induced transients. Rapid increases in oxygen tension also caused dramatic transient decreases in nitrogenase activity in all species. The magnitude of the transient decrease was related to the size of the O2 partial pressure (pO2) rise, to the proximity of the starting and ending oxygen tensions to the pO2 optimum, and to the time for which the plant was exposed to the lower pO2. Oxygen-induced transients, induced both by step jumps in pO2 and by O2 pulses, were also observed in cultures of Frankia. The effects seen in nodules are purely a response by the bacterium and not a nodule effect per se. Oxygen-induced nitrogenase transients in actinorhizal nodules from the plant genera tested here do not appear to be a result of changes in nodule diffusion resistance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dalton H., Postgate J. R. Growth and physiology of Azotobacter chroococcum in continuous culture. J Gen Microbiol. 1969 Jun;56(3):307–319. doi: 10.1099/00221287-56-3-307. [DOI] [PubMed] [Google Scholar]
- Davis L. C. Diffusion of Gases through Plant Tissues : Entry of Acetylene into Legume Nodules. Plant Physiol. 1984 Dec;76(4):854–857. doi: 10.1104/pp.76.4.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S., King B. J., Canvin D. T., Layzell D. B. Steady and nonsteady state gas exchange characteristics of soybean nodules in relation to the oxygen diffusion barrier. Plant Physiol. 1987 May;84(1):164–172. doi: 10.1104/pp.84.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B. J., Hunt S., Weagle G. E., Walsh K. B., Pottier R. H., Canvin D. T., Layzell D. B. Regulation of o(2) concentration in soybean nodules observed by in situ spectroscopic measurement of leghemoglobin oxygenation. Plant Physiol. 1988 Jun;87(2):296–299. doi: 10.1104/pp.87.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry M. A., Fontaine M. S., Tjepkema J. D. Oxygen protection of nitrogenase in Frankia sp. HFPArI3. Arch Microbiol. 1984 Oct;139(2-3):162–166. doi: 10.1007/BF00401993. [DOI] [PubMed] [Google Scholar]
- Parsons R., Silvester W. B., Harris S., Gruijters W. T., Bullivant S. Frankia vesicles provide inducible and absolute oxygen protection for nitrogenase. Plant Physiol. 1987 Apr;83(4):728–731. doi: 10.1104/pp.83.4.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Postgate J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol. 1980;34:183–207. doi: 10.1146/annurev.mi.34.100180.001151. [DOI] [PubMed] [Google Scholar]
- Tjepkema J. D., Schwintzer C. R., Monz C. A. Time course of acetylene reduction in nodules of five actinorhizal genera. Plant Physiol. 1988 Feb;86(2):581–583. doi: 10.1104/pp.86.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]


