Key Points
Question
What is the antitumor activity and safety of dostarlimab monotherapy for patients with mismatch repair deficient and microsatellite instability–high solid tumors?
Findings
In this nonrandomized, open-label, single-group, multicenter clinical trial with 327 participants, the objective response rate was 44.0% in patients with mismatch repair deficient solid tumors, with a median duration of response that was not reached at a median follow-up of 27.7 months. Safety was consistent with the drug class; no new safety signals were observed.
Meaning
In this study, dostarlimab monotherapy demonstrated durable antitumor activity across multiple tumor types in patients with mismatch repair deficient and microsatellite instability–high advanced or recurrent solid tumors.
This nonrandomized controlled trial assesses the antitumor activity and safety of dostarlimab in patients with advanced or recurrent mismatch repair deficiency (dMMR) solid tumors.
Abstract
Importance
Mismatch repair deficiency (dMMR) occurs in various cancers, and these tumors are attractive candidates for anti–programmed cell death 1 therapies, such as dostarlimab, a recently approved immune checkpoint inhibitor.
Objective
To assess the antitumor activity and safety of dostarlimab in patients with advanced or recurrent dMMR solid tumors.
Design, Setting, And Participants
The GARNET trial was a phase 1, open-label, single-group, multicenter study that began enrolling May 8, 2017. Participants had advanced or recurrent dMMR and microsatellite instability–high (MSI-H) or polymerase epsilon (POLE)–altered solid tumors. The data cut for this interim analysis was from November 1, 2021, with median follow-up of 27.7 months.
Interventions
Patients received 500 mg of dostarlimab intravenously every 3 weeks for 4 doses, then 1000 mg every 6 weeks until disease progression, discontinuation, or withdrawal.
Main Outcomes and Measures
The primary objective was to evaluate objective response rate and duration of response in patients with dMMR solid tumors by blinded independent central review using Response Evaluation Criteria in Solid Tumors, version 1.1.
Results
The efficacy population included 327 patients (median [range] age, 63 [24-85] years; 235 [71.9%] female; 7 [2.1%] Asian, 6 [1.8%] Black, and 206 [63.0%] White patients), with 141 patients (43.1%) with dMMR endometrial cancer, 105 patients (32.1%) with dMMR colorectal cancer, and 81 patients (24.8%) with other dMMR tumor types. All patients had at least 1 previous line of therapy. Objective response rate assessed per blinded independent central review for dMMR solid tumors was 44.0% (95% CI, 38.6% to 49.6%). Median duration of response was not reached (range, ≥1.18 to ≥47.21 months); 72.2% of responders (104 of 144) had a response lasting 12 or more months. Median progression-free survival was 6.9 months (95% CI, 4.2 to 13.6 months); probability of progression-free survival at 24 months was 40.6% (95% CI, 35.0% to 46.1%). Median overall survival was not reached (95% CI, 31.6 months to not reached). The most frequent immune-related adverse events were hypothyroidism (25 [6.9%]), alanine aminotransferase increase (21 [5.8%]), and arthralgia (17 [4.7%]). No new safety concerns were identified.
Conclusions And Relevance
In this nonrandomized controlled trial, dostarlimab was a well-tolerated treatment option with rapid, robust, and durable antitumor activity in patients with diverse dMMR solid tumors. These findings suggest that dostarlimab provides meaningful long-term benefit in a population with high unmet need.
Trial Registration
ClinicalTrials.gov Identifier: NCT02715284
Introduction
Classification and treatment of cancers have historically been based on tumor type and histological subtype. However, advancements in precision medicine have led to the development of biomarker-driven tumor-agnostic treatments.1 This change is reflected by biomarker-linked approvals, including the recent US Food and Drug Administration tissue-agnostic drug approvals for tumors characterized by defective mismatch repair (MMR) machinery.1
In MMR protein-deficient (dMMR) tumors, mismatches accumulate and lead to genome instability with many mutations in microsatellites, leading to microsatellite instability (MSI), which can have either a sporadic origin (somatic mutation or promoter hypermethylation) or an inherited origin (Lynch syndrome).2 Tumors with dMMR and/or high MSI (MSI-H) have been found to have increased tumor-infiltrating lymphocytes and increased expression of programmed cell death 1 (PD-1) receptor and its ligands (PD-L1 and PD-L2). Simultaneously, the MSI-driven oncogenic pathway leads to a high tumor mutational burden (TMB), with highly immunogenic neoantigens arising from frameshift mutations. Together, these features make these dMMR and MSI-H tumors attractive candidates for anti–PD-1 and anti–PD-L1 checkpoint inhibition.2,3,4,5 The highest incidence of dMMR and MSI-H has been reported in endometrial cancer (EC; 25%-30%) and colorectal cancer (CRC; 10%-15%) of all stages.4,5,6,7 However, dMMR and MSI-H is also found in other cancers, including gastric, small intestine, urothelial, central nervous system, and several other solid tumors.6,7 Regardless of tumor type, patients with advanced dMMR and MSI-H tumors that failed to respond to systemic therapy have limited treatment options, and there is a high unmet therapeutic need in these settings.8
Alterations in other DNA proofreading proteins can also be associated with hypermutagenesis and may lead to tumors that are susceptible to response to anti–PD-1/PD-L1 therapy.9,10,11 Defective proofreading polymerase epsilon (POLE) alterations are rare and have been predominantly described in MMR proficient (MMRp) CRC and EC as sporadic and germline events.9,10,11 Although patients with pathogenic POLE alterations have been shown to benefit from immune checkpoint inhibitor therapy, the overall number of patients with POLE alterations of advanced solid tumors in trials has been small, and thus there is a gap in knowledge of anti–PD-1 therapy in regards to POLE alteration.9,10,11
The Study of TSR-042, an Anti–PD-1 Monoclonal Antibody, in Participants With Advanced Solid Tumors (GARNET) Trial is a phase 1, multicenter, open-label, single-group study of dostarlimab monotherapy, an anti–PD-1 monoclonal antibody, in patients with advanced and recurrent solid tumors.12,13 GARNET has prospectively evaluated dostarlimab in patients with dMMR and MSI-H recurrent or advanced solid tumors.13,14 In the United States, dostarlimab is approved for patients with dMMR recurrent or advanced solid tumors that have progressed on or following prior treatment and who have no satisfactory alternative treatment options. It is also approved in adult patients with dMMR (United States) or dMMR and MSI-H (European Union and United Kingdom) recurrent or advanced EC that has progressed on or following prior treatment with a platinum-containing regimen.15,16,17,18 Here, we report on a prespecified population for an interim analysis of dostarlimab among patients with dMMR and MSI-H or POLE-altered solid tumors.
Methods
Study Design
GARNET is a phase 1, single-group study of dostarlimab monotherapy in patients with advanced and recurrent solid tumors. The trial protocol and statistical analysis plan are available in Supplement 1.
In parts 1 and 2A of the trial, the recommended therapeutic dose (RTD) was determined to be 500 mg intravenously every 3 weeks for 4 cycles, then 1000 mg intravenously every 6 weeks until discontinuation.19 Part 2B of the ongoing GARNET study explores antitumor activity and safety in prespecified tumor types using the RTD. Cohort A1 enrolled patients with dMMR and MSI-H EC, and cohort F enrolled patients with dMMR and MSI-H non-EC or POLE-altered solid tumors. Key inclusion and exclusion criteria, enrolling sites, sample size, and brief statistical analysis overview can be found in eAppendix 1 in Supplement 2. Data from the first and second prespecified interim analyses, which included the endometrial cancer cohorts (cohort A1 [dMMR and MSI-H EC] and cohort A2 [MMRp/microsatellite stable (MSS) EC]), were published prior to this prespecified third interim analysis.14,20
The study was initiated on April 10, 2017; enrollment in cohort A1 is complete, whereas cohort F is open for enrollment. Data analysis of this interim analysis was performed using a data cut date of November 1, 2021, with a median follow-up of 27.7 months.
Full sample size information for cohorts A1 and F can be found in eAppendix 1 in Supplement 2 and has been previously published for cohort A1.14 In short, a total sample size of 300 patients evaluable for antitumor activity from cohorts A1 and F combined allowed the lower-limit boundary of the exact 95% CI to exclude a response rate of 30% or less, assuming the observed objective response rate (ORR) was 35%.
The trial was performed in accordance with the principles of the Declaration of Helsinki, Good Clinical Practices, and all local laws. Part 2B of the study was overseen by an independent data and safety monitoring committee. The study protocol and/or other relevant documents received approval by the institutional ethics committee, institutional review board, and/or relevant competent authorities at each site. Written informed consent was obtained from all subjects or a legal surrogate. This trial follows the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guidelines for nonrandomized trial studies.
Biomarker Screening
Patients were screened prospectively for MMR/MSI status using immunohistochemistry (IHC), polymerase chain reaction, or next-generation sequencing. For patients enrolled after protocol amendment 5, eligibility was determined by IHC performed in a certified local laboratory or by central testing if local IHC testing was not available.7,21,22,23,24
Patients enrolled on the study based on POLE-alteration status must have had local results available showing tumor alteration in the exonuclease domain of the POLE gene (amino acid residues 268-471) prior to screening for assignment into cohort F (prospective POLE alteration). Additional biomarker screening information can be found in eAppendix 1 in Supplement 2.
Patients
A total of 363 patients with dMMR and MSI-H and/or POLE-altered tumors were enrolled and received dostarlimab (Figure 1). The efficacy population consisted of 347 patients with dMMR and MSI-H and/or POLE-altered tumors, which included 327 patients with dMMR tumors.
Figure 1. Enrollment and Outcomes.
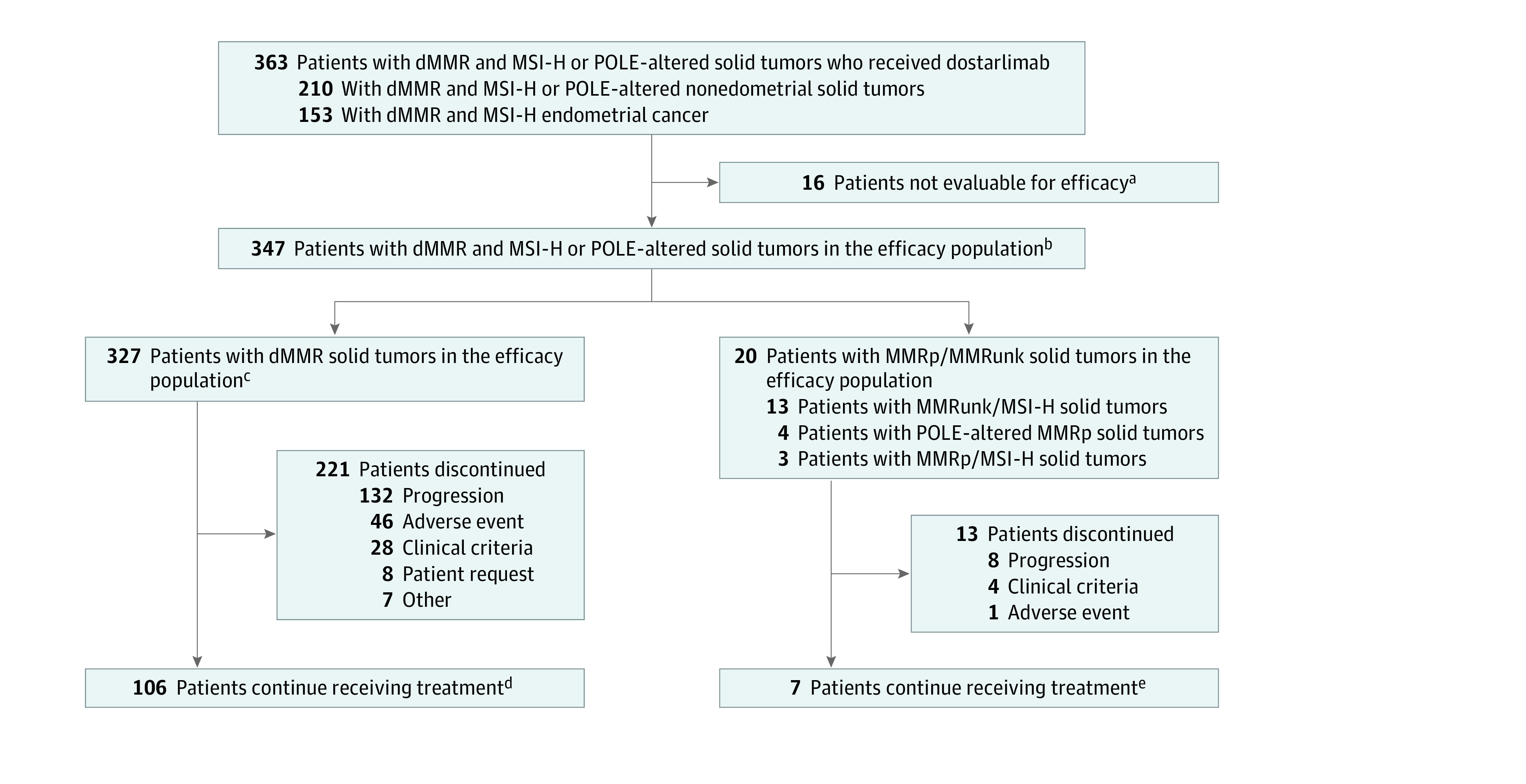
dMMR indicates mismatch repair deficient; MMRp, mismatch repair proficient; MMRunk, mismatch repair status unknown; MSI-H, microsatellite instability–high; POLE, polymerase epsilon.
aSixteen patients had no measurable disease per blinded independent central review (BICR) at baseline and were excluded from the efficacy population.
bAll patients with dMMR and MSI-H or POLE-altered solid tumors who had received at least 1 dose of dostarlimab, had at least 1 BICR-confirmed measurable lesion at baseline, and had the opportunity to be followed up for at least 6 months as of the data cutoff date were included in the efficacy population, regardless of whether the patient had a postbaseline tumor assessment.
cTotal of 327 patients with dMMR solid tumors (including 2 who were also POLE-altered).
dTotal of 106 patients with dMMR solid tumors (including 2 who also had POLE alterations).
eSeven patients with MMR unknown/MSI-H tumors or MMRp tumors (including 2 who had POLE alterations).
Objectives
Efficacy End Points
The primary end point for each cohort (A1 and F) was to evaluate the antitumor activity of dostarlimab in dMMR tumors in terms of ORR and duration of response (DOR) by blinded independent central review (BICR) using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 in the efficacy population. Durable responses were defined as those responses with a duration of 12 months or longer.
Prespecified secondary end points included a combined analysis of ORR and DOR by BICR using RECIST version 1.1 in dMMR tumors from cohorts A1 and F in the efficacy population. Additional prespecified secondary end points for cohorts A1 and F, individually, included disease control rate and progression-free survival (PFS), and overall survival (OS) in the efficacy population.
Post Hoc Analyses
Post hoc and exploratory analyses included evaluation of the antitumor activity of dostarlimab in terms of ORR, DOR, PFS, and OS by tumor type (EC, CRC, non-CRC/non-EC, gastrointestinal, pancreatic, small intestinal, and ovarian). These analyses were also conducted by biomarker status, including POLE-altered tumors (both prospective and retrospective POLE-altered tumors), PD-L1, and TMB status as well as MSI-H tumors with discordant or unknown MMR results.
Safety Analyses
Safety analyses included incidence of treatment-emergent adverse events, immune-related adverse events (irAEs) of interest, and serious adverse events occurring while patients were receiving treatment or up to 90 days after the end of treatment.
Statistical Analysis
Point estimates and exact 2-sided 95% CIs were provided for ORR; DOR was analyzed using the Kaplan-Meier method. Patients who did not achieve a confirmed response, either complete response (CR) or partial response (PR), were excluded from the DOR analysis. Median follow-up time was calculated using the reverse Kaplan-Meier method. Time-to-event analyses were performed using Kaplan-Meier methods. All statistical outputs were generated using SAS version 9.4 (SAS Institute). No formal hypothesis-testing analysis of adverse event incidence rates was performed. Additional information can be found in eAppendix 1 in Supplement 2 and the trial protocol (Supplement 1).
Results
Patients
Demographic and baseline characteristics of the efficacy population are included in Table 1. The median age was 63 years (range, 24-85 years), and all patients had received at least 1 prior line of therapy. Among the 327 patients with dMMR tumors, there were 235 (71.9%) female patients; 7 (2.1%) Asian patients, 6 (1.8%) Black patients, and 206 (63.0%) White patients. Overall, 141 (43.1%) had EC, 105 (32.1%) had CRC, and 81 (24.8%) had other tumor types.
Table 1. Combined Cohort Analysis: Demographics and Baseline Characteristics.
| Characteristic | Patients, No. (%) | |
|---|---|---|
| dMMR solid tumors (n = 327) | dMMR and MSI-H and/or POLE-altered (n = 347) | |
| Age, median (range), y | 63 (24-85) | 63 (24-85) |
| Sex | ||
| Female | 235 (71.9) | 241 (69.5) |
| Male | 92 (28.1) | 106 (30.5) |
| Race | ||
| American Indian or Alaska Native | 3 (0.9) | 3 (0.9) |
| Asian | 7 (2.1) | 7 (2.0) |
| Black | 6 (1.8) | 6 (1.7) |
| Native Hawaiian or Other Pacific Islander | 0 | 0 |
| White | 206 (63.0) | 223 (64.3) |
| Other, unknown, or not reported | 105 (32.1) | 108 (31.1) |
| Ethnicity | ||
| Hispanic or Latino | 10 (3.1) | 10 (2.9) |
| Not Hispanic or Latino | 205 (62.7) | 222 (64.0) |
| Unknown or not reported | 112 (34.3) | 115 (33.1) |
| ECOG performance status | ||
| 0 | 129 (39.4) | 138 (39.8) |
| 1 | 198 (60.6) | 208 (60.0) |
| Prior lines of therapya | ||
| 1 | 137 (41.9) | 141 (40.6) |
| 2 | 118 (36.1) | 131 (37.8) |
| ≥3 | 72 (22.0) | 75 (21.6) |
| Prior therapy type | ||
| Surgery | 279 (85.3) | 296 (85.3) |
| Radiotherapy | 140 (42.8) | 147 (42.4) |
| Tumor types | ||
| Endometrial cancer | 141 (43.1) | 143 (41.2) |
| Colorectal cancer | 105 (32.1) | 115 (33.1) |
| Gastric and gastroesophageal junction cancer | 21 (6.4) | 22 (6.3) |
| Small-intestinal cancer | 19 (5.8) | 23 (6.6) |
| Pancreatic carcinoma | 11 (3.4) | 12 (3.5) |
| Biliary neoplasm | 10 (3.1) | 11 (3.2) |
| Ovarian cancer | 7 (2.1) | 7 (2.0) |
| Otherb | 13 (4.0) | 14 (4.0)b |
| Biomarkers, No. | ||
| dMMR | 327c | 327c |
| MSI-H/MMRunk | NA | 13d |
| MSI-H/MMRp | NA | 3d |
| POLE alteration and MMRp | NA | 4 |
Abbreviations: dMMR, mismatch repair deficient; ECOG, Eastern Cooperative Oncology Group; MMRp, mismatch repair proficient; MMRunk, mismatch repair status unknown; MSI-H, microsatellite instability–high; NA, not applicable; POLE, polymerase epsilon.
Includes lines of therapy in the adjuvant setting.
Includes adrenal cortical carcinoma, cancer of unknown origin, esophageal cancer, mesothelioma, breast cancer, malignant neoplasm of the female genitals, renal cell carcinoma, sarcoma, and thymic tumor.
Includes 3 patients with endometrial cancer and 2 patients with nonendometrial cancer that was POLE altered and dMMR by IHC. The 3 patients with endometrial cancer were retrospectively identified as having POLE alterations.
Excludes patients with POLE alterations.
Because of a change in study design after protocol amendment 5, cohorts A1 and F also included patients with MSI-H/MMR unknown tumors or discordant MMR/MSI status. Cohort F also included patients prospectively enrolled based on POLE exonuclease domain alteration status. Cohorts A1 and F included a total of 347 patients in the efficacy population; specifically, in addition to the 327 dMMR tumors, there were 13 patients enrolled with MSI-H/MMR unknown tumors (2 EC and 11 non-EC), 3 patients with MSI-H/MMRp tumors, and 4 patients with POLE-altered tumors that were MMRp. Included among the 327 tumors that were dMMR, there were 5 tumors that were also POLE altered (Figure 1 and Table 1).
Antitumor Activity
Prespecified Efficacy Analyses
At the time of the data cut, the ORR as assessed per BICR for dMMR solid tumors (n = 327) was 44.0% (144 of 327; 95% CI, 38.6%-49.6%) (Table 2 and eTable 1 in Supplement 2): there were 43 CRs (13.1%) and 101 PRs (30.9%). The disease control rate was 58.4%.
Table 2. Efficacy Results by Tumor Type for Patients With dMMR and MSI-H or POLE-Altered Tumors in the Efficacy Population.
| Tumor type | Patients, No. | No. (%) | ORR, % (95% CI) | mDOR (95% CI), mo | mPFS (95% CI), mo | mOS (95% CI), mo | |
|---|---|---|---|---|---|---|---|
| CR | PR | ||||||
| Overall | 347 | 46 (13.3) | 107 (30.8) | 44.1 (38.8-49.5) | NR (NR-NR) | 7.0 (4.2-13.8) | NR (39.9-NR) |
| dMMR overall | 327 | 43 (13.1) | 101 (30.9) | 44.0 (38.6-49.6) | NR (NR-NR) | 6.9 (4.2-13.6) | NR (31.6-NR) |
| EC | 143 | 23 (16.1) | 42 (29.4) | 45.5 (37.1-54.0) | NR (38.9-NR) | 6.0 (4.1-18.0) | NR (25.7-NR) |
| Non-EC | 204 | 23 (11.3) | 65 (31.9) | 43.1 (36.2-50.2) | NR (NR-NR) | 7.1 (3.6-19.5) | NR (31.5-NR) |
| Colorectal cancer | 115 | 14 (12.2) | 36 (31.3) | 43.5 (34.3-53.0) | NR (NR-NR) | 8.4 (3.4-NR) | NR (NR-NR) |
| Gastric cancer | 22 | 1 (4.5) | 9 (40.9) | 45.5 (24.4-67.8) | NR (17.5-NR) | 5.5 (2.8-NR) | 20.1 (6.7-NR) |
| Small-intestinal cancer | 23 | 5 (21.7) | 4 (17.4) | 39.1 (19.7-61.5) | NR (8.3-NR) | 8.1 (2.5-16.5) | 31.6 (8.2-NR) |
| Pancreatic carcinoma | 12 | 0 | 5 (41.7) | 41.7 (15.2-72.3) | NR (NR-NR) | 3.3 (2.6-NR) | 12.7 (3.1-NR) |
| Ovarian cancer | 7 | 0 | 3 (42.9) | 42.9 (9.9-81.6) | NAa | NAa | NAa |
| Otherb | 25 | 3 (12.0) | 8 (32.0) | 44.0 (24.4-65.1) | 4.5 (2.5-NR) | NR (8.8-NR) | NR (13.5-NR) |
Abbreviations: CR, complete response; dMMR, mismatch repair deficient; EC, endometrial cancer; mDOR, median duration of response; mOS, median overall survival; mPFS, median progression-free survival; NA, not applicable; NR, not reached; ORR, objective response rate; POLE, polymerase epsilon; PR, partial response.
mDOR, mPFS, and mOS, all with 95% CIs, could not be calculated for the ovarian cancer subgroup because of the small size of the population. However, mDOR was not reached, with a range of 6.0 or greater to 36.4 or greater months.
Other includes adrenal cortical carcinoma, biliary neoplasm, brain cancer, breast cancer, cancer of unknown primary, esophageal cancer, malignant neoplasm of the female genitals, mesothelioma, prostate cancer, renal cell carcinoma, sarcoma, and thymic tumor.
The median DOR (mDOR) for patients with dMMR solid tumors (n = 327) was not reached (range, ≥1.18 to ≥47.21 months). Responses were durable, with 104 of 144 responders (72.2%) having a response that lasted 12 months or longer. The probability of remaining in response at 6 months was 95.7%, at 12 months was 92.4%, and at 24 months was 84.7% (eTable 1 and eFigure 1 in Supplement 2).
The mPFS as assessed per BICR for all patients with dMMR solid tumors (n = 327) was 6.9 months (95% CI, 4.2-13.6 months) (Figure 2 and eTable 2 in Supplement 2). The probabilities of PFS at 6, 12, 24, and 36 months were 50.5% (95% CI, 44.9%-55.9%), 45.8% (95% CI, 40.2%-51.2%), 40.6% (95% CI, 35.0%-46.1%), and 39.7% (95% CI, 33.9%-45.3%), respectively (Figure 2 and eTable 2 in Supplement 2). The median OS (mOS) for all patients with dMMR solid tumors (n = 327) was not reached (95% CI, 31.6 months to not reached) (Figure 2 and eTable 3 in Supplement 2). The probabilities of OS at 6, 12, 24, and 36 months were 82.6% (95% CI, 78.0%-86.2%), 70.6% (95% CI, 65.3%-75.3%), 58.4% (95% CI, 52.5%-63.9%), and 55.9% (95% CI, 49.7%-61.7%), respectively (Figure 2 and eTable 3 in Supplement 2).
Figure 2. Progression-Free Survival and Overall Survival for Patients With Mismatch Repair Deficient (dMMR) Solid Tumors.
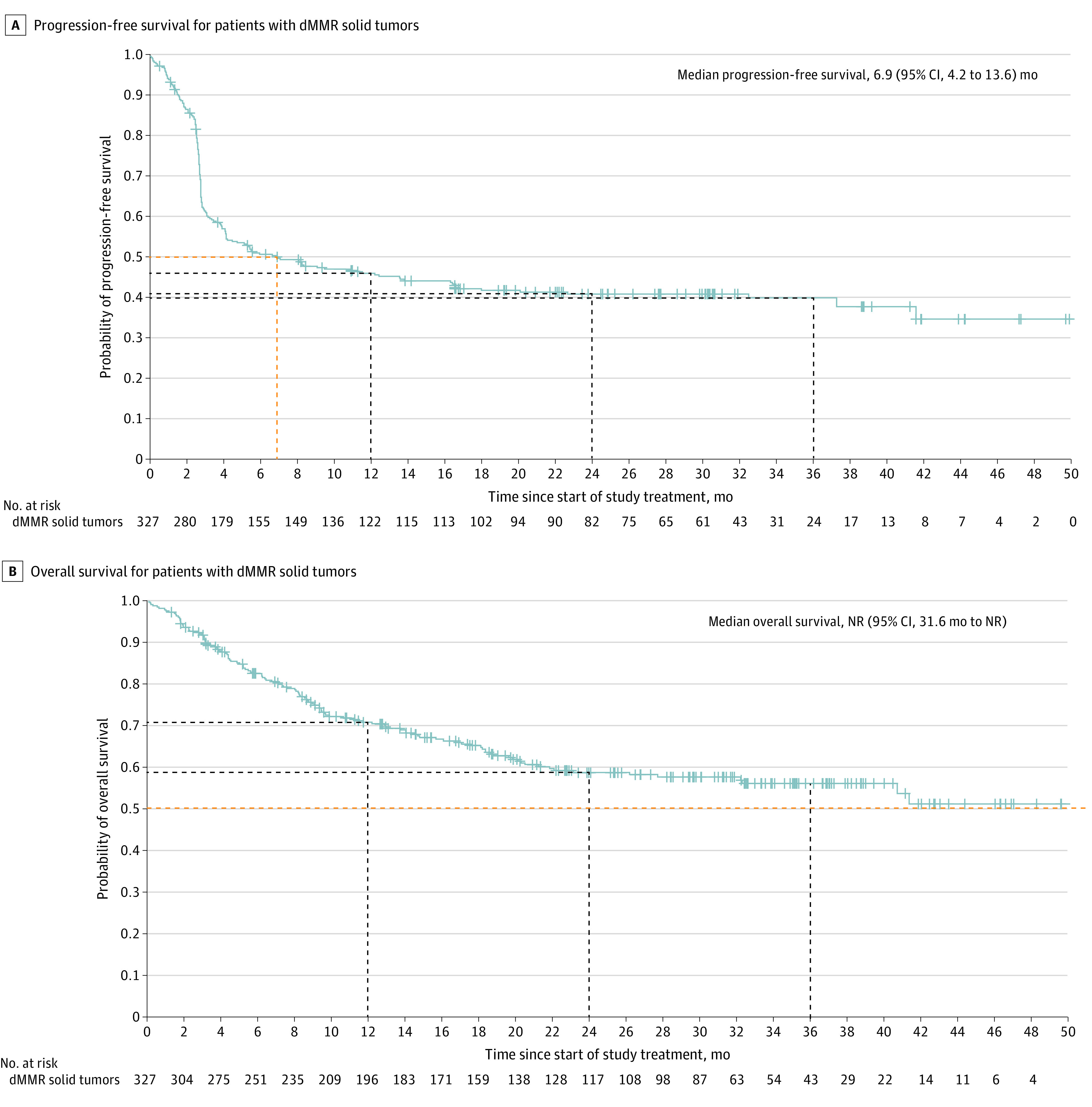
A, Dashed lines indicate study time points at 6, 12, 24, and 36 months. B, Dashed lines indicate study time points at 12, 24, and 36 months. Plus signs indicate censoring; NR, not reached.
Post Hoc Analyses
When included in the analysis, the results of the antitumor activity outcomes for the full efficacy population, which includes patients with dMMR and MSI-H or POLE-altered tumors (n = 347), were similar to the dMMR population, with an ORR of 44.1% (153 of 347; 95% CI, 38.8%-49.5%) (eTable 1 in Supplement 2). The mDOR was not reached (range, ≥1.18 to ≥47.21 months) (Table 2), mPFS was 7.0 months (95% CI, 4.2-13.8 months) (eTable 2 in Supplement 2), and mOS (n = 363) was not reached (95% CI, 39.9 months to not reached) (eTable 3 in Supplement 2).
Analyses were completed to determine the ORR, mDOR, mPFS, and mOS by tumor type in the full efficacy population (n = 347). ORR was similar across tumor types: 45.5% in patients with EC (65 of 143), 43.5% in patients with CRC (50 of 115), 45.5% in patients with gastric cancer (10 of 22), 39.1% in patients with small-intestinal cancer (9 of 23), 41.7% in patients with pancreatic cancer (5 of 12), 42.9% in patients with ovarian cancer (3 of 7), and 44.0% in a combined analysis of other tumor types (11 of 25) (Table 2). DOR, mPFS, and mOS were also similar across tumor types (Table 2 and eFigures 2 and 3 in Supplement 2). Because of the small number of patients with ovarian cancer (n = 7), an analysis of PFS and OS could not be completed; however, ORR was 42.9% (3 of 7; 95% CI, 9.9%-81.6%), and mDOR was not reached (range, ≥6.0 to ≥36.4 months). An analysis of PFS per best overall response can be found in eAppendix 2 and eTable 4 in Supplement 2.
Eleven patients with POLE alterations were enrolled (eFigure 4 and eTable 5 in Supplement 2). All had alterations in the exonuclease domain. The ORR for patients with POLE-altered solid tumors was 54.5% (6 of 11; 95% CI, 23.4%-83.3%). With a median duration of follow-up of 38.7 months, neither the mDOR (range, 16.9 to ≥44.4 months) or mOS (95% CI, 1.8 months to not reached) were reached (eTable 6 and eFigure 5 in Supplement 2). Median PFS was 19.5 months (95% CI, 1.2 months to not reached) (eTable 6 in Supplement 2). Three of 6 patients with MMRp/POLE-altered tumors achieved a response (including 1 patient with CRC who achieved a CR), and 3 of 5 patients with dMMR/POLE-altered tumors achieved a response (eFigure 5 in Supplement 2).
An exploratory analysis of the biomarkers TMB and PD-L1 was performed. It is included in eAppendix 3, eFigures 6 to 9, and eTable 7 in Supplement 2.
Safety
The safety profile of dostarlimab in dMMR and MSI-H or POLE-altered solid tumors was consistent with other anti–PD-(L)1 antibodies, and no new safety concerns were identified. Most treatment-related adverse events (TRAEs) were grade 1 or 2; 16.3% of patients (59 of 363) experienced a grade 3 or greater TRAE (Table 3). Twenty-five patients (6.9%) experienced a TRAE that led to discontinuation; the most frequent TRAEs leading to discontinuation were alanine aminotransferase increase (5 patients [1.4%]) and pneumonitis (4 [1.1%]). The most common grade 3 or greater TRAEs were anemia (9 [2.5%]), alanine aminotransferase increase (7 [1.9%]), and lipase increase (5 [1.4%]). irAEs were experienced by 34.2% of patients (124 of 363); 11.0% of these (40) were grade 3 or greater. The most frequent irAEs were hypothyroidism (25 [6.9%]), alanine aminotransferase increase (21 [5.8%]), and arthralgia (17 [4.7%]). A recent analysis showed no increase in toxic effects at the transition from receiving 500 mg every 3 weeks to receiving 1000 mg every 6 weeks dosing.25
Table 3. Safety Profile of Dostarlimab.
| Event | Patients with dMMR and MSI-H or POLE-altered solid tumors, No. (%) (n = 363)a |
|---|---|
| Any TEAE | 358 (98.6) |
| Grade ≥3 TEAE | 200 (55.1) |
| Any TRAE | 257 (70.8) |
| Grade ≥3 TRAE | 59 (16.3) |
| Any irAE | 124 (34.2) |
| Grade ≥3 irAE | 40 (11.0) |
| Treatment-related SAE | 35 (9.6) |
| Any TRAE leading to discontinuation | 25 (6.9) |
| TRAE leading to deathb | 2 (0.6) |
| TRAEs leading to discontinuation in ≥1% of patients | |
| Alanine aminotransferase increased | 5 (1.4) |
| Pneumonitis | 4 (1.1) |
| Any-grade TRAEs in ≥10% of patients | |
| Diarrhea | 56 (15.4) |
| Asthenia | 52 (14.3) |
| Pruritus | 47 (12.9) |
| Fatigue | 43 (11.8) |
| Hypothyroidism | 37 (10.2) |
| Grade ≥3 TRAEs in ≥1% of patients | |
| Anemia | 9 (2.5) |
| Alanine aminotransferase increased | 7 (1.9) |
| Lipase increased | 5 (1.3) |
| irAEs in ≥2% of patientsc | |
| Hypothyroidism | 25 (6.9) |
| Alanine aminotransferase increased | 21 (5.8) |
| Arthralgia | 17 (4.7) |
| Aspartate aminotransferase increased | 16 (4.4) |
| Pruritus | 12 (3.3) |
| Pneumonitis | 11 (3.0) |
| Rash | 11 (3.0) |
| Hyperthyroidism | 10 (2.8) |
Abbreviations: dMMR, mismatch repair deficient; irAE, immune-related adverse event; MSI-H, microsatellite instability–high; POLE, polymerase epsilon; SAE, serious adverse event; TEAE, treatment-emergent adverse event; TRAE, treatment-related adverse event.
Includes all patients with dMMR and MSI-H or POLE alterations who received at least 1 dose of dostarlimab.
One patient with biliary neoplasm had hepatic ischemia, and 1 patient with colorectal cancer died of suicide; these events were attributed by investigators to study treatment.
irAEs were defined as grade 2 and greater from a predefined list.
There were 2 deaths attributed by investigators to study treatment. One patient with biliary neoplasm had hepatic ischemia, and 1 patient with CRC died of suicide.
Discussion
This interim analysis provided a long-term follow-up of patients with dMMR and MSI-H and POLE-altered solid tumors who received dostarlimab. Dostarlimab demonstrated clinically meaningful antitumor activity in 327 patients with dMMR solid tumors across various tumor types in the efficacy population. This was a prespecified population based on previously seen responses by dMMR and MSI-H cancers to checkpoint inhibition.3,4,7,21 The study met its primary end point in dMMR solid tumors with an ORR of 44.0% (95% CI, 38.6%-49.6%). The lower limit of the 95% CI did not cross the prespecified threshold of 30%, allowing for rejection of the null hypothesis. The ORR was consistent in both EC and non-EC solid tumors. mDOR was not reached, with a probability of remaining in response at 2 years of 84.7%, demonstrating that for responders, the responses were durable. The PFS Kaplan-Meier curve begins to plateau just below the median, consistent with observation of sustained CR and PR in most patients who achieved an objective response.
This study demonstrated similar ORR, mDOR, and mPFS in all tumor types, regardless of histology or cell of origin. With a median follow-up of 27.7 months for the dMMR population and of 29.1 months in the full population (dMMR and MSI-H or POLE-altered), mOS was not reached in either population, which suggests meaningful clinical benefit in this biomarker-selected patient population. This finding builds on the body of evidence for dMMR as a meaningful tumor-agnostic biomarker, thus providing additional evidence on the importance of testing for dMMR status through the use of a companion test, such as IHC.7,24
Anti–PD-1 therapies have been tested in other studies of dMMR CRC or solid tumors (various primary origin), including pembrolizumab in KEYNOTE-164 and KEYNOTE-158 and nivolumab in CHECKMATE 142 and NCI-MATCH group Z1D.26,27,28,29,30 However, the trial designs and patient populations differed from GARNET. Specifically, KEYNOTE-158 and NCI-MATCH group Z1D did not include patients with CRC, whereas KEYNOTE-164 and CHECKMATE 142 were 100% CRC, and GARNET enrolled diverse tumor types for a combined analysis.26,27,28,29,30 Antitumor activity and safety comparisons across different studies have limitations and should be done with caution and awareness of the heterogeneity in study designs.
Few studies of immune checkpoint inhibitors have been conducted on patients with advanced disease harboring POLE alterations.9,10,11 Although only a small number of patients were included (n = 11), GARNET is one of the largest reports of immune checkpoint inhibitors in POLE-altered cancers in a prospective clinical study. Dostarlimab demonstrated encouraging antitumor activity in patients with pathogenic POLE alterations in the exonuclease domain in GARNET, with ORR of 54.5%, DOR not reached with 3 years or longer of median follow-up, and PFS of 1 year or longer, consistent with reports of other immune checkpoint inhibitors.9,10 The POLE-altered population in this trial was small, thus limiting the certainty of the results.
This study has several points to highlight relative to other published studies of anti–PD-1 therapies in patients with dMMR and MSI-H advanced metastatic tumors.3,5,26,27,28,30 First, MMR status, as assessed by IHC, is an appropriate biomarker to select patients for treatment with dostarlimab because of consistently high antitumor activity results across tumor types. Second, to qualify for GARNET, patients were required to have evidence of prior treatment progression by BICR, ensuring that most patients treated with dostarlimab in this study had actively progressing tumors. Finally, this study suggests that TMB and PD-L1 may be biomarkers that, when added to MMR status, identify a population who are more likely to respond to dostarlimab; however, this addition requires validation in a prospective analysis.
Limitations
There are several limitations to this study. GARNET is a single-group trial of dostarlimab and was not designed to assess superiority or equivalence with other therapies. Cohorts A1 (dMMR and MSI-H EC) and F (dMMR and MSI-H or POLE-altered non-EC solid tumors) were a predefined biomarker-selected populations with inclusion by local IHC, polymerase chain reaction, or next-generation sequencing testing, without centralized analysis before inclusion, which, although a limitation, reflects typical laboratory practice. Evaluation was by RECIST version 1.1, and therefore does not permit assessment of the rate of patients with pseudo-progression; consequently these patients would be considered as an event in the PFS evaluation, potentially underestimating the true treatment activity of dostarlimab.31 Furthermore, the sample sizes were small for some individual tumor types, and therefore the results observed in this study may not reflect their broader populations.
Conclusions
Advanced cancer that has progressed on or following prior chemotherapy with or without targeted therapy is typically associated with poor outcomes. The antitumor activity data presented here support the use of dostarlimab monotherapy in patients with dMMR solid tumors and provide evidence on the durability of response in this setting. The safety profile was manageable and consistent with prior reports. The additional post hoc tumor-specific and biomarker analyses provided in this report are of academic interest and suggest that prospective studies evaluating POLE alterations, as well as TMB and PD-L1 expression, may demonstrate that these biomarkers, in addition to dMMR and MSI-H, improve identification of patients most likely to benefit from dostarlimab. In summary, dostarlimab provided clinically meaningful long-term benefit with a tolerable safety profile in dMMR solid tumors with a high unmet need.
Trial Protocol and Statistical Analysis Plan
eAppendix 1. Sites and Investigators and Supplementary Methods
eFigure 1. Duration of Treatment for Responders With dMMR Solid Tumors
eTable 1. Antitumor Activity Analysis
eTable 2. Progression-Free Survival
eTable 3. Overall Survival
eFigure 2. Progression-Free Survival by Tumor Type
eFigure 3. Overall Survival by Tumor Type
eAppendix 2. Post Hoc Analysis of PFS by BOR
eTable 4. Progression-Free Survival by Best Overall Response per BICR in the Overall Efficacy Population
eFigure 4. Enrollment and Outcomes for Patients With POLE-Altered Tumors
eTable 5. Demographics and Baseline Characteristics of Patients With POLE Alterations
eTable 6. POLE-Altered Antitumor Activity Analysis by BICR
eFigure 5. Duration of Treatment for Patients With POLE-Altered Solid Tumors
eAppendix 3. Exploratory Analysis of Biomarkers
eFigure 6. Prevalence of TMB and PD-L1 in GARNET Cohorts A1 and F
eFigure 7. CPS and TMB Distribution by Cohort
eFigure 8. ORR by CPS as Continuous Variable
eFigure 9. ORR by TMB as Continuous Variable
eTable 7. Antitumor Activity Results by TMB and PD-L1 Status for dMMR EC and Non-EC Solid Tumors
Data Sharing Statement
References
- 1.Seligson ND, Knepper TC, Ragg S, Walko CM. Developing drugs for tissue-agnostic indications: a paradigm shift in leveraging cancer biology for precision medicine. Clin Pharmacol Ther. 2021,109(2):334-342. doi: 10.1002/cpt.1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kloor M, von Knebel Doeberitz M. The immune biology of microsatellite-unstable cancer. Trends Cancer. 2016,2(3):121-133. doi: 10.1016/j.trecan.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 3.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017,357(6349):409-413. doi: 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudley JC, Lin M-T, Le DT, Eshleman JR. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res. 2016,22(4):813-820. doi: 10.1158/1078-0432.CCR-15-1678 [DOI] [PubMed] [Google Scholar]
- 5.André T, Shiu KK, Kim TW, et al. , KEYNOTE-177 Investigators . Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020,383(23):2207-2218. doi: 10.1056/NEJMoa2017699 [DOI] [PubMed] [Google Scholar]
- 6.Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017,2017:1-15. doi: 10.1200/PO.17.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luchini C, Bibeau F, Ligtenberg MJL, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. 2019,30(8):1232-1243. doi: 10.1093/annonc/mdz116 [DOI] [PubMed] [Google Scholar]
- 8.Tougeron D, Sueur B, Zaanan A, et al. , Association des Gastro-entérologues Oncologues (AGEO) . Prognosis and chemosensitivity of deficient MMR phenotype in patients with metastatic colorectal cancer: an AGEO retrospective multicenter study. Int J Cancer. 2020,147(1):285-296. doi: 10.1002/ijc.32879 [DOI] [PubMed] [Google Scholar]
- 9.Garmezy B, Gheeya J, Lin HY, et al. Clinical and molecular characterization of POLE mutations as predictive biomarkers of response to immune checkpoint inhibitors in advanced cancers. JCO Precis Oncol. 2022,6:e2100267. doi: 10.1200/PO.21.00267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang F, Zhao Q, Wang YN, et al. Evaluation of POLE and POLD1 mutations as biomarkers for immunotherapy outcomes across multiple cancer types. JAMA Oncol. 2019,5(10):1504-1506. doi: 10.1001/jamaoncol.2019.2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rousseau B, Bieche I, Pasmant E, et al. PD-1 blockade in solid tumors with defects in polymerase epsilon. Cancer Discov. 2022,12(6):1435-1448. doi: 10.1158/2159-8290.CD-21-0521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tesaro. Study of TSR-042, an anti-programmed cell death-1 receptor (PD-1) monoclonal antibody, in participants with advanced solid tumors (GARNET). ClinicalTrials.gov. Updated August 22, 2023. Accessed September 27, 2023. https://clinicaltrials.gov/study/NCT02715284
- 13.Andre T, et al. Safety and efficacy of anti–PD-1 antibody dostarlimab in patients (pts) with mismatch repair-deficient (dMMR) solid cancers: Results from GARNET study. J Clin Oncol. 2021,39:9-9. doi: 10.1200/JCO.2021.39.3_suppl.9 [DOI] [Google Scholar]
- 14.Oaknin A, Tinker AV, Gilbert L, et al. Clinical activity and safety of the anti-programmed death 1 monoclonal antibody dostarlimab for patients with recurrent or advanced mismatch repair-deficient endometrial cancer: a nonrandomized phase 1 clinical trial. JAMA Oncol. 2020,6(11):1766-1772. doi: 10.1001/jamaoncol.2020.4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jemperli. GSK. Accessed September 27, 2023. https://jemperli.com/
- 16.European Medicines Agency . Jemperli. Accessed September 27, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jemperli
- 17.National Institute for Health and Care Excellence . Dostarlimab for previously treated advanced or recurrent endometrial cancer with high microsatellite instability or mismatch repair deficiency. March 16, 2022. Accessed September 27, 2023. https://www.nice.org.uk/guidance/ta779
- 18.Scottish Medicines Consortium. dostarlimab (Jemperli). Accessed September 27, 2023. https://www.scottishmedicines.org.uk/medicines-advice/dostarlimab-jemperli-full-smc2404/
- 19.Patnaik A, Weiss GJ, Rasco DW, et al. Safety, antitumor activity, and pharmacokinetics of dostarlimab, an anti-PD-1, in patients with advanced solid tumors: a dose-escalation phase 1 trial. Cancer Chemother Pharmacol. 2022,89(1):93-103. doi: 10.1007/s00280-021-04358-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oaknin A, Gilbert L, Tinker AV, et al. Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET-a phase I, single-arm study. J Immunother Cancer. 2022,10(1):e003777. doi: 10.1136/jitc-2021-003777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenzi M, Amonkar M, Zhang J, Mehta S, Liaw K-L. Epidemiology of microsatellite instability high (MSI-H) and deficient mismatch repair (dMMR) in solid tumors: a structured literature review. J Oncol. 2020,2020:1807929. doi: 10.1155/2020/1807929 [DOI] [Google Scholar]
- 22.Dedeurwaerdere F, Claes KB, Van Dorpe J, et al. Comparison of microsatellite instability detection by immunohistochemistry and molecular techniques in colorectal and endometrial cancer. Sci Rep. 2021,11(1):12880. doi: 10.1038/s41598-021-91974-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartley AN, Mills AM, Konnick E, et al. Mismatch repair and microsatellite instability testing for immune checkpoint inhibitor therapy: guideline from the College of American Pathologists in collaboration with the Association for Molecular Pathology and Fight Colorectal Cancer. Arch Pathol Lab Med. 2022,146(10):1194-1210. doi: 10.5858/arpa.2021-0632-CP [DOI] [PubMed] [Google Scholar]
- 24.Yoshino T, Pentheroudakis G, Mishima S, et al. JSCO-ESMO-ASCO-JSMO-TOS: international expert consensus recommendations for tumour-agnostic treatments in patients with solid tumours with microsatellite instability or NTRK fusions. Ann Oncol. 2020,31(7):861-872. doi: 10.1016/j.annonc.2020.03.299 [DOI] [PubMed] [Google Scholar]
- 25.Oaknin A, et al. 83 Time course of adverse events during dostarlimab treatment in patients with recurrent or advanced endometrial cancer in the garnet trial. Int J Gynecol Cancer. 2021,31:A74. doi: 10.1136/ijgc-2021-ESGO.113 [DOI] [Google Scholar]
- 26.Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2020,38(1):11-19. doi: 10.1200/JCO.19.02107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maio M, et al. Pembrolizumab in microsatellite instability high (MSI-H)/mismatch repair deficient (dMMR) cancers: updated analysis from phase 2 KEYNOTE-158 study. J Clin Oncol. Published online May 28, 2021. doi: 10.1200/JCO.2021.39.15_suppl.2565 [DOI] [PubMed] [Google Scholar]
- 28.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020,38(1):1-10. doi: 10.1200/JCO.19.02105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azad NS, Gray RJ, Overman MJ, et al. Nivolumab is effective in mismatch repair-deficient noncolorectal cancers: results from arm Z1D-A subprotocol of the NCI-MATCH (EAY131) study. J Clin Oncol. 2020,38(3):214-222. doi: 10.1200/JCO.19.00818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017,18(9):1182-1191. doi: 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colle R, Radzik A, Cohen R, et al. Pseudoprogression in patients treated with immune checkpoint inhibitors for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer. Eur J Cancer. 2021,144:9-16. doi: 10.1016/j.ejca.2020.11.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eAppendix 1. Sites and Investigators and Supplementary Methods
eFigure 1. Duration of Treatment for Responders With dMMR Solid Tumors
eTable 1. Antitumor Activity Analysis
eTable 2. Progression-Free Survival
eTable 3. Overall Survival
eFigure 2. Progression-Free Survival by Tumor Type
eFigure 3. Overall Survival by Tumor Type
eAppendix 2. Post Hoc Analysis of PFS by BOR
eTable 4. Progression-Free Survival by Best Overall Response per BICR in the Overall Efficacy Population
eFigure 4. Enrollment and Outcomes for Patients With POLE-Altered Tumors
eTable 5. Demographics and Baseline Characteristics of Patients With POLE Alterations
eTable 6. POLE-Altered Antitumor Activity Analysis by BICR
eFigure 5. Duration of Treatment for Patients With POLE-Altered Solid Tumors
eAppendix 3. Exploratory Analysis of Biomarkers
eFigure 6. Prevalence of TMB and PD-L1 in GARNET Cohorts A1 and F
eFigure 7. CPS and TMB Distribution by Cohort
eFigure 8. ORR by CPS as Continuous Variable
eFigure 9. ORR by TMB as Continuous Variable
eTable 7. Antitumor Activity Results by TMB and PD-L1 Status for dMMR EC and Non-EC Solid Tumors
Data Sharing Statement


