Abstract
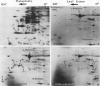
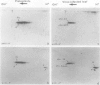
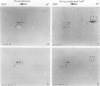
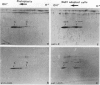
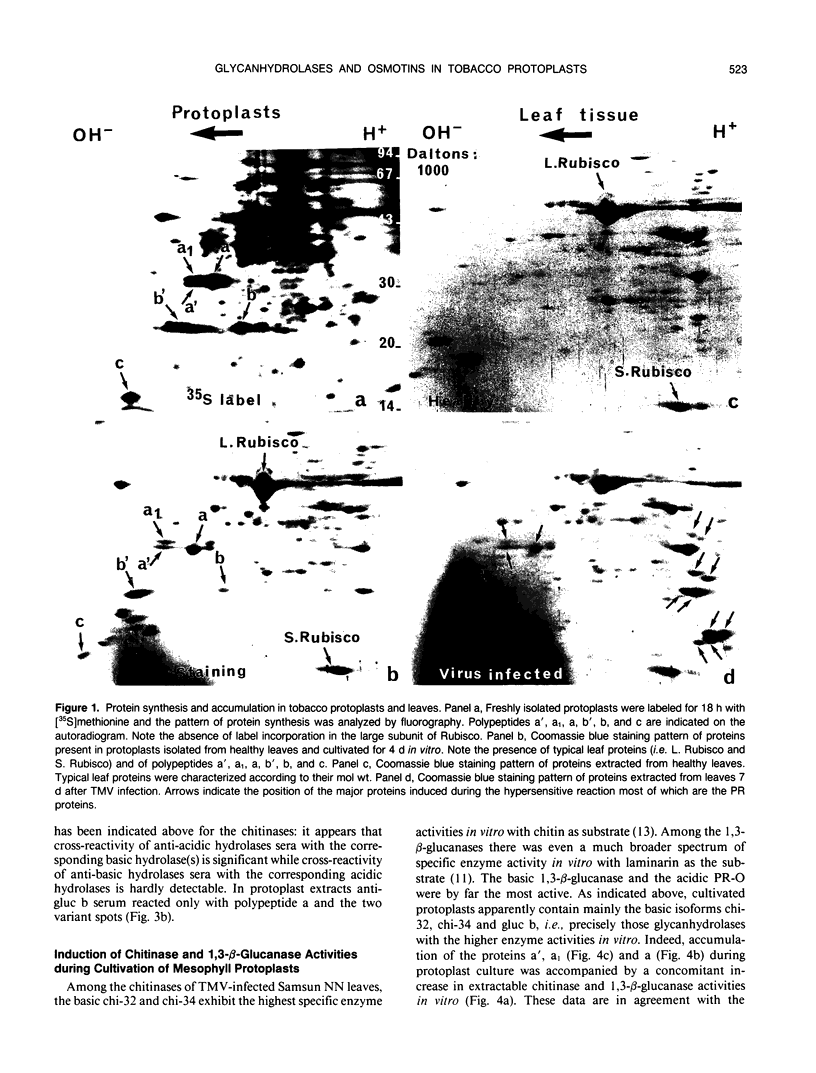
Tobacco (Nicotiana tabacum) mesophyll protoplasts synthesize six basic proteins (a, a′, a1, b, b′, and c) which are undetectable in the leaf and whose synthesis is reduced by auxin (Y Meyer, L Aspart, Y Chartier [1984] Plant Physiol 75: 1027-1033). Polypeptides a, a′, and a1 were shown to have similar mobilities on two-dimensional electrophoresis as one 1,3-β-glucanase and two chitinases from tobacco mosaic virus-infected leaves. In immunoblotting experiments, polypeptide a was recognized by specific antibodies raised against the 1,3-β-glucanase and a′ and a1 reacted with anti-chitinase antibodies. Similarly, b and b′ comigrated with osmotin and its neutral counterpart, two proteins characteristic of salt-adapted tobacco cells, and reacted with anti-osmotin antibodies. In addition it has been shown that 1,3-β-glucanase and chitinase activities increased at the same time as a, a′, and a1 accumulated in cultivated protoplasts. Finally, polypeptide c was also detected in tobacco mosaic virus-infected leaves but could not be identified as any of the pathogenesis-related proteins characterized so far in tobacco. Thus, cultivated tobacco protoplasts synthesize and accumulate typical stress proteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boller T., Vögeli U. Vacuolar localization of ethylene-induced chitinase in bean leaves. Plant Physiol. 1984 Feb;74(2):442–444. doi: 10.1104/pp.74.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bulcke M. V., Bauw G., Castresana C., Van Montagu M., Vandekerckhove J. Characterization of vacuolar and extracellular beta(1,3)-glucanases of tobacco: Evidence for a strictly compartmentalized plant defense system. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2673–2677. doi: 10.1073/pnas.86.8.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen B. J., Hooft van Huijsduijnen R. A., Bol J. F. A tobacco mosaic virus-induced tobacco protein is homologous to the sweet-tasting protein thaumatin. 1986 May 29-Jun 4Nature. 321(6069):531–532. doi: 10.1038/321531a0. [DOI] [PubMed] [Google Scholar]
- Holländer-Czytko H., Andersen J. K., Ryan C. A. Vacuolar localization of wound-induced carboxypeptidase inhibitor in potato leaves. Plant Physiol. 1985 May;78(1):76–79. doi: 10.1104/pp.78.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanneau J. P., Tandeau de Marsac N. Stepwise effects of cytokinin activity and DNA synthesis upon mitotic cycle events in partially synchronized tobacco cells. Exp Cell Res. 1973 Mar 15;77(1):167–174. doi: 10.1016/0014-4827(73)90565-x. [DOI] [PubMed] [Google Scholar]
- Kauffmann S., Legrand M., Geoffroy P., Fritig B. Biological function of ;pathogenesis-related' proteins: four PR proteins of tobacco have 1,3-beta-glucanase activity. EMBO J. 1987 Nov;6(11):3209–3212. doi: 10.1002/j.1460-2075.1987.tb02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand M., Kauffmann S., Geoffroy P., Fritig B. Biological function of pathogenesis-related proteins: Four tobacco pathogenesis-related proteins are chitinases. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6750–6754. doi: 10.1073/pnas.84.19.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch F., Mauch-Mani B., Boller T. Antifungal Hydrolases in Pea Tissue : II. Inhibition of Fungal Growth by Combinations of Chitinase and beta-1,3-Glucanase. Plant Physiol. 1988 Nov;88(3):936–942. doi: 10.1104/pp.88.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y., Chartier Y., Alibert G. Auxin reduces the synthesis of major vacuolar proteins in tobacco mesophyl protoplast. Plant Physiol. 1987 Mar;83(3):713–718. doi: 10.1104/pp.83.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y., Grosset J., Chartier Y., Cleyet-Marel J. C. Preparation by two-dimensional electrophoresis of proteins for antibody production: antibodies against proteins whose synthesis is reduced by auxin in tobacco mesophyll protoplasts. Electrophoresis. 1988 Nov;9(11):704–712. doi: 10.1002/elps.1150091105. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Schuster A. M., Davies E. Ribonucleic Acid and protein metabolism in pea epicotyls : I. The aging process. Plant Physiol. 1983 Nov;73(3):809–816. doi: 10.1104/pp.73.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinshi H., Mohnen D., Meins F. Regulation of a plant pathogenesis-related enzyme: Inhibition of chitinase and chitinase mRNA accumulation in cultured tobacco tissues by auxin and cytokinin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):89–93. doi: 10.1073/pnas.84.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinshi H., Wenzler H., Neuhaus J. M., Felix G., Hofsteenge J., Meins F. Evidence for N- and C-terminal processing of a plant defense-related enzyme: Primary structure of tobacco prepro-beta-1,3-glucanase. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5541–5545. doi: 10.1073/pnas.85.15.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Bracker C. A., Hasegawa P. M., Handa A. K., Buckel S., Hermodson M. A., Pfankoch E., Regnier F. E., Bressan R. A. Characterization of osmotin : a thaumatin-like protein associated with osmotic adaptation in plant cells. Plant Physiol. 1987 Oct;85(2):529–536. doi: 10.1104/pp.85.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Larosa P. C., Handa A. K., Hasegawa P. M., Bressan R. A. Hormonal regulation of protein synthesis associated with salt tolerance in plant cells. Proc Natl Acad Sci U S A. 1987 Feb;84(3):739–743. doi: 10.1073/pnas.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]