Abstract
Previous studies have found that β-Hydroxybutyrate (BHB), the main component of ketone bodies, is of physiological importance as a backup energy source during starvation or induces diabetic ketoacidosis when insulin deficiency occurs. Ketogenic diets (KD) have been used as metabolic therapy for over a hundred years, it is well known that ketone bodies and BHB not only serve as ancillary fuel substituting for glucose but also induce anti-oxidative, anti-inflammatory, and cardioprotective features via binding to several target proteins, including histone deacetylase (HDAC), or G protein-coupled receptors (GPCRs). Recent advances in epigenetics, especially novel histone post-translational modifications (HPTMs), have continuously updated our understanding of BHB, which also acts as a signal transduction molecule and modification substrate to regulate a series of epigenetic phenomena, such as histone acetylation, histone β-hydroxybutyrylation, histone methylation, DNA methylation, and microRNAs. These epigenetic events alter the activity of genes without changing the DNA structure and further participate in the pathogenesis of related diseases. This review focuses on the metabolic process of BHB and BHB-mediated epigenetics in cardiovascular diseases, diabetes and complications of diabetes, neuropsychiatric diseases, cancers, osteoporosis, liver and kidney injury, embryonic and fetal development, and intestinal homeostasis, and discusses potential molecular mechanisms, drug targets, and application prospects.
Keywords: β-hydroxybutyrate, Epigenetics, Histone post-translational modification, Ketogenic diet, DNA methylation, microRNAs
1. Introduction
β-Hydroxybutyrate (BHB) is the main component of ketone bodies, constituting around 70 % of the circulating ketone body pool. Previous studies suggested that physiological doses of BHB, in contrast to other ketone molecules, act as a backup energy source for the body, providing energy to extrahepatic tissues (e.g., the brain, heart, and skeletal muscle) when the glucose supply is insufficient or impaired [[1], [2], [3]], however, overgenerated BHB in diabetes induces diabetic ketoacidosis (DKA) when insulin deficiency occurs. Despite the mechanism of weight loss and the safety of long-term carbohydrate restriction still being debated [4,5], ketogenic diets (KD) have been utilized as metabolic therapy for over a hundred years. It is widely known that ketone bodies not only serve as ancillary fuel substituting for glucose but also induce anti-oxidative, anti-inflammatory, and cardioprotective features via binding to several target proteins, including histone deacetylase (HDAC), or G protein-coupled receptors (GPCRs) [[6], [7], [8]]. However, the underlying mechanisms are poorly understood, and it is not known whether there are other molecular mechanisms.
Epigenetics is a heritable change in gene expression or cell phenotype that does not involve nucleotide sequence changes and regulates how the genome and environment interact and includes histone post-translational modifications (HPTMs), DNA methylation, and non-coding RNAs [9]. HPTMs have increasingly gained importance in the context of epigenetics, affecting chromatin conformation to alter gene expression. With the application of high-sensitivity mass spectrometry, the current catalog of observed HPTMs is immense and growing [10,11]. In addition to histone acetylation (Kac), methylation (Kme), phosphorylation, and ubiquitination, HPTMs also include novel modifications such as propionylation, butyrylation, crotonylation, and β-hydroxybutyrylation, (Kbhb), and these different HPTMs can interact with each other and with DNA methylation in a dynamic and reversible regulatory process [12,13]. Recent advances in epigenetics have continuously updated our understanding of BHB. As an endogenous small molecule, BHB is regarded not only as an energy metabolite but also as a signal transduction molecule and modification substrate that regulates a series of epigenetic phenomena, such as Kme, Kac, and Kbhb, linking gene transcription and cell function to the external environment. This review focuses on the metabolic process of BHB and BHB-mediated epigenetics in cardiovascular diseases, diabetes and complications of diabetes, neuropsychiatric diseases, cancers, osteoporosis, liver and kidney injury, embryonic and fetal development, and intestinal homeostasis, and discusses possible molecular mechanisms, potential drug targets, and application prospects. The purpose of this review is not to provide an overview of the epigenetic mechanisms of BHB but to guide the reader to areas of current research interest.
2. Metabolism, regulation, and function of BHB
Ketone synthesis mainly occurs in the liver's Mitochondrial matrix. Fatty acids are first metabolized to acetyl CoA (Ac-CoA) through mitochondrial β oxidation, and the 3-hydroxy-3-methylglutaryl-CoA synthase 2 (HMGCS2) condenses Ac-CoA and acetoacetyl CoA (AcAc-CoA) to form 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA), which releases acetoacetate (AcAc) by HMG-CoA lyase (HMGCL). The majority of it is converted to BHB by the β-hydroxybutyrate dehydrogenase 1 (BDH1)-catalyzed NAD+/NADH-coupled equilibrium reaction [14], and a small amount of AcAc spontaneously decarboxylates to acetone. In mammals, ketogenesis occurs primarily in the liver, but the abnormal expression of HMGCS2 is sufficient for ketogenesis in other tissues [[15], [16], [17], [18]], of which the kidney is of the most concern. However, during fasting, only the liver is involved in the production of endogenous circulating ketone [19]. Thus, more research is needed to understand the function of HMGCS2 and its ketogenesis effects in extrahepatic tissue.
AcAc and BHB are released from the cell membrane by monocarboxylic acid transporters (MCT1 and 2) for transport to extrahepatic organs [20], however, it is uncertain how ketone bodies pass through the inner mitochondrial membrane. The most abundant circulating ketone body is BHB, which is also less likely than AcAc to spontaneously break down to acetone. Once BHB is absorbed by the target tissue, it will be converted into AcAc by BDH1. AcAc is activated as AcAc-CoA by 3-oxoacid CoA-transferase (SCOT), which is expressed in all mammalian cells containing mitochondria except hepatocytes, so the liver cannot use BHB for energy. This is one of only a few near-equilibrium reactions that occur in mammals. The hydrolysis of AcAc-CoA releases more free energy than succinyl-CoA, which is advantageous for the production of AcAc. Two molecules of Ac-CoA are produced by the reversible acetyl-CoA acetyltransferase (ACAT) reaction and enter into the TCA cycle as an energy source for oxidation or may be used for lipid synthesis.
GPR109A and GPR41 are two GPCRs that bind short-chain fatty acids, and BHB is a ligand for both of these GPCRs. BHB was shown to activate GPR109A to reduce lipolysis in adipocytes, avoid ketoacidosis, and encourage effective fat utilization [21]. Numerous cell types, including adipocytes, monocytes, macrophages, and vascular endothelial cells, express GPR109A. By activating GPR109A, BHB at a specific dosage can prevent immune cell-induced inflammation and atherosclerosis [22,23]. Recent studies have shown that BHB inhibited sympathetic nerve activity and regulated energy consumption by antagonizing GPR41, which may be related to the Gβγ-PLCβ-MAPK signaling pathway [24]. GPR41 was found to inhibit insulin secretion by β-cells and play a role in maintaining glucose homeostasis in the body [25]. Therefore, BHB plays a significant role in inflammatory, neurologic, and metabolic illnesses as an endogenous GPCRs ligand.
3. BHB regulates epigenetic phenomena
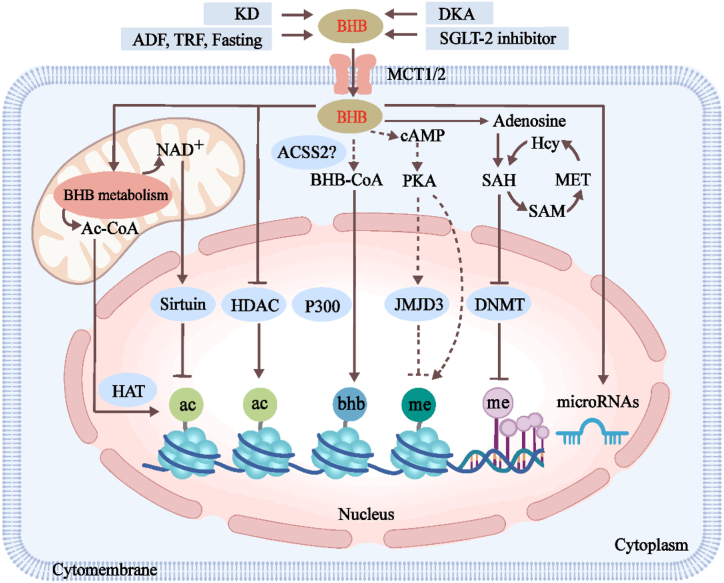
BHB is regarded as an energy carrier from the liver to peripheral tissues during fasting or exercise. As shown in Fig. 1, a growing number of studies have demonstrated that BHB also acts as a signal transduction molecule and modification substrate to regulate a series of epigenetic phenomena, such as Kme, Kac, and Kbhb, and microRNAs, to affect the transcription level of genes.
Fig. 1.
The BHB regulates epigenetics.
Ketogenic diets (KD), alternate-day fasting (ADF), time-restricted feeding (TRF), fasting, diabetic ketoacidosis (DKA), and SGLT-2 inhibitors cause an increase in BHB concentration. BHB metabolism in mitochondrion increases Ac-CoA, which is transported to the nucleus as a substrate for histone acetyltransferase (HAT) and promotes Kac. BHB also directly inhibits histone deacetylase (HDAC) and then increases Kac. However, excessive NAD+ during BHB metabolism activates Sirtuin and reduces Kac. BHB may be catalyzed by acyl-CoA synthetase 2 (ACSS2) to produce BHB-CoA and promote Kbhb under acyltransferase P300. BHB directly promotes Kme via cAMP/PKA signaling but indirectly inhibits Kme by enhancing the expression of histone demethylase JMJD3. BHB blocks DNA methylation by inhibiting DNA methyltransferase (DNMT). Furthermore, BHB also up-regulates microRNAs and affects gene expression. These BHB-regulated epigenetic effects are involved in the regulation of oxidative stress, inflammation, fibrosis, tumors, and neurobiological-related signaling. The “dotted lines” mean that the process needs to be further verified, and the solid lines mean that the process has been proven.
3.1. Histone deacetylases inhibition
HPTMs have increasingly gained importance in the context of epigenetics, affecting chromatin conformation to alter gene expression. BHB is known to act as a competitive enzyme inhibitor, including endogenous and specific class I and IIa HDAC inhibitors, increasing Kac, weakening DNA-histone connections, loosening chromatin structure, and activating transcription. Initially, treatment of mice with BHB by osmotic pump resulted in hyperacetylation of kidney histones, particularly lysines 9 and 14 of histone 3 (H3K9/K14). BHB increased Kac on FOXO3a and Mt2 promoters in human embryonic kidney 293 (HEK293) cells, and further increased the expression of these genes by inhibiting HDAC1 or HDAC2, suggesting a direct role for BHB as an HDAC inhibitor [26]. This is the first report of BHB as an epigenetic modifier. However, in other experiments, BHB was not detected to inhibit HDAC activity and did not increase Kac levels in vitro [27,28], which may be due to different cell lines. Perhaps, more experiments are needed to reevaluate BHB as an HDAC inhibitor. Additionally, non-histone proteins such as NF-κB, TP53, p53, c-Myc, and MyoD can also be deacetylated by HDAC [[29], [30], [31], [32]]. The conversion of BHB to Ac-CoA can increase the level of Ac-CoA in mitochondria, and the output of Ac-CoA from mitochondria is mainly mediated by citrate synthase (CS) and ATP-citrate lyase (ACLY) [33]. The increased Ac-CoA is transported to the nucleus as a substrate for histone acetyltransferase (HAT), pushing the equilibrium reaction toward Kac. On the other hand, the metabolism of two Ac-CoA molecules from a single molecule of BHB involves the conversion of only two NAD+ molecules to NADH, using fewer NAD+ molecules than glucose metabolism. KD causes an excess of NAD+ and regulates the activity of NAD+-dependent enzymes (including sirtuin) involved in deacetylation. In summary, BHB also indirectly affects Kac levels through the concentration of Ac-CoA and NAD+.
3.2. Histone β-hydroxybutyrylation
Professor Yingming Zhao's team first reported that the Kbhb marker was significantly induced in the livers of mice with DKA induced by prolonged fasting or streptozotocin (STZ) and that Kbhb levels were regulated by cellular BHB concentrations. Chromatin immunoprecipitation (ChIP)-seq and RNA-seq analysis showed that Kbhb was an enriched marker in active gene promoters, and an increased H3K9bhb level during starvation was associated with the upregulation of genes in metabolic pathways in response to starvation, aiding the body's quick adaptation to metabolic changes caused by an insufficient energy supply [34]. However, subsequent studies have found that Kbhb of the tumor suppressor protein p53 significantly reduced the level and activity of p53 Kac and promoted the growth of cancer cells [35]. Thus, the biological function of Kbhb should not be undervalued. The modification itself can up-regulate gene expression and indirectly regulate other modification levels. Many acyl-CoA enzymes can be derived from their homologous short-chain fatty acids (SCFAs) by acyl-CoA synthetase 2 (ACSS2), similar to how butyrate and crotonate are converted to their corresponding CoA derivatives [36,37]. However, it has not been confirmed that ACSS2 is the mediator of the conversion of BHB to BHB-CoA, which needs to be further confirmed by subsequent studies. Both in vivo and in vitro, acyltransferase p300 catalyzed Kbhb and promoted downstream transcription, while HDAC1 and HDAC2 mediated the removal of Kbhb by enzymatic activity. 3248 modification sites on 1397 proteins were identified by Kbhb modification omics of HEK293 cells, revealing key regulatory enzymes (" Writer ", “Reader”, “Eraser”) and protein substrates. It established a basis for investigating Kbhb's function in various biological processes [38]. Therefore, as a newly discovered epigenetic mark, Kbhb combines metabolism with gene expression and offers a new way to explore the chromatin regulation and various functions of BHB in significant human pathophysiological states.
3.3. Histone methylation
Part of the information about the effect of BHB on Kme comes from the transcriptional regulation of brain-derived neurotrophic factor (BDNF). BHB increased the expression of the H3K27me3-specific demethylase JMJD3 and decreased H3K27me3 binding on the BDNF promoter [39]. Additionally, the concentration of H3K4me3 on the BDNF promoter increased after BHB treatment, indicating that H3K4me3 was involved in the increased expression of BDNF stimulated by BHB [40], which may have a synergistic effect with H3K27me3. Both directly regulated BDNF transcription in an active or repressive manner, and both depended on the activation of the cAMP/PKA signaling pathways. Perhaps, the effect of BHB on the methylation state may also be related to the Ac-CoA pool, which together with glycine, is required for the synthesis of S-adenosylmethionine (SAM) [41]. It can be seen that this is a problem to be explored in the future.
3.4. DNA methylation
Changes in dietary patterns can modulate the methylation patterns of key metabolic genes, thereby regulating their expression [42]. Epigenome sequencing analysis showed that hippocampal DNA methylation levels were significantly increased in rats with chronic epilepsy. A ketogenic, high-fat, low-carb diet was administered, which inhibited the progression of seizures and ameliorated DNA methylation-mediated gene expression alterations [43]. Multiple studies have shown that these effects may be related to the BHB-induced increase in hippocampal adenosine [44,45]: increased adenosine promotes S-adenosine homocysteine (SAH) formation [46], which in turn inhibits DNA methyltransferase (DNMT) [47]and reduces DNA methylation level [46,48], and also affects the methionine cycle. These findings offer some proof of the biological significance of DNA methylation alterations linked to epilepsy.
3.5. MicroRNAs
MicroRNAs are a new class of short (∼22 nucleotides) non-coding RNAs that pair with the 3′- untranslated region (3′-UTR) of the target gene mRNA to cleave or disrupt the stability of the mRNA and inhibit the post-transcriptional expression of eukaryotic genes [49,50]. Serum levels of miR-16-5p, miR-196b-5p, and miR-218-5p were elevated in a caloric restriction mouse model, and miR-16-5p expression levels in the spleen, thymus, colon, and stomach were considerably elevated. Notably, miR-16-5p inhibited inflammatory cytokines, suggesting that changes in microRNAs levels during caloric restriction may maintain immune homeostasis and regulate inflammation [51]. In a study involving human volunteers, KD recipients showed microRNAs regulation of specific genes involved in nutritional metabolism and mTOR, PPAR, insulin, and cytokine signaling pathways [52]. The study team also found that obese participants had altered levels of microRNAs linked to antioxidant and anti-inflammatory signaling pathways, but that these levels were restored to normal levels after terminating the KD [53]. These results suggest that microRNAs regulate gene expression as an epigenetic mechanism, which needs to be further confirmed by more studies.
4. BHB as an epigenetic modifier in disease and therapeutics
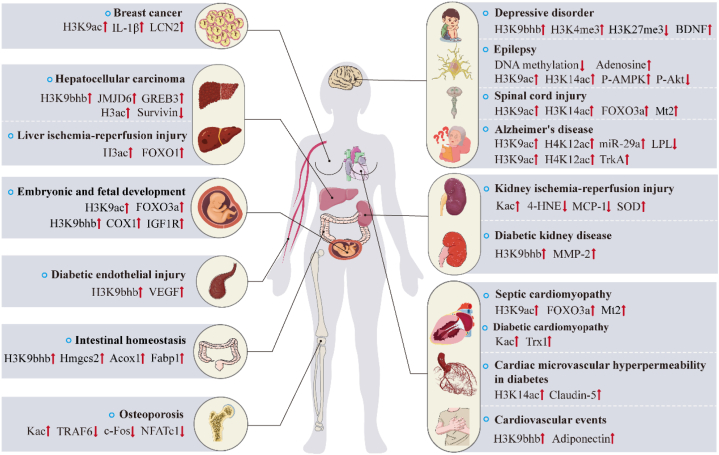
As shown in Fig. 2, studies have shown that BHB plays an important role as an epigenetic regulatory molecule in the pathogenesis and treatment of cardiovascular diseases, complications of diabetes, neuropsychiatric diseases, cancer, osteoporosis, liver and kidney injury, embryonic and fetal development and intestinal homeostasis. Next, we will explain the molecular mechanisms separately (see Table 1).
Fig. 2.
Overview of BHB-regulated epigenetics and target genes in the pathogenesis and treatment of diseases.
BHB, as an epigenetic modifier, on the one hand, regulates the transcription of the target genes by the histones post-translational modification in the promoter region of genes, or DNA methylation and microRNAs, which affect the transduction of disease-related signal pathways. On the other hand, BHB-mediated epigenetics exist in crosstalk, which jointly affects the regulation of gene transcription in cardiovascular diseases, diabetic complications, central nervous system diseases, cancers, osteoporosis, liver/kidney ischemia-reperfusion injury, embryonic and fetal development, and intestinal homeostasis.
Abbreviations ↑, upregulation; ↓, downregulation; IL-1β, interleukin-1β; LCN2, lipocalin 2; FOXO1, forkhead box O1; FOXO3a, forkhead box class O3a; IGF1R, insulin-like growth factor 1 receptor; VEGF, vascular endothelial growth factor; Acox1, acyl-Coenzyme A oxidase 1; Fabp1, fatty acid binding protein 1; TRAF6, tumor necrosis factor receptor-associated factor 6; NFATc1, T-cells cytoplasmic 1; BDNF, brain-derived neurotrophic factor; P-AMPK, phosphorylation-AMP-activated protein kinase; P-Akt, phosphorylated protein kinase B; Mt2, metallothionein 2; LPL, lipoprotein lipase; TrkA, tyrosine kinase receptor A; 4-HNE, 4-hydroxynonenal; SOD, superoxide dismutase; MCP-1, monocyte chemotactic protein 1; MMP-2, matrix metalloproteinase-2; Trx1, Thioredoxin1; JMJD6, jumonji domain containing 6; COX1, cytochrome c oxidase subunit 1.
Table 1.
BHB as epigenetic modifiers in vivo and in vitro models of human diseases.
| Type | Model Species | Intervention Dose |
Epigenetic Modification | Target Genes/Result | Ref |
|---|---|---|---|---|---|
| In vivo | LPS-induced mice (septic cardiomyopathy) | 1,3-Butanediol (3 mg/g/day) by i.g | H3K9ac | LVEF↑, FS↑, CO↑, SOD↑, Plasma CK-MB↓, Plasma LDH↓, MDA↓ | [56] |
| KKAy mice (obese, diabetes) | Dapagliflozin (0.02 mg/mL) by freely drinking | H3K9bhb | Adiponectin↑, Plasma insulin↓, HbA1c↓, Plasma leptin↓ | [58] | |
| STZ-induced rats (diabetes) | BHB (160, 200, and 240 mg/kg/day) by i.h | H3K14ac | Claudin-5↑, Microvascular permeability↓ | [59] | |
| STZ-induced rats (diabetes) | BHB (160, 200, and 240 mg/kg/day) by i.h | H3K9bhb | MMP-2↑, Serum creatinine↓, Urine protein↓, Collagen IV↓ | [62] | |
| STZ-induced rats (diabetes) | BHB (160, 200, and 240 mg/kg/day) by i.h | H3K9bhb | NO↑, VEGF↑ | [63] | |
| Pilocarpine-induced seizure rats | Time-restricted feeding (20hr/day) | H3K9ac, H3K14ac |
P-AMPK↑, P-Akt↓ | [66] | |
| Pilocarpine-induced seizure rats, Pentylenetetrazole-induced kindling mice | KD | DNA methylation | Adenosine↑, 5-methylcytosine↓ | [44] | |
| Dexamethasone and spatial restraint stress-induced depression mice | BHB (300 mg/kg/day) by i.p | H3K9bhb | BDNF↑ | [71] | |
| C57BL/6J mice | BHB (60 mg/kg, tiwce a day) by i.g | H3K27me3, H3K4me3 | BDNF↑ | [39,40] | |
| C57BL/6J mice | Fasting (48hr) | H3K9bhb | Plasma FFAs↑, Liver TG↑, Irs2↑, Sgk1↑, Adcy9↑, Idh2↑, Sult1a1↑, Hmgcs2↑, Subcutaneous Fat↓, P-Akt↓, PS6↓ | [72] | |
| APP/PS1 double-transgenic mice(Alzheimer's disease) | Alternate-day fasting | H3K9ac, H4K12ac, miR-29a | LPL↓ | [78] | |
| C5 hemi-contusion injury rats | KD; Every other day fasting; Every other day KD | H3K9ac, H3K14ac | FOXO3a↑, Mt2↑, Mn-SOD↑, Catalase↑, MDA↓ | [79] | |
| Hepatocellular carcinoma mice | BHB (100 mg/kg/day) by i.p | Kac | Cleaved caspase3/8↑, Survivin↓ | [84] | |
| Liver IRI mice | Fasting (12hr); BHB (1 mM/kg, 30 min before ischemia) by i.p |
H3ac | Serum ALT↓, Serum HMGB1↓, Cleaved caspase3↓, IL-6 ↓, IFNγ↓, TNF-α↓, IL-1β↓, NLRP3↓, 4-HNE↓, Bcl-2↑, FOXO1↑, HO-1↑, LC3B↑ | [95] | |
| Kidney IRI rats | KD | Kac | Plasma TG↓, Plasma BUN↓, Plasma creatinine↓, HSP70↓, p50↓, FN↓, α-SMA↓, 8-OHdG↓, 3-NT↓, 4-HNE↓, TNF-α↓, IL-6↓, MCP-1↓, GPx↑, SOD↑ | [97] | |
| Han: SPRD rats, PKD1 mice, Feline ADPKD (polycystic kidney disease) |
Time-restricted feeding (8hr/day); KD; Acute fasting (24hr, 48hr); BHB (157.5 mM) by freely drinking |
Not described | Serum creatinine↓, Cysts per section↓, Cyst size↓, SMA↓, Ki-67↓, P-AMPK↑, CPT1α↑ | [98] | |
| Dahl salt-sensitive rats (hypertension) | 1,3-Butanediol (20 %) by freely drinking | Not described | Serum Na+/K+ ratio↓, Serum microalbumin↓, NLRP3↓, Caspase1↓, IL-18↓, IL-1β↓, LCN2↓, Hif1α↓, Serpine1↓ | [100] | |
| Mice with conditionally deleted Hmgcs2 in intestinal epithelial cells | Feeding (24hr); Fasting (24hr) | H3K9bhb | Hmgcs2↓, Fabp1↓, Acox1↓, Acox2↓ | [111] | |
| In vitro | Rat myocardial (H9C2) cells | BHB (5 mM) | H3K9ac | FOXO3a↑, Mt2↑, SOD2↑, ROS↓ | [56] |
| Mouse adipocytes (3T3-L1) | BHB (10 mM) | H3K9bhb | Adiponectin↑, MCP-1↓, PAI-1↓, IL-6↓, TNF-α↓ | [58] | |
| Human cardiac microvascular endothelial cells (HCMECs) | BHB (2 mM) | H3K14ac | Claudin-5↑ | [59] | |
| Rat primary myocardial cells | BHB (0.1, 0.3, 1, 3, 10 mM) | Kac | Trx1↑, P–S6K↑, P-4EBP1↑, P-ACC↑ | [61] | |
| Mouse primary cortical neurons cells | BHB (5, 10, 20 mM) | H3K9bhb | BDNF↑ | [71] | |
| Mouse hippocampal neuron (HT22) cells, rat primary hippocampal neuron cells | BHB (2 mM) | H3K27me3, H3K4me3 | BDNF↑, P-CREB↑, P-CAMKII↑, JMJD3↑ | [39] [40] |
|
| Human neuroblastoma (SH-SY5Y) cells | BHB (5 mM) | H3K9ac, H4K12ac | TrkA↑ | [77] | |
| Rat adrenal pheochromocytoma (PC12) cells | BHB (1, 5, 20 mM) | H3K9ac, H3K14ac | Catalase↑, Mn-SOD↑, FOXO3a↑, Nox2/4↓, ROS↓ | [80] | |
| Human hepatocellular carcinoma (HepG2, HLE) cells | BHB (10 mM) | H3ac | Cleaved caspase3/8↑, Survivin↓ | [84] | |
| Human hepatocellular carcinoma (PLC-PRF-5) cells | BDH1 (knock-out) | H3K9bhb | JMJD6↑, GREB3↑, GTPBP4↑, NPM1↑, TIMM23↑ | [85] | |
| MCT2-expressing human breast cancer cells | BHB (1, 5, 10, 20 mM) | H3K9ac | IL-1β↑, LCN2↑ | [86] | |
| Mouse bone marrow-derived macrophages (BMDMs) | BHB (2, 4, 6 mM) | Kac | NFATc1↓, TRAF6↓, c-Fos↓, TRAF2↓ | [93] | |
| Mouse inner cell mass and trophectoderm (TE) cells | BHB (20 mM) | H3K27bhb | Glucose consumption↓, Glycolytic flux↑ |
[107] | |
| Bovine zygotes | BHB (6 mM) | H3K9ac | FOXO3a↑ | [28] | |
| Mouse TE cells | BHB (2 mM); AcAc (0.8 mM) | H3K27ac | Glucose consumption↑, Glycolytic flux↓, Rate of fetal development↓ | [108] | |
| Bovine cumulus cells | BHB (2, 4, 6 mM) | H3K9bhb | COX1↑, COX2↑, ATP8↑, IGF1R↑, SMAD4↑ | [109] |
↑: upregulation; ↓: downregulation.
4.1. Cardiovascular diseases
Ketone bodies, primarily BHB, have emerged as important regulators of the cardiovascular system, not only providing an important source of additional ATP production for the “energy-poor” but also promoting protective effects by anti-oxidative stress and another signal pathway in the heart [54]. BHB inhibits the production of reactive oxygen species (ROS) and alleviates the apoptosis induced by oxidative stress [55]. Lipopolysaccharide (LPS)-the stimulated macrophage-conditioned medium used to mimic the pathological process of septic cardiomyopathy showed that BHB inhibited HDAC, enhanced H3K9ac levels, and activated the antioxidant FOXO3a/Mt2 pathway, preventing ROS production and providing mitochondrial protection in septic cardiomyopathy [56]. The mechanisms by which Sodium/glucose cotransporter 2(SGLT-2)inhibitors reduce cardiovascular events are not fully understood but include improved blood glucose control, reduced weight, blood pressure, and serum uric acid. In addition to these elements, it has been suggested that the therapeutic effect of SGLT-2 inhibitors is related to elevated serum BHB levels [57]. Research revealed that dapagliflozin increased plasma and adipose BHB levels and upregulated adiponectin levels in 3T3-L1 adipocytes. The mechanism may be through the induction of H3K9bhb expression by adiponectin, independent of Kac and Kme, to play a protective role in the heart [58].
4.2. Diabetes and complications of diabetes
In the STZ-induced diabetic rat model, different concentrations of BHB promoted the production of claudin-5 for which dysfunction can lead to an increased diabetic microvascular hyperpermeability. BHB also antagonized the hyperpermeability of human cardiac microvascular endothelial cells (HCMECs). In vitro, inhibition of HDAC3 expression in HCMECs induced a specific increase in the H3K14ac concentration at the claudin-5 promoter, activating claudin-5 transcription. Notably, although the H3K9ac levels were decreased in the high glucose group, BHB treatment had no significant effect on the H3K9ac levels [59]. Increased oxidative stress and mitochondrial dysfunction are the main driving forces for the progression of diabetic cardiomyopathy [60]. BHB upregulated Thioredoxin1 (Trx1) in a dose and time-dependent manner, and induced Kac, enhancing the antioxidant defense ability of cardiomyocytes and protecting diabetic cardiomyopathy [61]. BHB treatment alleviated the changes to serum creatinine, 24-h urinary protein, and glomerular morphology in diabetic rats, and reduced the content of collagen IV. The mechanism was shown to be that BHB upregulated the H3K9bhb level in the matrix metalloproteinase-2 (MMP-2) promoter and enhanced the expression of MMP-2 [62]. Moreover, this team also reported that an appropriate concentration of BHB antagonized aortic endothelial injury and promoted the production of vascular endothelial growth factor (VEGF) in diabetic rats by upregulating the H3K9bhb level [63].
4.3. Neurological disorders
To date, various reports have suggested that some metabolic-based therapies, such as the KD or calorie restriction diet, have anticonvulsant effects [64,65] and can be used as metabolic replacement therapy in epilepsy. Time-restricted feeding (TRF) is a nutritional challenge that restricts the food supply to a short period during a mammal's waking phase. In the lithium-pilocarpine model to induce status epilepticus, TRF significantly increased BHB concentrations, which inhibited HDAC activity, resulting in increased H3K9ac and H3K14ac and changes in chromatin structure, promoting the transcription of a subset of genes that conferred anticonvulsant effects [66]. In addition, there is mounting evidence that KD therapy exerts epigenetic effects on DNA methylation. In recent studies, KD therapy was shown to prevent disease progression by increasing adenosine and decreasing DNA methylation levels. Notably, the downregulation of DNA methylation by KD treatment persisted after discontinuation of the diet [44]. Based on these findings, KD therapy is likely to exert its antiepileptic effects through adenosine-dependent DNA methylation regulation.
In addition to its use in the treatment of epilepsy, BHB plays a neuroprotective role in psychotic diseases [67,68] and has been demonstrated to have antidepressant effects on depression [69]. Antidepressants can restore downregulated BDNF levels in patients with depressive disorder [70]. Endogenous BHB and H3K9bhb were decreased in the depressed mouse brain, and exogenous BHB directly increased H3K9bhb levels. This modification activated the promoter of BDNF and then induced mRNA and protein level expression [71]. Studies have also shown that the induction of H3K4me3 and H3K27me3 by BHB can directly regulate the transcription of BDNF in an active or repressive manner, leading to promoter occupancy and BDNF expression [39,40]. Moreover, Cornuti et al. found significantly increased H3K9bhb content in the cortex of fasting mice. The dataset crossing identified H3K9bhb as an epigenetic marker associated with fasting-induced gene expression by ChIP-seq and RNA-seq and the regulated genes are involved not only in brain tissue metabolism but also in synaptic transmission and plasticity pathways. One of the richest functional annotations both at the epigenetic and transcriptional levels was “circadian rhythms''. Core-clock gene (e.g., Per1, Cry1, Cry2, Bmal1) expressions were observed to change with fasting in the visual cortex and suprachiasmatic nucleus, and fasting also disrupted the normal development of motor rhythms. Interestingly, no changes in cortical expression of these genes were observed compared to the control group after re-feeding. Thus, results suggest that BHB plays a powerful epigenetic molecular role through direct and specific histone markers in nervous tissue [72].
Moreover, neurological illnesses may benefit from using BHB in prevention and treatment [73,74]. One of the crucial elements of the intricate etiology of Alzheimer's disease (AD) is the deposition of β-amyloid peptide (Aβ) [75,76]. The study has demonstrated that BHB has a protective function on Aβ-induced neurotoxicity in Human neuroblastoma (SH-SY5Y) cells. The underlying mechanism may be related to the upregulation of H3K9ac and H4K12ac levels, increasing the expression of tyrosine kinase receptor A (TrkA), which is important in safeguarding cholinergic neurons in AD [77]. In another study, alternate-day fasting (ADF) also increased cortical H3K9ac and H4K12ac levels and decreased brain-derived lipoprotein lipase (LPL) expression in an APP/PS1 double-transgenic AD mouse model. Studies conducted in vitro found that miR-29a was essential for controlling LPL, in part by suppressing its expression through BHB. The impact of BHB on the expression of miR-29a may be connected to HDAC2/3 [78].
Spinal cord injury (SCI) usually results in long-term neurological disability, and there is currently no clinically proven treatment that targets SCI and promotes functional recovery beyond physical rehabilitation. However, Wang et al. found that feeding a KD, every other day fasting (EODF), and every other day KD (EODKD) significantly increased the levels of BHB in serum and cerebrospinal fluid, increased the expression of H3K9ac and H3K14ac, and increased the expression of anti-oxidative stress proteins FOXO3a and Mt2 and related proteins [79]. In addition, the team reported that BHB reduced HDAC activity and reduced H2O2-induced ROS production in rat adrenal pheochromocytoma (PC12) cells after SCI in rats [80].
4.4. Cancers
A KD has been used as adjunctive therapy for glioblastoma and also to prevent prostate, colon, pancreatic, and lung cancer [81,82]. Glucose serves as the primary energy source for tumor cells. Even in the presence of oxygen, tumor cells use glycolysis to meet the needs of rapid multiplication, this is known as the “Warburg effect”. Therefore, a KD affects both glucose metabolism and glucose-dependent signaling in malignant cells, and the decrease in circulating glucose levels appears to be the key reason for the antitumor effect of a KD [83]. However, it has been suggested that BHB, as a major component of ketone bodies, has anti-tumor properties mainly due to its role as an HDAC inhibitor. BHB significantly enhanced cisplatin-induced hepatocellular carcinoma cytotoxicity and apoptosis by reducing the expression of HDAC 3/6 and led to the reduction of survivin [84]. However, there are conflicting results. Recent studies indicate that metastasis-associated protein 2 (MTA2) interacted with HDAC2 and HDAC4 and inhibited BDH1 through R-loop transcription, upregulated and induced BHB accumulation in hepatocellular carcinoma, increased the level of H3K9bhb, and had a cascade effect on hepatocellular carcinoma formation and progression [85]. BHB treatment of MCT2-expressing human breast cancer cells enhances tumorigenic properties, resulting in increased H3K9ac and up-regulated transcription of tumor-promoting genes such as IL-1β and LCN2 [86]. Interestingly, Rodrigues et al. also confirmed that BHB administration accelerated tumor growth, but the tumors did not show increased Kac levels, explaining that this may be due to the oxidative metabolism of BHB reducing their concentration below the level at which tumor growth could be inhibited by inhibiting Kac [87]. Taken together, ketone bodies, such as BHB, perform metabolic and epigenetic roles in tumors collectively, yet their actions may work against or accelerate tumor growth.
4.5. Osteoporosis
The degradation product of polyhydroxybutyrate is BHB. It has been shown that BHB and its 3-hydroxybutyrate methyl ester (3HBME) reduce oxidative damage and rescue mitochondrial respiratory damage [88], and BHB also stimulates a rapid increase in intracellular calcium through L-type Ca2+ channels, which are necessary for bone repair and turn affect cell-cycle progression [89]. Although a KD has been shown to cause osteoporosis [4], BHB has been reported to have the ability to inhibit postmenopausal osteoporosis induced by ovariectomy [90]. However, AcAc enhanced alkaline phosphatase (ALP) activity in mouse embryo osteoblast precursor cells (MC3T3-E1) and mouse primary osteoblasts in a concentration-dependent manner, in contrast to BHB, which decreased ALP activity in the same experimental setup, but without affecting ALP mRNA or protein expression [91]. In osteoclasts, BHB downregulated the nuclear factor of activated T-cells cytoplasmic 1 (NFATc1) and inhibited osteoclast differentiation, which led to faster recovery of mice from osteoporosis induced by simulated microgravity [92]. Recent studies suggest that BHB regulation of osteoclasts may be primarily dependent on HDAC inhibitions rather than GPR109A receptor activation [93]. A485 is a highly selective catalytic p300 inhibitor. A485 attenuated RANKL-induced osteoclast differentiation and downregulated the expression of osteoclast differentiation-related genes in a time and dose-dependent manner. The molecular mechanism may be through inhibition of phosphorylation of the MAPK signaling pathway and activation of NFATc1 [94].
4.6. Liver and kidney injury
In the mouse liver ischemia-reperfusion injury (IRI) model, fasting for 12 hours significantly improved liver IRI. The reason may be that increased BHB induced H3ac, which led to increased FOXO1 expression and upregulation of antioxidant enzymes and autophagy activity, and improved liver IRI by reducing inflammation and apoptotic cell death. Moreover, Exogenous BHB also has this effect [95]. The study of a KD in renal medicine may be constrained by various contraindications, such as the possibility of kidney stones [96], but several studies in recent years have shown that KD can improve some kidney diseases. Three days following KD treatment, a model of IRI-induced kidney injury showed significant increases in blood ketones and Kac in the liver and kidney, which may be a factor in the powerful antioxidant and anti-inflammatory effects of a KD in ischemic kidney injury [97]. TRF and KD strongly inhibited cystogenesis and cyst expansion and reduced serum creatinine. SMA positive myofibroblasts and Ki-67 positive cyst-lining cells decreased in TRF and KD-fed polycystic kidney disease (PKD) rats, indicating collagen deposition decreases and Inhibition of proliferation. It was subsequently observed that treatment with BHB significantly reduced fibrosis, almost completely eliminated myofibroblasts, and improved renal function. Moreover, acute fasting-induced significant apoptosis of cyst-lining epithelial cells and significantly reversed renal cystic burden in mouse, rat, and feline models of PKD. Thus, induction ketosis delays PKD progression, the main reason may be BHB as a metabolite or signaling molecule affecting PKD cells [98]. Besides, exogenous continuous infusion of BHB alleviated ischemic acute kidney injury in mice [99]. Supplementing 1,3-butanediol, a precursor of BHB, reduced serum microalbumin and Na+/K+ ratio, and alleviated glomerular morphological changes in Dahl salt-sensitive rats. This protective effect of BHB on salt-sensitive hypertension was likely independent of gut-microbiota and Th17 activation but rather reduced the release of Caspase1, IL-1β, and IL-18 by inhibiting the overactivation of NLRP3 in the kidney. Treatment with 1,3-butanediol, which reduces hypertension and protects renal function, may be an interesting strategy for the clinical management of salt-sensitive hypertension [100].
4.7. Embryonic and fetal development
The relationship between BHB and embryonic development is gradually attracting academic attention. Although KD appears to improve polycystic ovary syndrome (PCOS) by correcting hormonal and metabolic imbalances [101,102], there is insufficient evidence to support its reproductive safety as a diet before and during pregnancy. Some studies have pointed out that KD in pregnancy adversely affects a woman's brain and fertility and impairs her offspring's physical and neurological development [103,104]. Notably, with prenatal exposure to KD, adult offspring showed reduced susceptibility to anxiety and depression [105]. A previous study has shown that BHB has teratogenic effects on embryos [106]. Pre-implantation BHB exposure impairs mouse embryonic development and posttransplant viability, limiting fetal growth, with female offspring most severely affected. In vitro, exposure to 2 mM BHB significantly reduced the number of trophectoderm (TE) cells and glucose consumption, and increased glycolytic flux. Superphysiological concentrations of BHB (20 mM) increased the H3K27bhb in inner cell mass and TE cell lineages, but are unlikely to contribute to the metabolic and morpho-developmental adaptations observed in this study [107]. Subsequently, the team also observed that combined exposure to 0.8 mM AcAc and 2 mM BHB did not affect blastocyst development and significantly increased H3K27ac of TE, but also reduced fetal development and delayed the development of female offspring [108]. Whatley et al. suggest that these differential effects are plausibly regulated by ACL/HAT-mediated (proliferation-promoting) Kac by AcAc, versus HDAC inhibitors (apoptosis-promoting) by BHB. 2–6 mM concentrations of BHB also significantly induced H3K9bhb in bovine fibroblasts and cumulus cells [109]. In addition, Sangalli et al. conducted a series of experiments in which bovine somatic cells, cumulus-oocyte complexes (COCs), were exposed to BHB, none of which changed Kac levels. Interestingly, treatment of somatic cell nuclear transfer zygotes with BHB caused an elevation of H3K9ac, which was maintained to the blastocyst stage, resulting in enhanced FOXO3a expression and blastocyst production [28]. Thus, exposure to KD or BHB before or during pregnancy is a very interesting hot research topic in the future.
4.8. Intestinal homeostasis
The intestine is the most frequently renewed organ in adult mammals and responds to dietary restrictions and the ketogenic process. Recent studies by Cheng et al. suggest that HMGCS2, a rate-limiting enzyme for the ketogenic process, is enriched in Lgr5+ intestinal stem cells (ISCs), HMGCS2 deletion depletes BHB levels and compromises ISCs self-renewal and differentiation in Lgr5+ ISCs, which can be rescued by supplementing exogenous BHB [110]. Moreover, the loss of HMGCS2 impairs the ability of fasting-induce H3K9bhb and alters the expression of fasting-related genes (such as HMGCS2, Fabp1, Acox1, and Acox2) in the small intestine crypts [111]. It is suggested that BHB maintains intestinal homeostasis and has an important role in reprogramming and regenerating by epigenetic mechanisms.
5. Conclusions and prospects
A large number of diseases are related to environmental factors, including diet and lifestyle, as well as to individual genetics and epigenetics. In addition to serving as a backup energy source, BHB also directly affects the activity of gene transcription as an epigenetic regulator without changing DNA structure and further participates in the pathogenesis of related diseases. BHB has been shown to mediate three histone modification types (Kac, Kbhb, and Kme), DNA methylation, and microRNAs, in the pathophysiological regulation mechanisms in cardiovascular diseases, diabetes and complications of diabetes, neuropsychiatric diseases, cancers, osteoporosis, liver and kidney injury, embryonic and fetal development and intestinal homeostasis. BHB has pleiotropic effects through these mechanisms in many physiological and pathological settings with potential therapeutic value, and endogenous ketosis and exogenous supplementation may be promising strategies for these diseases.
This article reviews the recent progress of epigenetic effects of BHB, which provides new directions for exploring the pathogenesis and therapeutic targets of related diseases. However, a large number of BHB-mediated epigenetic mechanisms are still only found in basic studies or animal models, while clinical studies are rare. Furthermore, whether there is competition or antagonism between BHB-mediated epigenetic mechanisms, and whether these epigenetic mechanisms intersect with BHB as a signal transduction mechanism (GPR109A, GPR41) or backup energy source remains to be determined. As the main source of BHB, a KD could cause negative effects, such as fatty liver, kidney stones, vitamin deficiency, hypoproteinemia, gastrointestinal dysfunction, and even potential cardiovascular side effects [112,113], which may be one of the factors limiting adherence to a KD. Whether BHB-mediated epigenetic mechanisms participate in the occurrence and development of these side effects, and how to balance BHB intervention dosages and organ specificity, are unanswered. These interesting issues and areas mentioned above need to be further studied.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82170834, No.81970676, and No.U22A20286), Sichuan Science and Technology Program (No. 2022YFS0617, 2022YFS0612), the Office of Science Technology and Talent Work of Luzhou (No. 2020LZXNYDP02, No. 2021LZXNYD-G01, and No. 2019LZXNYDJ40) and the Project of The Health Commission of Sichuan Province (No. 19PJ294).
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Contributor Information
Yong Xu, Email: xywyll@aliyun.com.
Wei Huang, Email: huangwei1212520@163.com.
References
- 1.Owen O.E., Morgan A.P., Kemp H.G., Sullivan J.M., Herrera M.G., Cahill G.F., Jr Brain metabolism during fasting. J. Clin. Invest. 1967;46(10):1589–1595. doi: 10.1172/jci105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbasi J. Ketone body supplementation-A potential new approach for heart disease. JAMA. 2021 doi: 10.1001/jama.2021.8789. [DOI] [PubMed] [Google Scholar]
- 3.Sultan A.M. D-3-hydroxybutyrate metabolism in the perfused rat heart. Mol. Cell. Biochem. 1988;79(2):113–118. doi: 10.1007/bf02424552. [DOI] [PubMed] [Google Scholar]
- 4.Zhu H., Bi D., Zhang Y., Kong C., Du J., Wu X., Wei Q., Qin H. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Signal Transduct. Targeted Ther. 2022;7(1):11. doi: 10.1038/s41392-021-00831-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolb H., Kempf K., Röhling M., Lenzen-Schulte M., Schloot N.C., Martin S. Ketone bodies: from enemy to friend and guardian angel. BMC Med. 2021;19(1):313. doi: 10.1186/s12916-021-02185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L., Chen P., Xiao W. β-Hydroxybutyrate as an anti-aging metabolite. Nutrients. 2021;13(10) doi: 10.3390/nu13103420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasser S., Vialichka V., Biesiekierska M., Balcerczyk A., Pirola L. Effects of ketogenic diet and ketone bodies on the cardiovascular system: concentration matters. World J. Diabetes. 2020;11(12):584–595. doi: 10.4239/wjd.v11.i12.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graff E.C., Fang H., Wanders D., Judd R.L. Anti-inflammatory effects of the hydroxycarboxylic acid receptor 2. Metabolism. 2016;65(2):102–113. doi: 10.1016/j.metabol.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Kim J.K., Samaranayake M., Pradhan S. Epigenetic mechanisms in mammals. Cell. Mol. Life Sci. 2009;66(4):596–612. doi: 10.1007/s00018-008-8432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu C., Coradin M., Porter E.G., Garcia B.A. Accelerating the field of epigenetic histone modification through mass spectrometry-based approaches. Mol. Cell. Proteomics. 2021;20 doi: 10.1074/mcp.R120.002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabari B.R., Zhang D., Allis C.D., Zhao Y. Metabolic regulation of gene expression through histone acylations. Nat. Rev. Mol. Cell Biol. 2017;18(2):90–101. doi: 10.1038/nrm.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Dobosy J.R., Selker E.U. Emerging connections between DNA methylation and histone acetylation. Cell. Mol. Life Sci. 2001;58(5–6):721–727. doi: 10.1007/pl00000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehninger A.L., Sudduth H.C., Wise J.B. D-beta-Hydroxybutyric dehydrogenase of muitochondria. J. Biol. Chem. 1960;235:2450–2455. [PubMed] [Google Scholar]
- 15.Nasser S., Solé T., Vega N., Thomas T., Balcerczyk A., Strigini M., Pirola L. Ketogenic diet administration to mice after a high-fat-diet regimen promotes weight loss, glycemic normalization and induces adaptations of ketogenic pathways in liver and kidney. Mol. Metabol. 2022;65 doi: 10.1016/j.molmet.2022.101578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang D., Yang H., Kong X., Wang K., Mao X., Yan X., Wang Y., Liu S., Zhang X., Li J., Chen L., Wu J., Wei M., Yang J., Guan Y. Proteomics analysis reveals diabetic kidney as a ketogenic organ in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2011;300(2):E287–E295. doi: 10.1152/ajpendo.00308.2010. [DOI] [PubMed] [Google Scholar]
- 17.Wentz A.E., D'avignon D.A., Weber M.L., Cotter D.G., Doherty J.M., Kerns R., Nagarajan R., Reddy N., Sambandam N., Crawford P.A. Adaptation of myocardial substrate metabolism to a ketogenic nutrient environment. J. Biol. Chem. 2010;285(32):24447–24456. doi: 10.1074/jbc.M110.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thumelin S., Forestier M., Girard J., Pegorier J.P. Developmental changes in mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene expression in rat liver, intestine and kidney. Biochem. J. 1993;292(Pt 2):493–496. doi: 10.1042/bj2920493. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venable A.H., Lee L.E., Feola K., Santoyo J., Broomfield T., Huen S.C. Fasting-induced HMGCS2 expression in the kidney does not contribute to circulating ketones. Am. J. Physiol. Ren. Physiol. 2022;322(4):F460–f467. doi: 10.1152/ajprenal.00447.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halestrap A.P., Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Archiv. 2004;447(5):619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 21.Taggart A.K., Kero J., Gan X., Cai T.Q., Cheng K., Ippolito M., Ren N., Kaplan R., Wu K., Wu T.J., Jin L., Liaw C., Chen R., Richman J., Connolly D., Offermanns S., Wright S.D., Waters M.G. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005;280(29):26649–26652. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S.J., Li Z.H., Zhang Y.D., Chen J., Li Y., Wu F.Q., Wang W., Cui Z.J., Chen G.Q. Ketone body 3-hydroxybutyrate ameliorates atherosclerosis via receptor gpr109a-mediated calcium influx. Adv. Sci. 2021;8(9) doi: 10.1002/advs.202003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee A.K., Kim D.H., Bang E., Choi Y.J., Chung H.Y. β-Hydroxybutyrate suppresses lipid accumulation in aged liver through gpr109a-mediated signaling. Aging Dis. 2020;11(4):777–790. doi: 10.14336/ad.2019.0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura I., Inoue D., Maeda T., Hara T., Ichimura A., Miyauchi S., Kobayashi M., Hirasawa A.Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc. Natl. Acad. Sci. U.S.A. 2011;108(19):8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang C., Ahmed K., Gille A., Lu S., Gröne H.J., Tunaru S., Offermanns S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat Med. 2015;21(2):173–177. doi: 10.1038/nm.3779. [DOI] [PubMed] [Google Scholar]
- 26.Shimazu T., Hirschey M.D., Newman J., He W., Shirakawa K., Le Moan N., Grueter C.A., Lim H., Saunders L.R., Stevens R.D., Newgard C.B., Farese R.V., Jr., De Cabo R., Ulrich S., Akassoglou K., Verdin E. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chriett S., Dąbek A., Wojtala M., Vidal H., Balcerczyk A., Pirola L. Prominent action of butyrate over β-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Sci. Rep. 2019;9(1):742. doi: 10.1038/s41598-018-36941-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sangalli J.R., Sampaio R.V., Del Collado M., Da Silveira J.C., De Bem T.H.C., Perecin F., Smith L.C., Meirelles F.V. Metabolic gene expression and epigenetic effects of the ketone body β-hydroxybutyrate on H3K9ac in bovine cells, oocytes and embryos. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-31822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glozak M.A., Sengupta N., Zhang X.Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 30.New M., Olzscha H.La, Thangue N.B. HDAC inhibitor-based therapies: can we interpret the code? Mol. Oncol. 2012;6(6):637–656. doi: 10.1016/j.molonc.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X.J., Seto E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat. Rev. Mol. Cell Biol. 2008;9(3):206–218. doi: 10.1038/nrm2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olzscha H., Bekheet M.E., Sheikh S.La, Thangue N.B. HDAC inhibitors. Methods Mol. Biol. 2016;1436:281–303. doi: 10.1007/978-1-4939-3667-0_19. [DOI] [PubMed] [Google Scholar]
- 33.Wellen K.E., Hatzivassiliou G., Sachdeva U.M., Bui T.V., Cross J.R., Thompson C.B. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324(5930):1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie Z., Zhang D., Chung D., Tang Z., Huang H., Dai L., Qi S., Li J., Colak G., Chen Y., Xia C., Peng C., Ruan H., Kirkey M., Wang D., Jensen L.M., Kwon O.K., Lee S., Pletcher S.D., Tan M., Lombard D.B., White K.P., Zhao H., Li J., Roeder R.G., Yang X., Zhao Y. Metabolic regulation of gene expression by histone lysine β-hydroxybutyrylation. Mol Cell. 2016;62(2):194–206. doi: 10.1016/j.molcel.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu K., Li F., Sun Q., Lin N., Han H., You K., Tian F., Mao Z., Li T., Tong T., Geng M., Zhao Y., Gu W., Zhao W. p53 β-hydroxybutyrylation attenuates p53 activity. Cell Death Dis. 2019;10(3):243. doi: 10.1038/s41419-019-1463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabari B.R., Tang Z., Huang H., Yong-Gonzalez V., Molina H., Kong H.E., Dai L., Shimada M., Cross J.R., Zhao Y., Roeder R.G., Allis C.D. Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation. Mol Cell. 2018;69(3):533. doi: 10.1016/j.molcel.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hao F., Tian M., Zhang X., Jin X., Jiang Y., Sun X., Wang Y., Peng P., Liu J., Xia C., Feng Y., Wei M. Butyrate enhances CPT1A activity to promote fatty acid oxidation and iTreg differentiation. Proc. Natl. Acad. Sci. U.S.A. 2021;118(22) doi: 10.1073/pnas.2014681118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang H., Zhang D., Weng Y., Delaney K., Tang Z., Yan C., Qi S., Peng C., Cole P.A., Roeder R.G., Zhao Y. The regulatory enzymes and protein substrates for the lysine β-hydroxybutyrylation pathway. Sci. Adv. 2021;7(9) doi: 10.1126/sciadv.abe2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu E., Du H., Zhu X., Wang L., Shang S., Wu X., Lu H., Lu X. Beta-hydroxybutyrate promotes the expression of BDNF in hippocampal neurons under adequate glucose supply. Neuroscience. 2018;386:315–325. doi: 10.1016/j.neuroscience.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 40.Hu E., Du H., Shang S., Zhang Y., Lu X. Beta-hydroxybutyrate enhances BDNF expression by increasing H3K4me3 and decreasing H2AK119ub in hippocampal neurons. Front. Neurosci. 2020;14 doi: 10.3389/fnins.2020.591177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dąbek A., Wojtala M., Pirola L., Balcerczyk A. Modulation of cellular biochemistry, epigenetics and metabolomics by ketone bodies. Implications of the ketogenic diet in the physiology of the organism and pathological states. Nutrients. 2020;12(3) doi: 10.3390/nu12030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allison J., Kaliszewska A., Uceda S., Reiriz M.Arias N. Targeting DNA methylation in the adult brain through diet. Nutrients. 2021;13(11) doi: 10.3390/nu13113979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobow K., Kaspi A., Harikrishnan K.N., Kiese K., Ziemann M., Khurana I., Fritzsche I., Hauke J., Hahnen E., Coras R., Mühlebner A., El-Osta A., Blümcke I. Deep sequencing reveals increased DNA methylation in chronic rat epilepsy. Acta Neuropathol. 2013;126(5):741–756. doi: 10.1007/s00401-013-1168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lusardi T.A., Akula K.K., Coffman S.Q., Ruskin D.N., Masino S.A., Boison D. Ketogenic diet prevents epileptogenesis and disease progression in adult mice and rats. Neuropharmacology. 2015;99:500–509. doi: 10.1016/j.neuropharm.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masino S.A., Li T., Theofilas P., Sandau U.S., Ruskin D.N., Fredholm B.B., Geiger J.D., Aronica E., Boison D. A ketogenic diet suppresses seizures in mice through adenosine A₁ receptors. J. Clin. Invest. 2011;121(7):2679–2683. doi: 10.1172/jci57813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams-Karnesky R.L., Sandau U.S., Lusardi T.A., Lytle N.K., Farrell J.M., Pritchard E.M., Kaplan D.L., Boison D. Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. J. Clin. Invest. 2013;123(8):3552–3563. doi: 10.1172/jci65636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.James S.J., Melnyk S., Pogribna M., Pogribny I.P., Caudill M.A. Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteine-related pathology. J. Nutr. 2002;132(8 Suppl):2361s–2366s. doi: 10.1093/jn/132.8.2361S. [DOI] [PubMed] [Google Scholar]
- 48.Chen F., He X., Luan G., Li T. Role of DNA methylation and adenosine in ketogenic diet for pharmacoresistant epilepsy: focus on epileptogenesis and associated comorbidities. Front. Neurol. 2019;10:119. doi: 10.3389/fneur.2019.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y., Wang Z., Gemeinhart R.A. Progress in microRNA delivery. J. Contr. Release. 2013;172(3):962–974. doi: 10.1016/j.jconrel.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fabian M.R., Sonenberg N., Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 51.Yamada K., Takizawa S., Ohgaku Y., Asami T., Furuya K., Yamamoto K., Takahashi F., Hamajima C., Inaba C., Endo K., Matsui R., Kitamura H., Tanaka S. MicroRNA 16-5p is upregulated in calorie-restricted mice and modulates inflammatory cytokines of macrophages. Gene. 2020;725 doi: 10.1016/j.gene.2019.144191. [DOI] [PubMed] [Google Scholar]
- 52.Cannataro R., Perri M., Gallelli L., Caroleo M.C., De Sarro G.Cione E. Ketogenic diet acts on body remodeling and MicroRNAs expression profile. MicroRNA. 2019;8(2):116–126. doi: 10.2174/2211536608666181126093903. [DOI] [PubMed] [Google Scholar]
- 53.Cannataro R., Caroleo M.C., Fazio A., La Torre C., Plastina P., Gallelli L., Lauria G.Cione E. Ketogenic diet and microRNAs linked to antioxidant biochemical homeostasis. Antioxidants. 2019;8(8) doi: 10.3390/antiox8080269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopaschuk Gary D., Dyck Jason R.B. Ketones and the cardiovascular system. Nature Cardiovascular Research. 2023;2(5):425–437. doi: 10.1038/s44161-023-00259-1. [DOI] [PubMed] [Google Scholar]
- 55.Nagao M., Toh R., Irino Y., Mori T., Nakajima H., Hara T., Honjo T., Satomi-Kobayashi S., Shinke T., Tanaka H., Ishida T., Hirata K. β-Hydroxybutyrate elevation as a compensatory response against oxidative stress in cardiomyocytes. Biochem. Biophys. Res. Commun. 2016;475(4):322–328. doi: 10.1016/j.bbrc.2016.05.097. [DOI] [PubMed] [Google Scholar]
- 56.Ji L., He Q., Liu Y., Deng Y., Xie M., Luo K., Cai X., Zuo Y., Wu W., Li Q., Zhou R.Li T. Ketone body β-hydroxybutyrate prevents myocardial oxidative stress in septic cardiomyopathy. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/2513837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferrannini E., Mark M., Mayoux E. CV protection in the EMPA-REG outcome trial: a "thrifty substrate" hypothesis. Diabetes Care. 2016;39(7):1108–1114. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 58.Nishitani S., Fukuhara A., Shin J., Okuno Y., Otsuki M., Shimomura I. Metabolomic and microarray analyses of adipose tissue of dapagliflozin-treated mice, and effects of 3-hydroxybutyrate on induction of adiponectin in adipocytes. Sci. Rep. 2018;8(1):8805. doi: 10.1038/s41598-018-27181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li B., Yu Y., Liu K., Zhang Y., Geng Q., Zhang F., Li Y., Qi J. β-Hydroxybutyrate inhibits histone deacetylase 3 to promote claudin-5 generation and attenuate cardiac microvascular hyperpermeability in diabetes. Diabetologia. 2021;64(1):226–239. doi: 10.1007/s00125-020-05305-2. [DOI] [PubMed] [Google Scholar]
- 60.Ritchie R.H., Abel E.D. Basic mechanisms of diabetic heart disease. Circ. Res. 2020;126(11):1501–1525. doi: 10.1161/circresaha.120.315913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oka S.I., Tang F., Chin A., Ralda G., Xu X., Hu C., Yang Z., Abdellatif M., Sadoshima J. β-Hydroxybutyrate, a ketone body, potentiates the antioxidant defense via Thioredoxin 1 upregulation in cardiomyocytes. Antioxidants. 2021;10(7) doi: 10.3390/antiox10071153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo W., Yu Y., Wang H., Liu K., Wang Y., Huang M., Xuan C., Li Y., Qi J. Up-regulation of MMP-2 by histone H3K9 β-hydroxybutyrylation to antagonize glomerulosclerosis in diabetic rat. Acta Diabetol. 2020;57(12):1501–1509. doi: 10.1007/s00592-020-01552-2. [DOI] [PubMed] [Google Scholar]
- 63.Wu X., Miao D., Liu Z., Liu K., Zhang B., Li J., Li Y., Qi J. β-hydroxybutyrate antagonizes aortic endothelial injury by promoting generation of VEGF in diabetic rats. Tissue Cell. 2020;64 doi: 10.1016/j.tice.2020.101345. [DOI] [PubMed] [Google Scholar]
- 64.Bough K.J., Schwartzkroin P.A., Rho J.M. Calorie restriction and ketogenic diet diminish neuronal excitability in rat dentate gyrus in vivo. Epilepsia. 2003;44(6):752–760. doi: 10.1046/j.1528-1157.2003.55502.x. [DOI] [PubMed] [Google Scholar]
- 65.Stafstrom C.E., Rho J.M. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front. Pharmacol. 2012;3:59. doi: 10.3389/fphar.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Landgrave-Gómez J., Mercado-Gómez O.F., Vázquez-García M., Rodríguez-Molina V., Córdova-Dávalos L., Arriaga-Ávila V., Miranda-Martínez A., Guevara-Guzmán R. Anticonvulsant effect of time-restricted feeding in a pilocarpine-induced seizure model: metabolic and epigenetic implications. Front. Cell. Neurosci. 2016;10:7. doi: 10.3389/fncel.2016.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kashiwaya Y., Takeshima T., Mori N., Nakashima K., Clarke K., Veech R.L. D-beta-hydroxybutyrate protects neurons in models of Alzheimer's and Parkinson's disease. Proc. Natl. Acad. Sci. U.S.A. 2000;97(10):5440–5444. doi: 10.1073/pnas.97.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Achanta L.B., Rae C.D. β-Hydroxybutyrate in the brain: one molecule, multiple mechanisms. Neurochem. Res. 2017;42(1):35–49. doi: 10.1007/s11064-016-2099-2. [DOI] [PubMed] [Google Scholar]
- 69.Murphy P., Likhodii S., Nylen K., Burnham W.M. The antidepressant properties of the ketogenic diet. Biol. Psychiatr. 2004;56(12):981–983. doi: 10.1016/j.biopsych.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 70.Gersner R., Gal R., Levit O., Moshe H., Zangen A. Inherited behaviors, BDNF expression and response to treatment in a novel multifactorial rat model for depression. Int. J. Neuropsychopharmacol. 2014;17(6):945–955. doi: 10.1017/s1461145714000030. [DOI] [PubMed] [Google Scholar]
- 71.Chen L., Miao Z., Xu X. β-hydroxybutyrate alleviates depressive behaviors in mice possibly by increasing the histone3-lysine9-β-hydroxybutyrylation. Biochem. Biophys. Res. Commun. 2017;490(2):117–122. doi: 10.1016/j.bbrc.2017.05.184. [DOI] [PubMed] [Google Scholar]
- 72.Cornuti S., Chen S., Lupori L., Finamore F., Carli F., Samad M., Fenizia S., Caldarelli M., Damiani F., Raimondi F., Mazziotti R., Magnan C., Rocchiccioli S., Gastaldelli A., Baldi P., Tognini P. Brain histone beta-hydroxybutyrylation couples metabolism with gene expression. Cell. Mol. Life Sci. 2023;80(1):28. doi: 10.1007/s00018-022-04673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clarke K., Tchabanenko K., Pawlosky R., Carter E., Todd King M., Musa-Veloso K., Ho M., Roberts A., Robertson J., Vanitallie T.B., Veech R.L. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul. Toxicol. Pharmacol. 2012;63(3):401–408. doi: 10.1016/j.yrtph.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soto-Mota A., Norwitz N.G., Clarke K. Why a d-β-hydroxybutyrate monoester? Biochem. Soc. Trans. 2020;48(1):51–59. doi: 10.1042/bst20190240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blennow K., De Leon M.J., Zetterberg H. Alzheimer's disease. Lancet. 2006;368(9533):387–403. doi: 10.1016/s0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 76.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 77.Li X., Zhan Z., Zhang J., Zhou F. An L., β-hydroxybutyrate ameliorates aβ-induced downregulation of TrkA expression by inhibiting HDAC1/3 in SH-SY5Y cells. Am J Alzheimers Dis Other Demen. 2020;35 doi: 10.1177/1533317519883496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang J., Li X., Ren Y., Zhao Y., Xing A., Jiang C., Chen Y.An L. Intermittent fasting alleviates the increase of lipoprotein lipase expression in brain of a mouse model of alzheimer's disease: possibly mediated by β-hydroxybutyrate. Front. Cell. Neurosci. 2018;12:1. doi: 10.3389/fncel.2018.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X., Wu X., Liu Q., Kong G., Zhou J., Jiang J., Wu X., Huang Z., Su W., Zhu Q. Ketogenic metabolism inhibits histone deacetylase (HDAC) and reduces oxidative stress after spinal cord injury in rats. Neuroscience. 2017;366:36–43. doi: 10.1016/j.neuroscience.2017.09.056. [DOI] [PubMed] [Google Scholar]
- 80.Kong G., Huang Z., Ji W., Wang X., Liu J., Wu X., Huang Z., Li R., Zhu Q. The ketone metabolite β-hydroxybutyrate attenuates oxidative stress in spinal cord injury by suppression of class I histone deacetylases. J. Neurotrauma. 2017;34(18):2645–2655. doi: 10.1089/neu.2017.5192. [DOI] [PubMed] [Google Scholar]
- 81.Weber D.D., Aminzadeh-Gohari S., Tulipan J., Catalano L., Feichtinger R.G., Kofler B. Ketogenic diet in the treatment of cancer - where do we stand? Mol. Metabol. 2020;33:102–121. doi: 10.1016/j.molmet.2019.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klement R.J. Beneficial effects of ketogenic diets for cancer patients: a realist review with focus on evidence and confirmation. Med. Oncol. 2017;34(8):132. doi: 10.1007/s12032-017-0991-5. [DOI] [PubMed] [Google Scholar]
- 83.Seyfried T.N., Sanderson T.M., El-Abbadi M.M., Mcgowan R., Mukherjee P. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br. J. Cancer. 2003;89(7):1375–1382. doi: 10.1038/sj.bjc.6601269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mikami D., Kobayashi M., Uwada J., Yazawa T., Kamiyama K., Nishimori K., Nishikawa Y., Nishikawa S., Yokoi S., Taniguchi T., Iwano M. β-Hydroxybutyrate enhances the cytotoxic effect of cisplatin via the inhibition of HDAC/survivin axis in human hepatocellular carcinoma cells. J. Pharmacol. Sci. 2020;142(1):1–8. doi: 10.1016/j.jphs.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 85.Zhang H., Chang Z., Qin L.N., Liang B., Han J.X., Qiao K.L., Yang C., Liu Y.R., Zhou H.G., Sun T. MTA2 triggered R-loop trans-regulates BDH1-mediated β-hydroxybutyrylation and potentiates propagation of hepatocellular carcinoma stem cells. Signal Transduct. Targeted Ther. 2021;6(1):135. doi: 10.1038/s41392-021-00464-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang C.K., Chang P.H., Kuo W.H., Chen C.L., Jeng Y.M., Chang K.J., Shew J.Y., Hu C.M., Lee W.H. Adipocytes promote malignant growth of breast tumours with monocarboxylate transporter 2 expression via β-hydroxybutyrate. Nat. Commun. 2017;8 doi: 10.1038/ncomms14706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rodrigues L.M., Uribe-Lewis S., Madhu B., Honess D.J., Stubbs M., Griffiths J.R. The action of β-hydroxybutyrate on the growth, metabolism and global histone H3 acetylation of spontaneous mouse mammary tumours: evidence of a β-hydroxybutyrate paradox. Cancer Metab. 2017;5:4. doi: 10.1186/s40170-017-0166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang J., Cao Q., Li S., Lu X., Zhao Y., Guan J.S., Chen J.C., Wu Q., Chen G.Q. 3-Hydroxybutyrate methyl ester as a potential drug against Alzheimer's disease via mitochondria protection mechanism. Biomaterials. 2013;34(30):7552–7562. doi: 10.1016/j.biomaterials.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 89.Cheng S., Wu Q., Yang F., Xu M., Leski M., Chen G.Q. Influence of DL-beta-hydroxybutyric acid on cell proliferation and calcium influx. Biomacromolecules. 2005;6(2):593–597. doi: 10.1021/bm049465y. [DOI] [PubMed] [Google Scholar]
- 90.Zhao Y., Zou B., Shi Z., Wu Q., Chen G.Q. The effect of 3-hydroxybutyrate on the in vitro differentiation of murine osteoblast MC3T3-E1 and in vivo bone formation in ovariectomized rats. Biomaterials. 2007;28(20):3063–3073. doi: 10.1016/j.biomaterials.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 91.Saito A., Yoshimura K., Miyamoto Y., Kaneko K., Chikazu D., Yamamoto M., Kamijo R. Enhanced and suppressed mineralization by acetoacetate and β-hydroxybutyrate in osteoblast cultures. Biochem. Biophys. Res. Commun. 2016;473(2):537–544. doi: 10.1016/j.bbrc.2016.03.109. [DOI] [PubMed] [Google Scholar]
- 92.Cao Q., Zhang J., Liu H., Wu Q., Chen J., Chen G.Q. The mechanism of anti-osteoporosis effects of 3-hydroxybutyrate and derivatives under simulated microgravity. Biomaterials. 2014;35(28):8273–8283. doi: 10.1016/j.biomaterials.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 93.Wu Y., Teng Y., Zhang C., Pan Y., Zhang Q., Zhu X., Liu N., Su X., Lin J. The ketone body β-hydroxybutyrate alleviates CoCrMo alloy particles induced osteolysis by regulating NLRP3 inflammasome and osteoclast differentiation. J Nanobiotechnology. 2022;20(1):120. doi: 10.1186/s12951-022-01320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huo S., Liu X., Zhang S., Lyu Z., Zhang J., Wang Y., Nie B.Yue B. p300/CBP inhibitor A-485 inhibits the differentiation of osteoclasts and protects against osteoporotic bone loss. Int Immunopharmacol. 2021;94 doi: 10.1016/j.intimp.2021.107458. [DOI] [PubMed] [Google Scholar]
- 95.Miyauchi T., Uchida Y., Kadono K., Hirao H., Kawasoe J., Watanabe T., Ueda S., Okajima H., Terajima H., Uemoto S. Up-regulation of FOXO1 and reduced inflammation by β-hydroxybutyric acid are essential diet restriction benefits against liver injury. Proc. Natl. Acad. Sci. U.S.A. 2019;116(27):13533–13542. doi: 10.1073/pnas.1820282116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Acharya P., Acharya C., Thongprayoon C., Hansrivijit P., Kanduri S.R., Kovvuru K., Medaura J., Vaitla P., Garcia Anton D.F., Mekraksakit P., Pattharanitima P., Bathini T., Cheungpasitporn W. Incidence and characteristics of kidney stones in patients on ketogenic diet: a systematic review and meta-analysis. Diseases. 2021;9(2) doi: 10.3390/diseases9020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rojas-Morales P., León-Contreras J.C., Sánchez-Tapia M., Silva-Palacios A., Cano-Martínez A., González-Reyes S., Jiménez-Osorio A.S., Hernández-Pando R., Osorio-Alonso H., Sánchez-Lozada L.G., Tovar A.R., Pedraza-Chaverri J.Tapia E. A ketogenic diet attenuates acute and chronic ischemic kidney injury and reduces markers of oxidative stress and inflammation. Life Sci. 2022;289 doi: 10.1016/j.lfs.2021.120227. [DOI] [PubMed] [Google Scholar]
- 98.Torres J.A., Kruger S.L., Broderick C., Amarlkhagva T., Agrawal S., Dodam J.R., Mrug M., Lyons L.A., Weimbs T. Ketosis ameliorates renal cyst growth in polycystic kidney disease. Cell Metab. 2019;30(6):1007–1023.e5. doi: 10.1016/j.cmet.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tajima T., Yoshifuji A., Matsui A., Itoh T., Uchiyama K., Kanda T., Tokuyama H., Wakino S., Itoh H. β-hydroxybutyrate attenuates renal ischemia-reperfusion injury through its anti-pyroptotic effects. Kidney Int. 2019;95(5):1120–1137. doi: 10.1016/j.kint.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 100.Chakraborty S., Galla S., Cheng X., Yeo J.Y., Mell B., Singh V., Yeoh B., Saha P., Mathew A.V., Vijay-Kumar M.Joe B. Salt-responsive metabolite, β-hydroxybutyrate, attenuates hypertension. Cell Rep. 2018;25(3):677–689.e4. doi: 10.1016/j.celrep.2018.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mavropoulos J.C., Yancy W.S., Hepburn J.Westman E.C. The effects of a low-carbohydrate, ketogenic diet on the polycystic ovary syndrome: a pilot study. Nutr. Metab. 2005;2:35. doi: 10.1186/1743-7075-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Paoli A., Mancin L., Giacona M.C., Bianco A., Caprio M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J. Transl. Med. 2020;18(1):104. doi: 10.1186/s12967-020-02277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kosiek W., Rauk Z., Szulc P., Cichy A., Rugieł M., Chwiej J., Janeczko K., Setkowicz Z. Ketogenic diet impairs neurological development of neonatal rats and affects biochemical composition of maternal brains: evidence of functional recovery in pups. Brain Struct. Funct. 2022;227(3):1099–1113. doi: 10.1007/s00429-021-02450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sussman D., Ellegood J., Henkelman M. A gestational ketogenic diet alters maternal metabolic status as well as offspring physiological growth and brain structure in the neonatal mouse. BMC Pregnancy Childbirth. 2013;13:198. doi: 10.1186/1471-2393-13-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sussman D., Germann J., Henkelman M. Gestational ketogenic diet programs brain structure and susceptibility to depression & anxiety in the adult mouse offspring. Brain Behav. 2015;5(2) doi: 10.1002/brb3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shum L., Sadler T.W. Biochemical basis for D,L,-beta-hydroxybutyrate-induced teratogenesis. Teratology. 1990;42(5):553–563. doi: 10.1002/tera.1420420512. [DOI] [PubMed] [Google Scholar]
- 107.Whatley E.G., Truong T.T., Wilhelm D., Harvey A.J., Gardner D.K. β-hydroxybutyrate reduces blastocyst viability via trophectoderm-mediated metabolic aberrations in mice. Hum. Reprod. 2022;37(9):1994–2011. doi: 10.1093/humrep/deac153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Whatley E.G., Truong T.T., Harvey A.J., Gardner D.K. Acetoacetate and β-hydroxybutyrate reduce mouse embryo viability via differential metabolic and epigenetic mechanisms. Reprod. Biomed. Online. 2023;46(1):20–33. doi: 10.1016/j.rbmo.2022.09.018. [DOI] [PubMed] [Google Scholar]
- 109.Sangalli J.R., Nociti R.P., Del Collado M., Sampaio R.V., Da Silveira J.C., Perecin F., Smith L.C., Ross P.J., Meirelles F.V. Characterization of histone lysine β-hydroxybutyrylation in bovine tissues, cells, and cumulus-oocyte complexes. Mol. Reprod. Dev. 2022;89(9):375–398. doi: 10.1002/mrd.23630. [DOI] [PubMed] [Google Scholar]
- 110.Cheng C.W., Biton M., Haber A.L., Gunduz N., Eng G., Gaynor L.T., Tripathi S., Calibasi-Kocal G., Rickelt S., Butty V.L., Moreno-Serrano M., Iqbal A.M., Bauer-Rowe K.E., Imada S., Ulutas M.S., Mylonas C., Whary M.T., Levine S.S., Basbinar Y., Hynes R.O., Mino-Kenudson M., Deshpande V., Boyer L.A., Fox J.G., Terranova C., Rai K., Piwnica-Worms H., Mihaylova M.M., Regev A., Yilmaz Ö H. Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell. 2019;178(5):1115–1131.e15. doi: 10.1016/j.cell.2019.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Terranova C.J., Stemler K.M., Barrodia P., Jeter-Jones S.L., Ge Z., De La Cruz Bonilla M., Raman A., Cheng C.W., Allton K.L., Arslan E., Yilmaz Ö H., Barton M.C., Rai K., Piwnica-Worms H. Reprogramming of H3K9bhb at regulatory elements is a key feature of fasting in the small intestine. Cell Rep. 2021;37(8) doi: 10.1016/j.celrep.2021.110044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Luong T.V., Abild C.B., Bangshaab M., Gormsen L.C., Søndergaard E. Ketogenic diet and cardiac substrate metabolism. Nutrients. 2022;14(7) doi: 10.3390/nu14071322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.O'neill B., Raggi P. The ketogenic diet: pros and cons. Atherosclerosis. 2020;292:119–126. doi: 10.1016/j.atherosclerosis.2019.11.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.