Abstract
Background:
Tea is frequently consumed worldwide, but the association of tea drinking with mortality risk remains inconclusive in populations where black tea is the main type consumed.
Objective:
To evaluate the associations of tea consumption with all-cause and cause-specific mortality and potential effect modification by genetic variation in caffeine metabolism.
Design:
Prospective cohort study.
Setting:
The UK Biobank.
Participants:
498 043 men and women aged 40 to 69 years who completed the baseline touchscreen questionnaire from 2006 to 2010.
Measurements:
Self-reported tea intake and mortality from all causes and leading causes of death, including cancer, all cardiovascular disease (CVD), ischemic heart disease, stroke, and respiratory disease.
Results:
During a median follow-up of 11.2 years, higher tea intake was modestly associated with lower all-cause mortality risk among those who drank 2 or more cups per day. Relative to no tea drinking, the hazard ratios (95% CIs) for participants drinking 1 or fewer, 2 to 3, 4 to 5, 6 to 7, 8 to 9, and 10 or more cups per day were 0.95 (95% CI, 0.91 to 1.00), 0.87 (CI, 0.84 to 0.91), 0.88 (CI, 0.84 to 0.92), 0.88 (CI, 0.84 to 0.92), 0.91 (CI, 0.86 to 0.97), and 0.89 (CI, 0.84 to 0.95), respectively. Inverse associations were seen for mortality from all CVD, ischemic heart disease, and stroke. Findings were similar regardless of whether participants also drank coffee or not or of genetic score for caffeine metabolism.
Limitation:
Potentially important aspects of tea intake (for example, portion size and tea strength) were not assessed.
Conclusion:
Higher tea intake was associated with lower mortality risk among those drinking 2 or more cups per day, regardless of genetic variation in caffeine metabolism. These findings suggest that tea, even at higher levels of intake, can be part of a healthy diet.
Primary Funding Source:
National Cancer Institute Intramural Research Program.
Tea is one of the most frequently consumed beverages in the United Kingdom and worldwide, but whether tea intake is associated with overall mortality or mortality from common causes of death is unclear. As an extract made from the leaves of the plant Camellia sinensis, tea has high concentrations of flavonoids and other antioxidants with posited beneficial properties (1, 2). Black and green, the predominant forms, differ in their constituents. Previous studies have suggested a modest inverse association for tea drinking and mortality (3–9), which has largely been seen in populations where green tea drinking is common, such as in China and Japan. In contrast, published studies in populations where black tea drinking is more common are limited with inconsistent findings (6–12).
Tea also contains caffeine, and concerns have been raised about high caffeine intake, particularly among persons genetically predisposed to impaired caffeine metabolism (13–15). Though generally lower in caffeine than coffee, tea is a major contributor to dietary caffeine in populations with high tea consumption (16). The extraction of flavonoids and other bioactive compounds from tea leaves differs by water temperature and steeping time, and use of additives such as milk alters the concentration of bioactive compounds; these differences may impact the association of tea consumption with mortality (1). However, previous studies have not assessed whether associations between tea consumption and mortality differ by genetic variation in caffeine metabolism, tea temperature, or use of additives.
We investigated the association of tea consumption with all-cause and cause-specific mortality in the UK Biobank, a prospective cohort study of half a million participants residing in the United Kingdom where black tea consumption is common. Furthermore, we address novel questions by evaluating potential differences by use of common additives such as milk and sugar, genetic variation in caffeine metabolism, and tea temperature.
Methods
Study Population
The UK Biobank study design was previously described in detail (17). In brief, approximately 9.2 million persons aged 40 to 69 years who were registered with the UK National Health Service (NHS) and resided within 40 km of 1 of 22 assessment centers across the United Kingdom (England, Wales, and Scotland) were invited to participate in the study. A total of 502 488 persons consented to participate and completed comprehensive questionnaires assessing sociodemographic, lifestyle, and health-related information using a touchscreen at assessment centers from 2006 to 2010. Participants received physical examinations and provided blood, urine, and saliva samples. We excluded participants with missing or incomplete tea intake information (n = 2193), those who were pregnant (n = 368), or those with missing or incomplete smoking data (n = 1884), yielding an analytic cohort of 498 043 participants. The UK Biobank study was approved by the National Information Governance Board for Health and Social Care and the NHS North West Multi-centre Research Ethics Committee. All participants provided electronically signed consent.
Exposure Assessment
On the baseline touchscreen questionnaire, participants entered the number of cups of tea they drank each day. If participants reported drinking more than 20 cups per day, they were asked to confirm their response. For the main analysis, we categorized tea intake into 7 categories: 0, 1 or fewer, 2 to 3, 4 to 5, 6 to 7, 8 to 9, and 10 or more cups per day. Participants also selected how they like their hot drinks, such as coffee or tea. Accordingly, we categorized tea drinkers by their preferred temperature group (very hot, hot, and warm). The baseline touchscreen questionnaire did not include questions about type of tea consumed or use of tea additives; instead, these factors were assessed via a 24-hour dietary recall questionnaire in a subset of 70 699 participants who were recruited in 2009 and 2010. Four additional 24-hour dietary recall questionnaires were sent by e-mail between 2011 and 2015. Among participants who completed the baseline 24-hour dietary recall questionnaire, 56 066 participants (79%) reported drinking tea. Among tea drinkers, 89% drank black tea (n = 49 997) and 7% drank green tea (n = 4050) (18). In a subset of 122 283 participants who completed 2 or more of the five 24-hour recall questionnaires, the correlation coefficient (r) between baseline touchscreen questionnaire and mean 24-hour recall tea intake was 0.81 (19). Within the subset of 20 348 participants who repeated the baseline touchscreen questionnaire approximately 4 years after recruitment, the weighted κ for tea intake across the 2 assessments was 0.83 (20). Tea intake data from the baseline questionnaire and the 24-hour dietary recall questionnaires are described in Supplement Methods 1 (available at Annals.org).
Potential confounders, including age, sex, race and ethnicity, education, body mass index (BMI), general health, comorbid conditions, smoking, alcohol drinking, coffee intake, and dietary intake, were assessed at baseline. Participants reported perceived general health and comorbid conditions, such as cancer, diabetes, and cardiovascular disease (CVD). The BMI was constructed from height and weight measured during the initial assessment center visit. Participants were assigned a Townsend deprivation score as a socioeconomic status indicator (21). For smoking, we combined data on current and past smoking, type of tobacco currently smoked, type of tobacco previously smoked, number of cigarettes currently smoked per day, number of cigarettes previously smoked per day, and the age at which they stopped smoking (for former smokers), resulting in a 25-level detailed smoking variable. For alcohol intake, we created a 6-level variable by combining alcohol intake status and amount of alcohol consumed on a typical drinking day (Supplement Table 1, available at Annals.org). For dietary intake, we created variables for major food groups, including vegetables (cooked and raw; tablespoons per day), fruits (fresh and dried; pieces per day), red meat (beef, lamb, and pork combined; 0 to 1, 1.5, 2, 2.5, 3 to 21 times per week as quintiles), and processed meat (0, <1, 1, 2 to 4, 5 to 6, and ≥7 times per week).
Genetic Caffeine Metabolism Score
Genetic data were available for 403 780 participants in our analytic cohort after excluding sample outliers based on heterozygosity and missingness, participants whose reported sex did not agree with X-chromosome heterozygosity, and those potentially related to other participants, based on estimated kinship coefficients for all pairs of samples (22). Using 4 common single-nucleotide polymorphisms previously associated with blood caffeine metabolite levels and located in or near genes involved in caffeine metabolism (rs2472297, rs56113850, rs6968554, and rs17685), we created a genetic caffeine metabolism score (CMSG4) by adding the number of alleles and weighted CMSG4 (wCMSG4), which was then calibrated between 0 and 8 (Supplement Methods 2, available at Annals.org) (23–25). Four categories (0 to 2, >2 to 3, >3 to 4, and >4) were created for wCMSG4 based on the score distribution. Persons with higher scores are predicted to have higher caffeine metabolism.
Mortality Ascertainment
Vital status, date of death, and underlying primary cause of death were provided by the NHS Information Centre (England and Wales) and the NHS Central Register (Scotland). Detailed information on the linkage procedure is available online (http://biobank.ctsu.ox.ac.uk/crystal/ukb/docs/DeathLinkage.pdf). Specific causes of death were defined using the following codes from the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10): cancer (C00-D48), CVD (I00-I79), ischemic heart diseases (I20-I25), stroke (I60-I69), and respiratory disease (J09-J18 and J40-J47).
Statistical Analysis
Follow-up time was computed from the date of assessment center visit until the date of death or the end of follow-up (26 April 2020), whichever came first. Hazard ratios (HRs) and 95% CIs were estimated for tea intake as categorical and continuous scales (cups per day) using Cox proportional hazards regression models and no tea intake (“0”) as the referent group. Person-time was the underlying time metric. Covariates in the multivariable-adjusted model included age, sex, race and ethnicity (White, Black, Asian, mixed, or other race), Townsend deprivation score, BMI (kg/m2), general health (excellent, good, fair, or poor), physical activity (>10 minutes of moderate or vigorous activity; days per week), alcohol intake (6-level categorical), tobacco smoking (25-level categorical), coffee intake (cups per day), fruit intake (pieces per day), vegetable intake (tablespoons per day), red meat intake (0 to 1, 1.5, 2, 2.5, 3 to 21 times per week as quintiles), and processed meat intake (never, <1, 1, 2 to 4, 5 to 6 times per week, or daily). Approximately 18% of participants were missing values for 1 or more variable; to account for missing data, we used multiple imputation with 5 data sets. We tested the proportional hazards assumption by comparing multivariable-adjusted models with and without the cross-product term between person-years and tea intake, and saw no violation of the assumption (P = 0.98). We performed a sensitivity analysis excluding 387 707 participants who reported drinking coffee. To examine potential reverse causality, we performed a lag analysis excluding 8578 deaths occurring within 5 years after baseline. To assess a dose–response association for tea with all-cause mortality, we produced a spline curve, excluding outliers who reported tea intake above the 99th percentile (that is, >12 cups per day). To address absolute risk, we produced cumulative mortality curves by tea consumption, adjusted for the covariates included in the final Cox regression model (26, 27).
Among 210 058 participants who completed at least one 24-hour dietary recall questionnaire, we conducted a stratified analysis of tea and all-cause mortality by use of sugar or milk in tea. As tea intake is a major source of caffeine in the United Kingdom, potential effect modification by genetically predicted caffeine metabolism was assessed. Genetic analyses were also restricted to the subset of participants who did not drink coffee (n = 88 643) and separately to 335 465 White participants. We conducted a stratified analysis by preferred tea temperature (warm, hot, and very hot) after excluding 310 tea drinkers who did not report a temperature preference and then restricting to the 103 700 perticipants who did not drink coffee or other hot beverages. Analyses were conducted using the National Institutes of Health’s (NIH) High-Performance Computing Biowulf cluster with SAS version 9.4 (SAS Institute).
Results
Our analysis included 498 043 participants (227 149 men and 270 894 women), with a mean baseline age of 56.5 years (SD, 8.1 years). About 94% of participants self-reported being White. Approximately 85% of participants reported drinking tea, and most drank 2 to 3 (29%), 4 to 5 (26%), or 6 to 7 (12%) cups per day. The relationship between tea drinking and some potential risk factors was complex (Table 1). For example, moderate tea drinkers were less likely to be current smokers than nontea drinkers or heavy tea drinkers (that is, ≥8 cups per day). Heavy tea drinkers were more likely to be men, obese, and to live in England. They also tended to drink less coffee and eat more red and processed meat compared with less frequent and nontea drinkers. Heavy tea drinkers were more likely to report fair or poor general health than less frequent or nontea drinkers, but history of cancer, CVD, and diabetes did not differ. More frequent tea drinkers tended to prefer a hotter tea temperature than less frequent drinkers.
Table 1.
Demographic and Lifestyle Characteristics of 498 043 Persons by Tea Intake Category in the UK Biobank Study
| Characteristics | Tea Intake, Cups per Day |
||||||
|---|---|---|---|---|---|---|---|
| 0 | ≤1 | 2–3 | 4–5 | 6–7 | 8–9 | ≥10 | |
|
| |||||||
| Total, n (%) | 73 216 (14.7) | 57 510 (11.5) | 146 181 (29.4) | 126 921 (25.5) | 58 686 (11.8) | 18 618 (3.7) | 16 911 (3.4) |
| Median age (IQR), y | 56 (48 to 62) | 56 (48 to 63) | 58 (50 to 63) | 59 (51 to 64) | 58 (51 to 64) | 58 (51 to 63) | 57 (50 to 63) |
| Sex, n (%) | |||||||
| Male | 31 911 (43.6) | 27 544 (47.9) | 66 901 (45.8) | 56 848 (44.8) | 26 072 (44.4) | 8731 (46.9) | 9142 (54.1) |
| Female | 41 305 (56.4) | 29 966 (52.1) | 79 280 (54.2) | 70 073 (55.2) | 32 614 (55.6) | 9887 (53.1) | 7769 (45.9) |
| Race and ethnicity, n (%)* | |||||||
| White | 69 785 (95.3) | 52 625 (91.5) | 134 472 (92.0) | 121 313 (95.6) | 57 103 (97.3) | 18 136 (97.4) | 16 323 (96.5) |
| Black | 1258 (1.7) | 1641 (2.9) | 3105 (2.1) | 1353 (1.1) | 327 (0.6) | 94 (0.5) | 121 (0.7) |
| Asian | 863 (1.2) | 1661 (2.9) | 5489 (3.8) | 2368 (1.9) | 555 (1.0) | 149 (0.8) | 190 (1.1) |
| Mixed | 490 (0.7) | 477 (0.8) | 890 (0.6) | 634 (0.5) | 250 (0.4) | 91 (0.5) | 98 (0.6) |
| Other | 548 (0.8) | 843 (1.5) | 1742 (1.2) | 880 (0.7) | 280 (0.5) | 83 (0.5) | 108 (0.6) |
| Education, n (%) | |||||||
| College or university degree | 22 020 (30.1) | 22 956 (39.9) | 50 611 (34.6) | 39 050 (30.8) | 16 431 (28.0) | 5081 (27.3) | 4328 (25.6) |
| A/AS levels or equivalent | 8410 (11.5) | 7109 (12.4) | 16 519 (11.3) | 13 476 (10.6) | 6153 (10.5) | 1901 (10.2) | 1563 (9.2) |
| O/AS levels or equivalent | 16 347 (22.3) | 11 533 (20.1) | 30 640 (21.0) | 26 877 (21.2) | 12 372 (21.1) | 3754 (20.2) | 3186 (18.8) |
| CSEs or equivalent | 4630 (6.3) | 2570 (4.5) | 7271 (5.0) | 6796 (5.4) | 3363 (5.7) | 1009 (5.4) | 1063 (6.3) |
| NVQ/HND/HNC equivalent | 4822 (6.6) | 3131 (5.4) | 8824 (6.0) | 8631 (6.8) | 4286 (7.3) | 1418 (7.6) | 1362 (8.1) |
| Other professional qualifications | 3693 (5.0) | 2662 (4.6) | 7387 (5.1) | 6742 (5.3) | 3224 (5.5) | 1060 (5.7) | 886 (5.2) |
| Median Townsend deprivation index (IQR) | −1.9 (−3.5 to 0.9) | −2.0 (−3.6 to 0.8) | −2.2 (−3.7 to 0.4) | −2.3 (−3.7 to 0.2) | −2.2 (−3.7 to 0.3) | −2.0 (−3.6 to 0.9) | −1.4 (−3.3 to 1.7) |
| Study center region, n (%) | |||||||
| Scotland | 5618 (7.7) | 4973 (8.6) | 11 062 (7.6) | 8348 (6.6) | 3492 (6.0) | 1107 (5.9) | 980 (5.8) |
| England | 64 140 (87.6) | 50 435 (87.7) | 129 572 (88.6) | 113 252 (89.2) | 52 604 (89.6) | 16 678 (89.6) | 15 112 (89.4) |
| Wales | 3458 (4.7) | 2102 (3.7) | 5547 (3.8) | 5321 (4.2) | 2590 (4.4) | 833 (4.5) | 819 (4.8) |
| General health, n (%) | |||||||
| Excellent | 11 817 (16.1) | 10 430 (18.1) | 25 161 (17.2) | 20 350 (16.0) | 8852 (15.1) | 2609 (14.0) | 2273 (13.4) |
| Good | 40 358 (55.1) | 32 965 (57.3) | 86 440 (59.1) | 74 714 (58.9) | 33 956 (57.9) | 10 221 (54.9) | 8705 (51.5) |
| Fair | 16 507 (22.6) | 11 392 (19.8) | 28 475 (19.5) | 26 275 (20.7) | 12 881 (22.0) | 4549 (24.4) | 4337 (25.7) |
| Poor | 4189 (5.7) | 2416 (4.2) | 5426 (3.7) | 5042 (4.0) | 2751 (4.7) | 1142 (6.1) | 1495 (8.8) |
| Cancer, n (%) | 5125 (7.0) | 3718 (6.5) | 10 249 (7.0) | 9419 (7.4) | 4523 (7.7) | 1446 (7.8) | 1250 (7.4) |
| Cardiovascular disease, n (%) | 2577 (3.5) | 1718 (3.0) | 4363 (3.0) | 4049 (3.2) | 2000 (3.4) | 705 (3.8) | 817 (4.8) |
| Diabetes, n (%) | 4574 (6.3) | 3067 (5.3) | 7577 (5.2) | 6120 (4.8) | 2783 (4.7) | 968 (5.2) | 1013 (6.0) |
| Median body mass index (IQR) | 27.4 (24.5 to 30.9) | 26.7 (24.1 to 29.9) | 26.5 (24.0 to 29.6) | 26.6 (24.1 to 29.6) | 26.7 (24.2 to 29.8) | 26.9 (24.3 to 30.1) | 27.1 (24.3 to 30.2) |
| Smoking status, n (%) | |||||||
| Never | 38 146 (52.1) | 31 370 (54.6) | 82 892 (56.7) | 71 398 (56.2) | 32 142 (54.8) | 9421 (50.6) | 7367 (43.6) |
| Former | 24 996 (34.1) | 19 845 (34.5) | 51 041 (34.9) | 44 519 (35.1) | 20 272 (34.5) | 6336 (34.0) | 5620 (33.2) |
| Current | 10 074 (13.8) | 6295 (10.9) | 12 248 (8.4) | 11 004 (8.7) | 6272 (10.7) | 2861 (15.4) | 3924 (23.2) |
| Physical activity,† days per week, n (%) | |||||||
| 0 | 9614 (13.1) | 6225 (10.8) | 14 014 (9.6) | 12 266 (9.7) | 6128 (10.4) | 2070 (11.1) | 2042 (12.1) |
| 1–2 | 9628 (13.2) | 8008 (13.9) | 18 719 (12.8) | 15 603 (12.3) | 7299 (12.4) | 2227 (12.0) | 1835 (10.9) |
| 2–4 | 11 458 (15.7) | 9824 (17.1) | 24 894 (17.0) | 20 900 (16.5) | 9386 (16.0) | 2839 (15.3) | 2263 (13.4) |
| ≥5 | 36 499 (49.9) | 29 404 (51.1) | 77 219 (52.8) | 67 707 (53.4) | 30 845 (52.6) | 9710 (52.2) | 9022 (53.4) |
| Alcohol intake, drinks per week, n (%) | |||||||
| Never | 3911 (5.3) | 2406 (4.2) | 6748 (4.6) | 4958 (3.9) | 2423 (4.1) | 823 (4.4) | 938 (5.6) |
| Former | 3757 (5.1) | 1706 (3.0) | 3927 (2.7) | 4071 (3.2) | 2270 (3.9) | 963 (5.2) | 1186 (7.0) |
| Current, < 1 | 18 119 (24.8) | 11 876 (20.7) | 29 269 (20.0) | 28 668 (22.6) | 14 929 (25.4) | 5239 (28.1) | 4823 (28.5) |
| Current, >1 to <7 | 16 091 (22.0) | 13 704 (23.8) | 37 783 (25.9) | 33 777 (26.6) | 15 051 (25.7) | 4303 (23.1) | 3331 (19.7) |
| Current, 1–3 | 24 281(33.2) | 21 857 (38.0) | 55 765 (38.2) | 45 909 (36.2) | 19 840 (33.8) | 5905 (31.7) | 5127 (30.3) |
| Current, >3 | 6988 (9.5) | 5905 (10.3) | 12 522 (8.6) | 9440 (7.4) | 4116 (7.0) | 1365 (7.3) | 1482 (8.8) |
| Hot drink temperature, n (%) | |||||||
| Warm | 14 520 (19.8) | 10 748 (18.7) | 22 570 (15.4) | 17 436 (13.7) | 7723 (13.2) | 2367 (12.7) | 2211 (13.1) |
| Hot | 43 157 (58.9) | 38 321 (66.6) | 100 744 (68.9) | 86 998 (68.6) | 38 916 (66.3) | 11 886 (63.8) | 10 328 (61.1) |
| Very hot | 10 380 (14.2) | 8301 (14.4) | 22 770 (15.6) | 22 441 (17.7) | 12 030 (20.5) | 4359 (23.4) | 4369 (25.8) |
| Does not drink | 5059 (6.9) | 121 (0.2) | 75 (0.1) | 31 (0.0) | 13 (0.0) | 5 (0.0) | 3 (0.0) |
| Median coffee intake (IQR), cups per day | 3 (1 to 5) | 2 (1 to 4) | 2 (1 to 3) | 1 (0 to 2) | 1 (0 to 2) | 0 (0 to 2) | 0 (0 to 2) |
| Median vegetable intake (IQR), tablespoons per day | 4 (3 to 6) | 4 (3 to 6) | 4 (3 to 6) | 4 (3 to 6) | 4 (3 to 6) | 4 (3 to 6) | 4 (3 to 6) |
| Median fruit intake (IQR), pieces per day | 2 (1 to 4) | 2.5 (1 to 4) | 3 (1.5 to 4) | 3 (1.5 to 4) | 3 (1 to 4) | 2.5 (1 to 4) | 2 (1 to 4) |
| Red meat intake, times per week, n (%)‡ | |||||||
| Q1 (0–1) | 16 210 (22.1) | 10 855 (18.9) | 27 051 (18.5) | 22 315 (17.58) | 10 425 (17.8) | 3523 (18.9) | 3204 (19.0) |
| Q2 (1.5) | 21 710 (29.7) | 18 228 (31.7) | 46 104 (31.5) | 39 475 (31.10) | 17 997 (30.7) | 5627 (30.2) | 4818 (28.5) |
| Q3 (2) | 12 547 (17.1) | 10 553 (18.4) | 27 154 (18.6) | 24 118 (19.00) | 11 036 (18.8) | 3254 (17.5) | 2918 (17.3) |
| Q4 (2.5) | 6246 (8.5) | 5316 (9.2) | 14 072 (9.6) | 12 564 (9.90) | 5806 (9.9) | 1722 (9.3) | 1515 (9.0) |
| Q5 (3–21) | 16 368 (22.4) | 12 487 (21.7) | 31 586 (21.6) | 28 290 (22.29) | 13 356 (22.8) | 4464 (24.0) | 4418 (26.1) |
| Processed meat intake, times per week, n (%) | |||||||
| 0 | 8317 (11.4) | 5697 (9.9) | 13 676 (9.4) | 10 780 (8.5) | 4824 (8.2) | 1610 (8.7) | 1529 (9.0) |
| <1 | 23 020 (31.4) | 18 561 (32.3) | 45 569 (31.2) | 38 019 (30.0) | 16 805 (28.6) | 5080 (27.3) | 4365 (25.8) |
| 1 | 19 817 (27.1) | 16 077 (28.0) | 43 312 (29.6) | 38 235 (30.1) | 17 566 (29.9) | 5438 (29.2) | 4590 (27.1) |
| 2–4 | 18 574 (25.4) | 14 790 (25.7) | 38 237 (26.2) | 35 058 (27.6) | 17 003 (29.0) | 5566 (29.9) | 5277 (31.2) |
| 5–6 | 2540 (3.5) | 1780 (3.1) | 4054 (2.8) | 3724 (2.9) | 1907 (3.3) | 713 (3.8) | 852 (5.0) |
| ≥7 | 797 (1.1) | 470 (0.8) | 957 (0.7) | 881 (0.7) | 495 (0.8) | 182 (1.0) | 271 (1.6) |
| Median wCMSG4 (IQR)§ | 3.7 (2.6 to 4.9) | 3.6 (2.5 to 4.7) | 3.6 (2.5 to 4.7) | 3.7 (2.6 to 4.9) | 3.8 (2.6 to 5.0) | 3.9 (2.6 to 5.00) | 3.8 (2.6 to 5.0) |
A/AS = advanced/advanced subsidiary; CSE = certificate of secondary education; HNC = higher national certificate; HND = higher national diploma; IQR = interquartile range; NVQ = national vocational qualification; O/AS = ordinary/advanced subsidiary; Q1/2/3/4/5 = quintiles 1/2/3/4/5; wCMSG4 = weighted genetic caffeine metabolism score.
Percentages were computed including those with a missing value.
More than 10 minutes of moderate or vigorous activity per day.
Quintiles were not evenly distributed because many participants were in 1.5 and 2 times per week categories.
Among 483 349 persons who had genetic data.
During the 14 years (median, 11.2 years) and 5 463 896 person-years of follow-up, 29 783 deaths occurred. Higher tea intake was associated with modestly lower mortality risk in our multivariable-adjusted model (Table 2). Relative to nontea drinkers, HRs (95% CIs) for participants who reported drinking 1 or fewer, 2 to 3, 4 to 5, 6 to 7, 8 to 9, and 10 or more cups per day were 0.95 (95% CI, 0.91 to 1.00), 0.87 (CI, 0.84 to 0.91), 0.88 (CI, 0.84 to 0.91), 0.88 (CI, 0.84 to 0.92), 0.91 (CI, 0.86 to 0.97), and 0.89 (CI, 0.84 to 0.95), respectively. Similar associations were seen in analyses excluding coffee drinkers. When limiting to tea drinkers, the lower risk was seen across categories of 2 to 3 cups or higher per day compared with those of 1 cup or fewer per day. The spline analysis revealed a nonlinear association between tea intake and all-cause mortality, with risk decreasing with higher tea intake but plateauing at 2 to 3 cups per day (Figure 1). Similarly, better survival was seen with increasing tea intake relative to nontea drinking, but the cumulative mortalities were similar at intake levels of 2 to 3 cups per day and above (Figure 2).
Table 2.
Hazard Ratios (95% CIs) for All-Cause and Cause-Specific Mortality by Tea Intake Among 498 043 Persons in the UK Biobank Study
| Cause of death | Tea Intake, Cups per Day |
||||||
|---|---|---|---|---|---|---|---|
| 0 | ≤1 | 2–3 | 4–5 | 6–7 | 8–9 | ≥10 | |
|
| |||||||
| All causes | |||||||
| n | 73 216 | 57 510 | 146 181 | 126 921 | 58 686 | 18 618 | 16 911 |
| Death,n | 4887 | 3263 | 7927 | 7360 | 3668 | 1317 | 1361 |
| Age and sex-adjusted | 1.00 | 0.82 (0.78–0.86) | 0.72 (0.70–0.75) | 0.75 (0.72–0.78) | 0.81 (0.78–0.85) | 0.95 (0.89–1.01) | 1.08 (1.02–1.15) |
| Age, sex, and general health-adjusted | 1.00 | 0.87 (0.83–0.90) | 0.77 (0.74–0.80) | 0.78 (0.76–0.81) | 0.83 (0.79–0.86) | 0.93 (0.87–0.98) | 1.01 (0.95–1.07) |
| Age, sex, and smoking-adjusted | 1.00 | 0.89 (0.85–0.93) | 0.82 (0.79–0.85) | 0.84 (0.81–0.87) | 0.87 (0.83–0.91) | 0.94 (0.88–1.00) | 0.96 (0.90–1.02) |
| Multivariable-adjusted* | 1.00 | 0.95 (0.91–1.00) | 0.87 (0.84–0.91) | 0.88 (0.84–0.91) | 0.88 (0.84–0.92) | 0.91 (0.86–0.97) | 0.89 (0.84–0.95) |
| Sensitivity analysis (all cause) | |||||||
| Tea drinkers only (total n = 424 827) | |||||||
| Multivariable-adjusted | – | 1.00 (ref) | 0.92 (0.88–0.96) | 0.92 (0.88–0.96) | 0.92 (0.88–0.97) | 0.95 (0.89–1.02) | 0.93 (0.87–0.99) |
| Excluding coffee drinkers (total n = 110 336) | |||||||
| n | 11 571 | 5757 | 24 399 | 33 135 | 20 579 | 7560 | 7335 |
| Death, n | 634 | 319 | 1380 | 2035 | 1323 | 535 | 604 |
| Multivariable-adjusted | 1.00 | 0.97 (0.84–1.11) | 0.86 (0.78–0.94) | 0.83 (0.76–0.91) | 0.81 (0.74–0.89) | 0.80 (0.71–0.90) | 0.77 (0.69–0.87) |
| Excluding deaths in the first 5 y (total n = 489 465) | |||||||
| n | 71 825 | 56 542 | 143 968 | 124 776 | 57 626 | 18 203 | 16 525 |
| Death, n | 3496 | 2295 | 5714 | 5215 | 2608 | 902 | 975 |
| Multivariable-adjusted | 1.00 | 0.93 (0.89–0.99) | 0.88 (0.84–0.92) | 0.87 (0.83–0.91) | 0.89 (0.84–0.94) | 0.89 (0.83–0.96) | 0.92 (0.86–0.99) |
| All cancer | |||||||
| Death, n | 2448 | 1746 | 4254 | 3943 | 1968 | 708 | 723 |
| Age and sex-adjusted | 1.00 | 0.88 (0.83–0.94) | 0.78 (0.74–0.82) | 0.81 (0.77–0.85) | 0.88 (0.83–0.93) | 1.03 (0.95–1.12) | 1.18 (1.09–1.29) |
| Multivariable-adjusted | 1.00 | 0.99 (0.93–1.06) | 0.91 (0.86–0.96) | 0.92 (0.87–0.97) | 0.93 (0.87–0.99) | 1.00 (0.91–1.09) | 1.00 (0.92–1.09) |
| All cardiovascular diseases | |||||||
| Death, n | 1019 | 675 | 1547 | 1444 | 718 | 251 | 254 |
| Age and sex-adjusted | 1.00 | 0.79 (0.72–0.88) | 0.66 (0.61–0.71) | 0.69 (0.64–0.75) | 0.75 (0.68–0.82) | 0.85 (0.74–0.97) | 0.92 (0.80–1.06) |
| Multivariable-adjusted | 1.00 | 0.98 (0.89–1.08) | 0.85 (0.79–0.93) | 0.86 (0.79–0.94) | 0.86 (0.78–0.95) | 0.83 (0.72–0.95) | 0.76 (0.66–0.88) |
| Ischemic heart disease | |||||||
| Death, n | 283 | 192 | 403 | 410 | 199 | 65 | 75 |
| Age and sex-adjusted | 1.00 | 0.81 (0.67–0.97) | 0.62 (0.53–0.72) | 0.71 (0.61–0.83) | 0.75 (0.63–0.90) | 0.78 (0.60–1.03) | 0.94 (0.73–1.21) |
| Multivariable-adjusted | 1.00 | 1.03 (0.85–1.24) | 0.83 (0.70–0.97) | 0.90 (0.77–1.06) | 0.87 (0.72–1.06) | 0.74 (0.56–0.98) | 0.74 (0.56–0.96) |
| Stroke | |||||||
| Death, n | 93 | 59 | 141 | 131 | 75 | 27 | 15 |
| Age and sex-adjusted | 1.00 | 0.79 (0.57–1.09) | 0.68 (0.52–0.88) | 0.70 (0.54–0.91) | 0.86 (0.64–1.17) | 1.02 (0.66–1.57) | 0.63 (0.37–1.10) |
| Multivariable-adjusted | 1.00 | 0.91 (0.65–1.26) | 0.81 (0.61–1.06) | 0.80 (0.60–1.06) | 0.90 (0.65–1.25) | 0.92 (0.59–1.44) | 0.48 (0.28–0.85) |
| Respiratory disease | |||||||
| Death, n | 290 | 131 | 332 | 350 | 219 | 93 | 98 |
| Age and sex-adjusted | 1.00 | 0.55 (0.45–0.68) | 0.50 (0.42–0.58) | 0.58 (0.50–0.68) | 0.79 (0.66–0.94) | 1.10 (0.87–1.39) | 1.28 (1.02–1.61) |
| Multivariable-adjusted | 1.00 | 0.78 (0.63–0.96) | 0.78 (0.66–0.92) | 0.83 (0.70–0.98) | 0.95 (0.78–1.14) | 0.97 (0.76–1.24) | 0.79 (0.62–1.01) |
= not applicable; ref = reference.
Adjusted for age; sex; race and ethnicity (White, Black, Asian, mixed, or other race), Townsend deprivation score, general health status (excellent, good, fair, or poor), cancer (yes/no), cardiovascular disease (yes/no), diabetes (yes/no), body mass index (kg/m2), tobacco smoking (25-level variable including current smoking status, smoking intensity [current and former smokers], time since quitting [former smokers], and cigar and pipe use [current and former smokers]); physical activity (>10 minutes of moderate or vigorous activity; days per week); alcohol intake (never drinker, former drinker, infrequent drinker [<1 drink per week], occasional drinker [>1 drink per week but <1 drink per day], moderate daily drinker [1 to 3 drinks per day]), or heavy drinker [>3 drinks per day]; coffee intake (cups per day); and dietary intake including vegetables (tablespoons per day), fruits (pieces per day), red meat (beef, lamb, and pork; 0 to 1, 1.5, 2, 2.5, 3 to 21 times per week as quintiles), and processed meat (0, <1, 1, 2 to 4, 5 to 6, and ≥7 times per week). The baseline risk was stratified by assessment centers.
Figure 1. Dose–response association of tea consumption and all-cause mortalitya in the UK Biobank.
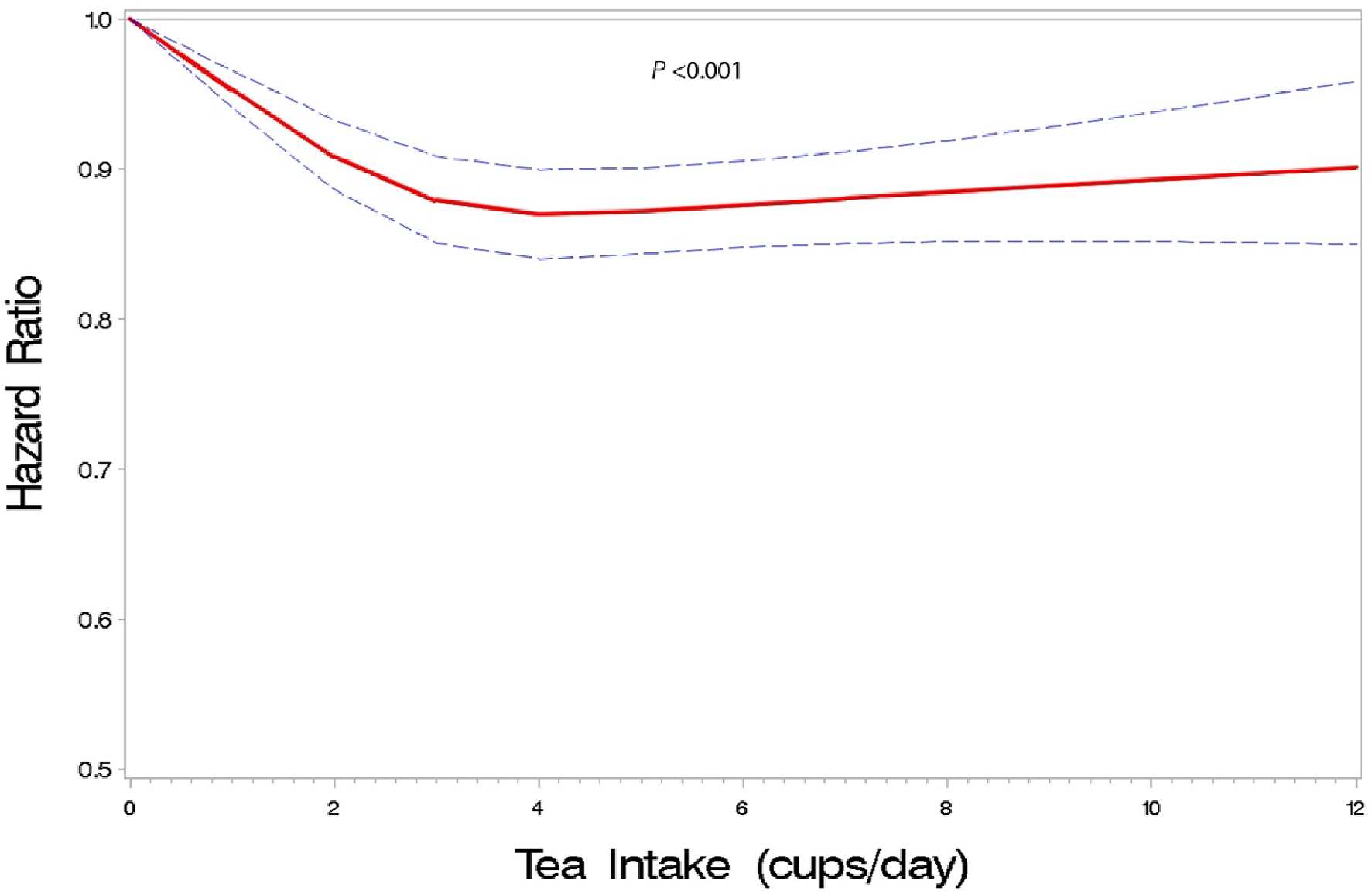
a Hazard ratio was adjusted for age; sex; race and ethnicity (White, Black, Asian, mixed, or other race), assessment center, Townsend deprivation score, general health status (excellent, good, fair, or poor), cancer (yes or no), cardiovascular disease (yes or no), diabetes (yes or no), BMI (kg/m2), tobacco smoking (25-level variable including current smoking status, smoking intensity [current and former smokers], time since quitting [former smokers], and cigar and pipe use [current and former smokers]); physical activity (>10 minutes of moderate or vigorous activity; days per week); alcohol intake (never drinker, former drinker, infrequent drinker [<1 drink per week], occasional drinker [>1 drink per week but <1 drink per day], moderate daily drinker [1 to 3 drinks per day]), or heavy drinker [>3 drinks per day]; coffee intake (cups per day); and dietary intake including vegetables (tablespoons per day), fruits (pieces per day), red meat (beef, lamb, and pork; 0 to 1, 1.5, 2, 2.5, 3 to 21 times per week), and processed meat (0, <1, 1, 2 to 4, 5 to 6, and ≥7 times per week).
Figure 2.
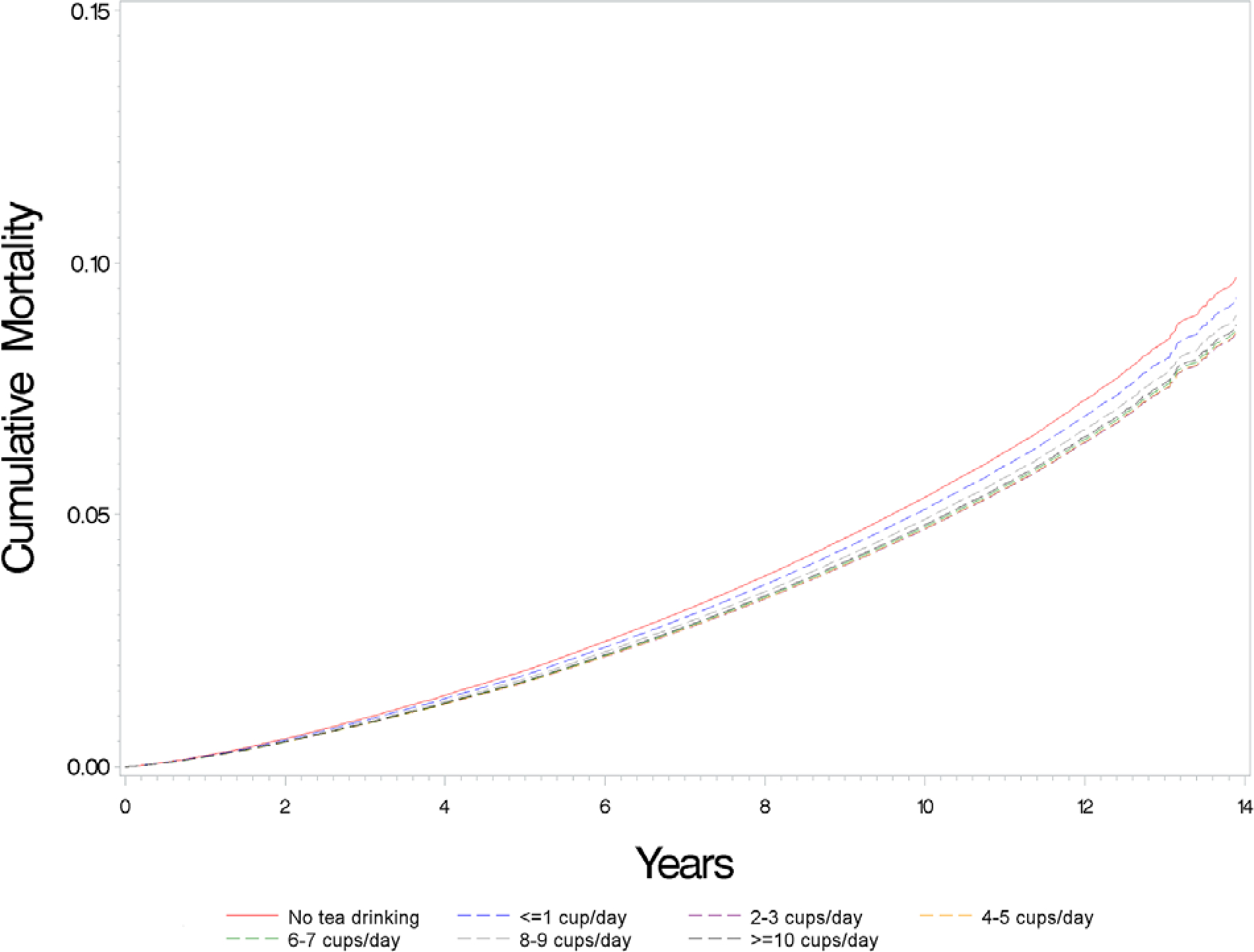
Adjusted cumulative mortality curvesa by tea intake in the UK Biobank.
a Adjusted for age; sex; race and ethnicity (White, Black, Asian, mixed, or other race), assessment center, Townsend deprivation score, general health status (excellent, good, fair, or poor), cancer (yes/no), cardiovascular disease (yes/no), diabetes (yes/no), BMI (kg/m2), tobacco smoking (25-level variable including current smoking status, smoking intensity [current and former smokers], time since quitting [former smokers], and cigar and pipe use [current and former smokers]); physical activity (>10 minutes of moderate or vigorous activity; days per week); alcohol intake (never drinker, former drinker, infrequent drinker [<1 drink per week], occasional drinker [>1 drink per week but <1 drink per day], moderate daily drinker [1 to 3 drinks per day]), or heavy drinker [>3 drinks per day]; coffee intake (cups per day); and dietary intake including vegetables (tablespoons per day), fruits (pieces per day), red meat (beef, lamb, and pork; 0 to 1, 1.5, 2, 2.5, 3 to 21 times per week), and processed meat (0, <1, 1, 2 to 4, 5 to 6, and ≥7 times per week).
Given potential concerns about confounding, we present HRs from all-cause mortality models adjusted for age and sex alone, additionally for smoking or general health, and a more comprehensive multivariable model. Associations for categories of tea drinking up to 6 to 7 cups per day were attenuated after multivariable adjustment. For 8 to 9 cups per day, the inverse associations in age- and sex-adjusted models (HR, 0.95 [CI, 0.89 to 1.01]) were strengthened after comprehensive multivariable adjustment (HR, 0.91 [CI, 0.86 to 0.97]); smoking and general health were shown to be the most meaningful confounders, shifting HRs by 7% and 2%, respectively. The HR (95% CI) for drinking 10 or more cups per day flipped from 1.08 (CI, 1.02 to 1.15) in age- and sex-adjusted models to 0.89 (CI, 0.84 to 0.95) after multivariable adjustment, resulting in a stronger dose–response association across tea intake categories. Inverse associations were also seen for deaths from all CVD, ischemic heart disease, and stroke. No clear trend was seen for cancer or respiratory disease mortality, with associations among higher intake categories tending toward the null. Multivariable adjustment similarly impacted overall and cause-specific mortality risk estimates.
Among 210 058 participants who completed at least one 24-hour dietary recall questionnaire, 74% and 13% of tea drinkers reported adding milk and sugar, respectively. We saw a dose–response inverse association between tea consumption and all-cause mortality among those who reported adding milk to their tea but not among those who did not (Table 3). No associations were seen above 4 to 5 cups per day among those who did not add milk, but their sample size was limited and CIs were wide. Higher tea consumption was associated with lower risk for mortality regardless of whether sugar was added.
Table 3.
All-Cause Mortality Risk by Tea Intake Stratified by Added Milk or Sugar in Tea in 210 058 Persons Who Completed at Least One 24-h Dietary Recall Questionnaires in the UK Biobank Study
| Characteristics Tea additives | Tea Intake, Cups per Day |
||||||
|---|---|---|---|---|---|---|---|
| 0 | ≤1 | 2–3 | 4–5 | 6–7 | 8–9 | ≥10 | |
|
| |||||||
| No tea intake * | |||||||
| n | 30 578 | – | – | – | – | – | – |
| Death | 1348 | – | – | – | – | – | – |
| HR | 1.00 (ref) | – | – | – | – | – | – |
| Milk | |||||||
| n | – | 10 949 | 47 709 | 42 963 | 19 633 | 6062 | 4932 |
| Death | – | 443 | 1716 | 1691 | 785 | 255 | 214 |
| HR(95% CI)† | – | 0.98 (0.88–1.09) | 0.85 (0.79–0.92) | 0.90 (0.83–0.97) | 0.86 (0.78–0.94) | 0.84 (0.73–0.97) | 0.78 (0.67–0.91) |
| No milk | |||||||
| n | – | 15 449 | 15 856 | 9552 | 3992 | 1206 | 1177 |
| Death | – | 604 | 582 | 340 | 170 | 58 | 58 |
| HR (95% CI) | – | 0.95 (0.86–1.04) | 0.86 (0.78–0.95) | 0.82 (0.73–0.93) | 0.94 (0.80–1.10) | 1.11 (0.85–1.45) | 1.00 (0.77–1.31) |
| Sugar | |||||||
| n | – | 2434 | 8471 | 6843 | 2950 | 908 | 915 |
| Death | – | 105 | 344 | 316 | 159 | 64 | 50 |
| HR (95% CI) | – | 0.96 (0.78–1.17) | 0.89 (0.79–1.00) | 0.91 (0.80–1.03) | 0.95 (0.80–1.13) | 1.06 (0.82–1.37) | 0.73 (0.54–0.97) |
| No sugar | |||||||
| n | – | 23 964 | 55 094 | 45 672 | 20 675 | 6360 | 5194 |
| Death | – | 942 | 1954 | 1715 | 796 | 249 | 222 |
| HR (95% CI) | – | 0.96 (0.88–1.04) | 0.85 (0.79–0.91) | 0.88 (0.82–0.95) | 0.86 (0.79–0.94) | 0.85 (0.74–0.97) | 0.85 (0.74–0.98) |
= not applicable; HR = hazard ratio; ref = reference.
Participants who reported not drinking tea served as a universal referent group.
Adjusted for age; sex; race and ethnicity (White, Black, Asian, mixed, or other race); Townsend deprivation score; general health status (excellent, good, fair, or poor); cancer (yes/no); cardiovascular disease (yes/no); diabetes (yes/no); body mass index (kg/m2); tobacco smoking (25-level variable including current smoking status, smoking intensity [current and former smokers], time since quitting [former smokers], and cigar and pipe use [current and former smokers]); physical activity (>10 minutes of moderate or vigorous activity; days per week); alcohol intake (never drinker, former drinker, infrequent drinker [<1 drink per week], occasional drinker [>1 drink per week but <1 drink per day], moderate daily drinker [1 to 3 drinks per day]), or heavy drinker [>3 drinks per day]; coffee intake (cups per day); and dietary intake including vegetables (table spoons per day), fruits (pieces per day), red meat (beef, lamb, and pork; 0 to 1, 1.5, 2, 2.5, 3 to 21 times per week as quintiles), and processed meat (0, <1, 1, 2 to 4, 5 to 6, and ≥7 times per week). The baseline risk was stratified by assessment centers.
In analyses stratified by a genetic score for caffeine metabolism, similar associations were found across strata of wCMSG4 (Table 4). For those in the lowest wCMSG4 category (0 to 2; inferring slow caffeine metabolism), HRs (95% CIs) were 0.88 (CI, 0.76 to 1.02), 0.80 (CI, 0.71 to 0.91), 0.77 (CI, 0.67 to 0.87), 0.77 (CI, 0.66 to 0.91), 0.86 (CI, 0.68 to 1.08), and 0.73 (CI, 0.58 to 0.94) for 1 or fewer, 2 to 3, 4 to 5, 6 to 7, 8 to 9, and 10 or more cups per day, respectively, whereas counterparts for those in the highest wCMSG4 category (>4; inferring rapid caffeine metabolism) were 0.99 (CI, 0.91 to 1.08), 0.85 (CI, 0.79 to 0.91), 0.81 (CI, 0.75 to 0.87), 0.83 (CI, 0.77 to 0.91), 0.89 (CI, 0.80 to 1.00), and 0.81 (CI, 0.73 to 0.91) for 1 or fewer, 2 to 3, 4 to 5, 6 to 7, 8 to 9, and 10 or more cups per day, respectively. Generally similar findings were seen in sensitivity analyses excluding coffee drinkers or limiting to White participants (Supplement Tables 2 and 3, available at Annals.org). Little difference was seen by temperature. Higher tea intake was associated with lower all-cause mortality, and a similar but somewhat stronger association was seen when excluding coffee and other nontea hot beverage drinkers (Supplement Table 4, available at Annals.org).
Table 4.
All-Cause Mortality Risk by Tea Intake Stratified by wCMSG4 in 403 780 Persons Who Had Genetic Data in the UK Biobank Study
| Risk wCMSG4 | Tea Intake, Cups per Day |
||||||
|---|---|---|---|---|---|---|---|
| 0 | ≤1 | 2–3 | 4–5 | 6–7 | 8–9 | ≥10 | |
|
| |||||||
| 0–2 | |||||||
| n | 6708 | 6273 | 15 597 | 11 397 | 4388 | 1232 | 1170 |
| Death | 430 | 322 | 797 | 605 | 257 | 94 | 84 |
| HR (95% CI)* | 1.00 | 0.88 (0.76–1.02) | 0.80 (0.71–0.91) | 0.77 (0.67–0.87) | 0.77 (0.66–0.91) | 0.86 (0.68–1.08) | 0.73 (0.58–0.94) |
| >2–3 | |||||||
| n | 14 755 | 12 363 | 31 225 | 25 205 | 10 858 | 3265 | 2948 |
| Death | 916 | 641 | 1627 | 1483 | 684 | 223 | 241 |
| HR (95% CI) | 1.00 | 0.93 (0.84–1.02) | 0.90 (0.82–0.98) | 0.95 (0.87–1.04) | 0.97 (0.88–1.08) | 0.89 (0.77–1.04) | 0.97 (0.83–1.12) |
| >3–4 | |||||||
| n | 17 927 | 14 100 | 35 936 | 31 018 | 14 256 | 4406 | 4064 |
| Death | 1183 | 792 | 1911 | 1766 | 890 | 280 | 332 |
| HR (95% CI) | 1.00 | 0.96 (0.87–1.05) | 0.86 (0.79–0.92) | 0.88 (0.81–0.95) | 0.89 (0.82–0.98) | 0.87 (0.76–0.99) | 0.92 (0.81–1.05) |
| >4 | |||||||
| n | 19 964 | 14 500 | 36 868 | 34 709 | 17 437 | 5880 | 5331 |
| Death | 1399 | 913 | 2022 | 1942 | 1070 | 414 | 407 |
| HR (95% CI) | 1.00 | 0.99 (0.91–1.08) | 0.85 (0.79–0.91) | 0.81 (0.75–0.87) | 0.83 (0.77–0.91) | 0.89 (0.80–1.00) | 0.81 (0.73–0.91) |
HR, hazard ratio; wCMSG4, weighted genetic caffeine metabolism score.
Adjusted for age; sex; race and ethnicity (White, Black, Asian, mixed, or other race); Townsend deprivation score; general health status (excellent, good, fair, or poor); cancer (yes/no); cardiovascular disease (yes/no); diabetes (yes/no); body mass index (kg/m2); tobacco smoking (25-level variable including current smoking status, smoking intensity [current and former smokers], time since quitting [former smokers], and cigar and pipe use [current and former smokers]); physical activity (>10 minutes of moderate or vigorous activity; days per week); alcohol intake (never drinker, former drinker, infrequent drinker [<1 drink per week], occasional drinker [>1 drink per week but <1 drink per day], moderate daily drinker [1 to 3 drinks per day]), or heavy drinker [>3 drinks per day]; coffee intake (cups per day); and dietary intake including vegetables (table spoons per day), fruits (pieces per day), red meat (beef, lamb, and pork; 0 to 1, 1.5, 2, 2.5, 3 to 21 times per week as quintiles), and processed meat (0, <1, 1, 2 to 4, 5 to 6, and ≥7 times per week). The baseline risk was stratified by assessment centers.
Discussion
In this prospective analysis of almost 500 000 participants of the UK Biobank, where more than 4 of 5 adults habitually drank tea, higher tea intake was modestly associated with lower mortality risk during a median of 11.2 years of follow-up, including among participants who reported adding milk or sugar to tea and among those with a slower genetically inferred caffeine metabolism rate. Lower risks with higher tea intake were also seen for mortality from overall CVD, ischemic heart disease, and stroke.
Tea is a major source of caffeine in the United Kingdom, and concerns remain about high caffeine intake, particularly among people with slower caffeine metabolism (13–15, 19). Yet, similar associations for tea drinking were seen among participants who had both lower and higher genetic capacities for caffeine metabolism. This finding suggests that caffeine does not underlie the observed relationship between tea and mortality, which is a difficult research question to address in traditional epidemiologic studies because consumption of decaffeinated tea is uncommon. Very hot tea intake has been associated with increased risk for esophageal cancer in previous studies (1, 28). However, we saw similar inverse associations for all-cause mortality among participants who reported drinking their tea warm, hot, or very hot. We note that esophageal cancer deaths were a very small proportion of total deaths in the cohort.
The UK Biobank cohort includes more participants who drink black tea regularly and with a wider range of intake than previous studies (29–31). With 19% of the analytic cohort drinking 6 or more cups per day and about 90% of habitual tea drinkers consuming black tea, the current study is well suited to furthering knowledge of black tea and mortality risk. In contrast, many prior studies of frequent tea drinking were set in Asian populations, such as China and Japan, where green tea drinking is more common and relatively few people drink black tea. These studies have generally seen stronger and more consistent associations for green tea than for black tea (3–5, 9). A recent study including 12 prospective cohorts in Asia found evidence for an inverse association between drinking green tea and overall mortality and mortality from cardiovascular disease but no association for black tea (9). Fewer studies have assessed tea intake and mortality in populations where black tea is predominantly consumed, such as in the United States and Europe, and results have varied across studies (6–12). A recent meta-analysis reported that black tea intake was not associated with all-cause mortality or CVD mortality (12). However, there was significant heterogeneity across studies. Five studies performed in the Netherlands or United Kingdom did not assess tea type. Of those, 3 studies reported no association between tea intake and all-cause, CVD, and cancer mortality. One study reported a positive association with all-cause, cancer, and ischemic heart disease mortality (30), and the last study reported a U-shaped association with coronary heart disease mortality (6). Five US studies included in the meta-analysis did not assess tea type, and only 1 study reported inverse associations with all-cause mortality (HR, 0.87 [CI, 0.80 to 0.95] for 1 cup per day increment) and cancer mortality (HR, 0.69 [CI, 0.54 to 0.88]) but not CVD mortality (32). One Australian study assessed black tea intake specifically and found an inverse association between black tea and all-cause mortality (HR, 0.91[CI, 0.90 to 0.99] for 1 cup per day increment) (33). Our study provides robust results indicating that the inverse association between tea and mortality extends to populations where black tea intake predominates.
Our study has several strengths. In addition to a high prevalence and wide range of tea intake, the UK Biobank has a large sample size, extended follow-up, information on tea additives and temperature, genetic data, and comprehensive assessment of lifestyle and disease risk factors. Limitations include that tea intake was assessed in the whole cohort at baseline only, and changes in tea intake during follow-up could introduce exposure misclassification. However, among the subset of cohort participants who recorded their tea intake on 2 touchscreen questionnaires administered 4 years apart, the concordance of tea intake was high (weighted κ = 0.83) (20). There was also a good correlation in tea intake from the baseline questionnaire and a mean tea intake of 24-hour dietary recall questionnaires in a subset of participants who completed 2 or more 24-hour dietary recall questionnaires (correlation coefficient r = 0.81). Teacup size was not indicated on either dietary questionnaire used in the UK Biobank, but other European studies suggest that average volume is likely in the range of 125 to 150 mL (7, 30). Other aspects of tea drinking (for example, steeping time) were also not assessed. In addition, the UK Biobank had a low participation rate (5.5%) and tended to enroll healthier participants than the general population (17). Nonetheless, valid assessments of association do not require population representativeness. The cohort was also predominantly White. Given the sparsity of prior studies of frequent black tea intake, studies in other populations with distinct lifestyle and genetic ancestry are needed.
Residual confounding is a concern in observational studies. Smoking and general health status were the strongest confounders in our analysis and had complicated relationships with tea intake. Participants who drank modest amounts of tea (≤2 to 3 cups per day) were more likely to be never smokers and report better health whereas those who drank very high amounts of tea (≥8 cups per day) were more likely to be smokers and report worse health. Accordingly, adjustment for these factors attenuated associations at lower tea intake levels but strengthened associations at higher intake levels, suggesting that the observed dose–response associations could be stronger without residual confounding. We have performed a formal quantitative sensitivity analysis and computed the E-value, which is the minimum strength of association with both the exposure and outcome needed for an unmeasured confounder to explain an observed risk (34, 35). The observed HR of 0.89 for 10 or more cups per day of tea and all-cause mortality could be explained away by an unmeasured confounder with an HR of 1.50, above and beyond the measured confounders, for both tea consumption and mortality. However, the CI could be moved to include the null by an unmeasured confounder that was associated with an HR of 1.29, above and beyond the measured confounders, for both tea consumption and mortality.
Although our results should be interpreted cautiously, many biologically plausible mechanisms link tea intake to disease. Black, green, and red tea are rich in polyphenols and flavonoids, namely catechins and their oxidated products. These and other bioactive compounds in tea have the potential to reduce oxidative stress and inflammation, which may promote carcinogenesis, and improve endothelial function (1, 36–38). A recent study in the UK Biobank also found that higher tea consumption (black and green tea) was associated with more favorable levels of cardiometabolic biomarkers, including lower total and low-density lipoprotein cholesterol, apolipoprotein B, and fasting triglycerides but higher high-density lipoprotein cholesterol (19). A Mendelian randomization study showed that a 1-cup increase in genetically predicted tea consumption was associated with 21% lower risk for small vessel stroke (39). Most short-term randomized controlled trials of tea or tea extracts have been small, and although results are inconsistent, some have found evidence supporting favorable effects of black and green tea on CVD risk factors (19, 40–42). Finally, some studies have suggested that habitual tea consumption is associated with lower risk for type 2 diabetes (43, 44).
In this study of nearly 500 000 participants in the UK Biobank where black tea drinking was common, higher tea intake was associated with modestly lower risk for all-cause mortality and mortality from all CVD, ischemic heart disease, and stroke, with lower risks seen for drinking 2 or more cups per day. Similar inverse associations were seen among participants who added milk or sugar to their tea and among those with slower genetically predicted caffeine metabolism. These findings provide reassurance to tea drinkers and suggest that black tea can be part of a healthy diet.
Supplementary Material
Acknowledgment:
This study was conducted using the UK Biobank resource (applications 18623 and 21394). The UK Biobank was established by the Wellcome Trust, the Medical Research Council, the UK Department of Health, and the Scottish Government. The UK Biobank has also received funding from the Welsh Assembly Government, the British Heart Foundation, and Diabetes United Kingdom. This work used the computational resources of the NIH High-Performance Computing Biowulf cluster.
Grant Support:
By the NIH National Cancer Institute Intramural Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, the Department of Health and Human Services.
Footnotes
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-0041.
Reproducible Research Statement: Study protocol and Statistical code: Not available. Data set: The data underlying this article were provided by UK Biobank by proposal approval and are available from the UK Biobank on request (www.ukbiobank.ac.uk).
Contributor Information
Maki Inoue-Choi, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Yesenia Ramirez, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Marilyn C. Cornelis, Department of Preventive Medicine, Northwestern University Feinberg School of Medicine, Chicago, Illinois.
Amy Berrington de González, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Neal D. Freedman, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Erikka Loftfield, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
References
- 1.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Coffee, Tea, Mate, Methylxanthines and Methylglyoxal. vol. 51. World Health Organization; 1991. [PMC free article] [PubMed] [Google Scholar]
- 2.Raman G, Avendano EE, Chen S, et al. Dietary intakes of flavan-3-ols and cardiometabolic health: systematic review and meta-analysis of randomized trials and prospective cohort studies. Am J Clin Nutr. 2019;110:1067–1078. doi: 10.1093/ajcn/nqz178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao LG, Li HL, Sun JW, et al. Green tea consumption and cause-specific mortality: results from two prospective cohort studies in China. J Epidemiol. 2017;27:36–41. doi: 10.1016/j.je.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Liu S, Zhou H, et al. Association of green tea consumption with mortality from all-cause, cardiovascular disease and cancer in a Chinese cohort of 165,000 adult men. Eur J Epidemiol. 2016;31:853–65. doi: 10.1007/s10654-016-0173-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abe SK, Saito E, Sawada N, et al. ; Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan. Green tea consumption and mortality in Japanese men and women: a pooled analysis of eight population-based cohort studies in Japan. Eur J Epidemiol. 2019;34:917–926. doi: 10.1007/s10654-019-00545-y [DOI] [PubMed] [Google Scholar]
- 6.de Koning Gans JM, Uiterwaal CS, van der Schouw YT, et al. Tea and coffee consumption and cardiovascular morbidity and mortality. Arterioscler Thromb Vasc Biol. 2010;30:1665–71. doi: 10.1161/ATVBAHA.109.201939 [DOI] [PubMed] [Google Scholar]
- 7.van den Brandt PA. Coffee or tea? A prospective cohort study on the associations of coffee and tea intake with overall and cause-specific mortality in men versus women. Eur J Epidemiol. 2018;33:183–200. doi: 10.1007/s10654-018-0359-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C, Qin YY, Wei X, et al. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. Eur J Epidemiol. 2015;30:103–13. doi: 10.1007/s10654-014-9960-x [DOI] [PubMed] [Google Scholar]
- 9.Shin S, Lee JE, Loftfield E, et al. Coffee and tea consumption and mortality from all causes, cardiovascular disease and cancer: a pooled analysis of prospective studies from the Asia Cohort Consortium. Int J Epidemiol. 2022;51:626–640. doi: 10.1093/ije/dyab161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang ZM, Zhou B, Wang YS, et al. Black and green tea consumption and the risk of coronary artery disease: a meta-analysis. Am J Clin Nutr. 2011;93:506–15. doi: 10.3945/ajcn.110.005363 [DOI] [PubMed] [Google Scholar]
- 11.Tang J, Zheng JS, Fang L, et al. Tea consumption and mortality of all cancers, CVD and all causes: a meta-analysis of eighteen prospective cohort studies. Br J Nutr. 2015;114:673–83. doi: 10.1017/S0007114515002329 [DOI] [PubMed] [Google Scholar]
- 12.Chung M, Zhao N, Wang D, et al. Dose-response relation between tea consumption and risk of cardiovascular disease and all-cause mortality: a systematic review and meta-analysis of population-based studies. Adv Nutr. 2020;11:790–814. doi: 10.1093/advances/nmaa010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelis MC, El-Sohemy A, Kabagambe EK, et al. Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA. 2006;295:1135–41. doi: 10.1001/jama.295.10.1135 [DOI] [PubMed] [Google Scholar]
- 14.Palatini P, Ceolotto G, Ragazzo F, et al. CYP1A2 genotype modifies the association between coffee intake and the risk of hypertension. J Hypertens. 2009;27:1594–601. doi: 10.1097/HJH.0b013e32832ba850 [DOI] [PubMed] [Google Scholar]
- 15.Zhou A, Hyppönen E. Long-term coffee consumption, caffeine metabolism genetics, and risk of cardiovascular disease: a prospective analysis of up to 347,077 individuals and 8368 cases. Am J Clin Nutr. 2019;109:509–516. doi: 10.1093/ajcn/nqy297 [DOI] [PubMed] [Google Scholar]
- 16.Reyes CM, Cornelis MC. Caffeine in the diet: country-level consumption and guidelines. Nutrients. 2018;10. doi: 10.3390/nu10111772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health-related characteristics of UK biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.UK Biobank. Online showcase of resources. Accessed at https://biobank.ndph.ox.ac.uk/showcase/index.cgi on 31 May 2021.
- 19.Cornelis MC, van Dam RM. Habitual coffee and tea consumption and cardiometabolic biomarkers in the UK biobank: the role of beverage types and genetic variation. J Nutr. 2020;150:2772–2788. doi: 10.1093/jn/nxaa212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradbury KE, Young HJ, Guo W, et al. Dietary assessment in UK Biobank: an evaluation of the performance of the touchscreen dietary questionnaire. J Nutr Sci. 2018;7:e6. doi: 10.1017/jns.2017.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster HME, Celis-Morales CA, Nicholl BI, et al. The effect of socioeconomic deprivation on the association between an extended measurement of unhealthy lifestyle factors and health outcomes: a prospective analysis of the UK Biobank cohort. Lancet Public Health. 2018;3:e576–e585. doi: 10.1016/S2468-2667(18)30200-7 [DOI] [PubMed] [Google Scholar]
- 22.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornelis MC, Kacprowski T, Menni C, et al. ; Swiss Kidney Project on Genes in Hypertension (SKIPOGH) team. Genome-wide association study of caffeine metabolites provides new insights to caffeine metabolism and dietary caffeine-consumption behavior. Hum Mol Genet. 2016;25:5472–5482. doi: 10.1093/hmg/ddw334 [DOI] [PubMed] [Google Scholar]
- 24.Cornelis MC, Byrne EM, Esko T, et al. ; Coffee and Caffeine Genetics Consortium. Genome-wide meta-analysis identifies six novel loci associated with habitual coffee consumption. Mol Psychiatry. 2015;20:647–656. doi: 10.1038/mp.2014.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loftfield E, Cornelis MC, Caporaso N, et al. Association of coffee drinking with mortality by genetic variation in caffeine metabolism: findings from the UK Biobank. JAMA Intern Med. 2018;178:1086–1097. doi: 10.1001/jamainternmed.2018.2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gail MH, Bryar DP. Variance and calculations for direct adjusted survival curves, wtih applications to testing for no treatment effect. Biometrical J. 1986;28:587–99. doi: 10.1002/bimj.4710280508 [DOI] [Google Scholar]
- 27.Zhang X, Zhang MJ. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101:87–93. doi: 10.1016/j.cmpb.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Islami F, Poustchi H, Pourshams A, et al. A prospective study of tea drinking temperature and risk of esophageal squamous cell carcinoma. Int J Cancer. 2020;146:18–25. doi: 10.1002/ijc.32220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan Y, Sui X, Yao B, et al. Is there a dose-response relationship between tea consumption and all-cause, CVD, and cancer mortality? J Am Coll Nutr. 2017;36:281–286. doi: 10.1080/07315724.2016.1261054 [DOI] [PubMed] [Google Scholar]
- 30.Hertog MG, Sweetnam PM, Fehily AM, et al. Antioxidant flavonols and ischemic heart disease in a Welsh population of men: the Caerphilly Study. Am J Clin Nutr. 1997;65:1489–94. doi: 10.1093/ajcn/65.5.1489 [DOI] [PubMed] [Google Scholar]
- 31.Klatsky AL, Armstrong MA, Friedman GD. Coffee, tea, and mortality. Ann Epidemiol. 1993;3:375–81. doi: 10.1016/1047-2797(93)90064-b [DOI] [PubMed] [Google Scholar]
- 32.Gardener H, Rundek T, Wright CB, et al. Coffee and tea consumption are inversely associated with mortality in a multiethnic urban population. J Nutr. 2013;143:1299–308. doi: 10.3945/jn.112.173807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim WH, Wong G, Lewis JR, et al. Total volume and composition of fluid intake and mortality in older women: a cohort study. BMJ Open. 2017;7:e011720. doi: 10.1136/bmjopen-2016-011720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith LH, VanderWeele TJ. Bounding bias due to selection. Epidemiology. 2019;30:509–516. doi: 10.1097/EDE.0000000000001032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 36.Donà M, Dell’Aica I, Calabrese F, et al. Neutrophil restraint by green tea: inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J Immunol. 2003;170:4335–41. doi: 10.4049/jimmunol.170.8.4335 [DOI] [PubMed] [Google Scholar]
- 37.Xing L, Zhang H, Qi R, et al. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J Agric Food Chem. 2019;67:1029–1043. doi: 10.1021/acs.jafc.8b06146 [DOI] [PubMed] [Google Scholar]
- 38.Agoston AT, Argani P, Yegnasubramanian S, et al. Increased protein stability causes DNA methyltransferase 1 dysregulation in breast cancer. J Biol Chem. 2005;280:18302–10. [DOI] [PubMed] [Google Scholar]
- 39.Wang M, Bai Y, Wang Z, et al. Higher tea consumption is associated with decreased risk of small vessel stroke. Clin Nutr. 2021;40:1430–1435. doi: 10.1016/j.clnu.2020.08.039 [DOI] [PubMed] [Google Scholar]
- 40.Hartley L, Flowers N, Holmes J, et al. Green and black tea for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD009934. doi: 10.1002/14651858.CD009934.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asbaghi O, Fouladvand F, Gonzalez MJ, et al. The effect of green tea on C-reactive protein and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Complement Ther Med. 2019;46:210–216. doi: 10.1016/j.ctim.2019.08.019 [DOI] [PubMed] [Google Scholar]
- 42.Igho-Osagie E, Cara K, Wang D, et al. Short-term tea consumption is not associated with a reduction in blood lipids or pressure: a systematic review and meta-analysis of randomized controlled trials. J Nutr. 2020;150:3269–3279. doi: 10.1093/jn/nxaa295 [DOI] [PubMed] [Google Scholar]
- 43.Yang WS, Wang WY, Fan WY, et al. Tea consumption and risk of type 2 diabetes: a dose-response meta-analysis of cohort studies. Br J Nutr. 2014;111:1329–39. doi: 10.1017/S0007114513003887 [DOI] [PubMed] [Google Scholar]
- 44.Nie J, Yu C, Guo Y, et al. Tea consumption and long-term risk of type 2 diabetes and diabetic complications: a cohort study of 0.5 million Chinese adults. Am J Clin Nutr. 2021;114:194–202. doi: 10.1093/ajcn/nqab006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


