Abstract
Objectives
Non-high-density lipoprotein cholesterol (non-HDL-C) has attracted attention because it is associated with a variety of diseases and is easy to measure. However, the relationship between non-HDL-C and depression is still unclear. Our aim was to assess the relationship between non-HDL-C and depression using the cross-sectional NHANES survey from 2005 to 2018.
Methods
We examined the association between non-HDL-C and depression using weighted multivariable logistic regression models and subgroup analysis. Sensitivity analysis demonstrated the robustness of the results.
Results
There were 42,143 participants in this study and 8.6% had depression (weighted 7.53%). Non-HDL-C was higher in participants with depression compared to those without depression (weighted mean 3.64 vs. 3.73, p < 0.01). There was a positive association between non-HDL-C and depression with a 95% OR of 1.22 adjusted for multifactorial (95% CI,1.03–1.45). In subgroup analyses, non-HDL-C was positively associated with depression in men (OR, 1.31; 95% CI, 1.01–1.70), normal BMI (OR: 0.93; 95% CI: 0.66–1.32) and in participants without hypertension (OR, 1.29; 95% CI, 1.01–1.66).
Conclusion
Non-HDL-C positively correlated with depression, and further research may be better for clinical service.
Keywords: non-HDL-C, depression, NHANES, cholesterol, survey
Introduction
More than 280 million individuals worldwide suffer from depression, positioning it as one of the most prevalent mental and psychological disorders (1). Depression not only negatively impacts patients’ quality of life but also increases the risk of physical illness (2), mortality (3), and imposes a significant economic burden (4–6). In fact, the World Health Organization predicts that depression will become the primary global disease burden by 2030 (7). Moreover, depression amplifies the risk of numerous diseases, including coronary heart disease (8), inflammatory bowel disease (9), diabetes (10), nonalcoholic fatty liver disease (11), osteoarthritis (12), and breast cancer (13). Adverse childhood experiences (14), poor social support (15), and irregular sleep schedules (16) are among the multitude of risk factors associated with depression. Furthermore, several studies have indicated a potential link between cholesterol and depression (17, 18).
Cholesterol plays a crucial role in maintaining cell membrane function and hormone production, and abnormal cholesterol levels predispose individuals to psychiatric disorders, including anxiety and depression (19). The lipid-lowering drug simvastatin may attenuate high-fat diet-induced depressive behavior in mice by reducing hippocampal neuritis (20). Additionally, cytokines that are activated contribute significantly to the pathophysiological process of depression by affecting cholesterol synthesis (21, 22). Non-high-density lipoprotein cholesterol (non-HDL-C), calculated as the difference between total cholesterol and HDL-C, can be measured during both fasting and non-fasting states (23). The association between non-HDL-C and diabetes and coronary heart disease has intrigued researchers (24, 25). Multiple investigations have demonstrated the relationship between non-HDL-C and atherosclerosis (26), stroke (27), retinal artery occlusion (28), and Alzheimer’s disease (29). Furthermore, non-HDL-C has been implicated in psychiatric disorders (30). However, the specific association between non-HDL-C and depression remains elusive. Therefore, this study aims to investigate the relationship between non-HDL-C and depression by analyzing data from a representative population in the United States.
Methods
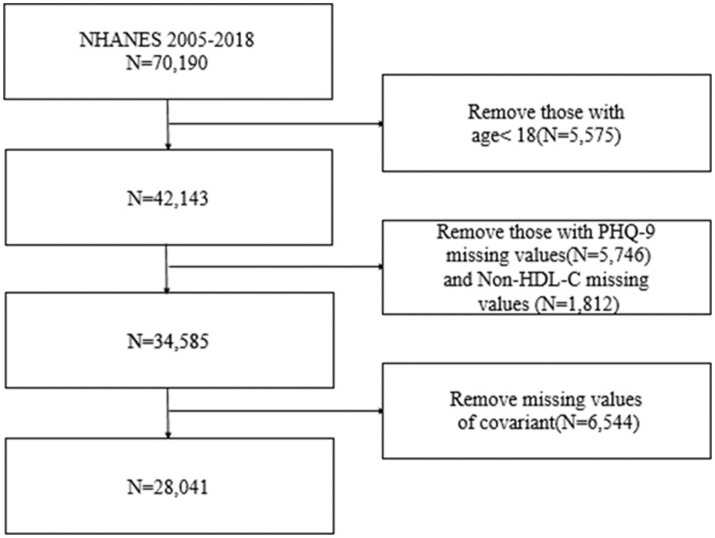
NHANES is a survey database managed by the National Center for Health Statistics (NCHS) that provides a comprehensive representation of the national health and nutrition status in the United States. This study utilized seven cycles of the NHANES database (2005–2018) and employed interviews and physical examinations as part of its sampling methodology. The selection of these seven cycles was based on their suitability for assessing the association between non-HDL-C and depression. The National Center for Health Statistics Research Ethics Review Committee approved NHANES ‘ethics program (Protocol #2005–06, Protocol #2011–17, and Protocol #2018–01) (31). The exclusion criteria consisted of patients with missing total cholesterol (TC) and high-density lipoprotein cholesterol (HDL-C) data, as well as incomplete responses to the Patient Health Questionnaire-9 (PHQ-9). Additionally, individuals under the age of 18 were also excluded. The specific procedure is outlined in Figure 1.
Figure 1.
Flow chart of the screening process for the selection of eligible participants.
Depression
In the NHANES dataset from 2005–06 to 2017–18, depression was assessed using the Patient Health Questionnaire-9 (PHQ-9). Participants were interviewed at a mobile examination center to evaluate the frequency of depressive symptoms over the past two weeks. For the purposes of this study, we defined major depression as a PHQ-9 score of 10 or higher (32).
Non-HDL-C
Non-HDL-C is calculated on the basis of fasting subjects’ standard lipids, which are total cholesterol (TC) minus high-density lipoprotein cholesterol (HDL-C).
Covariates
Several covariates were taken into consideration as potential confounding variables, including age, sex, race, marital status, family income below poverty level (PIR), educational level, body mass index (BMI), smoking status, alcohol consumption, history of cardiovascular disease (CVD), diabetes, and hypertension. Race was categorized as non-Hispanic white, Mexican American, non-Hispanic black, or other races. Marital status was classified as married or cohabiting, never married, widowed, or divorced or separated. Educational level was grouped as college graduate or higher, some college or associate’s degree, high school graduate or equivalent, or less than high school. Smoking status and alcohol status were classified as never, former, or current. The history of CVD encompassed heart failure, angina, myocardial infarction, and stroke.
Statistical analyses
The statistical analysis was conducted using R version 4.3.0. To ensure accurate results, appropriate 14-year sample weights were constructed following NHANES recommendations. All statistical tests were two-tailed, and a significance level of p < 0.05 was used. Categorical variables were represented as percentages, while continuous variables were presented as means plus and minus standard error (SE). Initially, logistic regression was employed to calculate the ratio of the 95% confidence interval between depression and non-HDL-C, with the first quartile serving as the reference. Furthermore, the relationship between non-HDL-C and depression was examined within subgroups defined by different covariates. Three regression models were developed for the analysis. Model 1 adjusted for demographics (age, sex, race), Model 2 adjusted for age, sex, race, marital status, PIR, educational level, BMI, smoking status, and alcohol consumption, and Model 3 further adjusted for CVD, diabetes, and hypertension in addition to the variables in Model 2. Sensitivity analyses were also conducted to assess the robustness of the results. Firstly, the unweighted raw data were analyzed using inverse probability of treatment weighting (IPTW) to address potential confounders. Secondly, individuals who were using antidepressants at baseline were excluded from the analysis.
Results
Characteristics of the participant
The NHANES 2005–2018 sample consisted of a total of 42,143 adult participants. After excluding missing data for depression, non-HDL-C, and covariates, the remaining number of participants was 28,041. Out of these participants, 2,419 (8.6%) were found to have depression, while 25,622 (91.4%) did not have depression when unweighted. The weighted percentages for depression and non-depression were 7.53% and 92.47%, respectively. The demographic characteristics, weighted according to the sample, are provided in Table 1. The mean age of the participants was 47.26 years, and no significant difference was observed between age and depression. Statistically significant differences were found in poverty, BMI, non-HDL-C, race, marital status, educational level, hypertension, diabetes, smoking, alcohol use, history of cardiovascular disease, and depression.
Table 1.
Weighted baseline characteristics of patients with or without depression.
| Variable | Total | Without depression | With depression | p-value |
|---|---|---|---|---|
| Age (years) | 47.26 ± 0.25 | 47.33 ± 0.26 | 46.41 ± 0.44 | 0.06 |
| PIR | 3.06 ± 0.03 | 3.13 ± 0.03 | 2.14 ± 0.06 | < 0.01 |
| BMI (kg/m2) | 29.08 ± 0.08 | 28.94 ± 0.09 | 30.81 ± 0.23 | < 0.01 |
| HDL-C (mmol/L) | 1.38 ± 0.01 | 1.39 ± 0.01 | 1.33 ± 0.03 | < 0.01 |
| Non-HDL-C (mmol/L) | 3.64 ± 0.01 | 3.64 ± 0.01 | 3.73 ± 0.03 | < 0.01 |
| Sex, % | < 0.01 | |||
| Female | 50.69 | 49.63 | 63.80 | |
| Male | 49.31 | 50.37 | 36.20 | |
| Race, % | < 0.01 | |||
| Mexican American | 8.00 | 8.03 | 7.56 | |
| Non-Hispanic Black | 10.20 | 9.99 | 12.79 | |
| Non-Hispanic White | 69.87 | 70.21 | 65.73 | |
| Other race | 11.93 | 11.77 | 13.92 | |
| Marital status, % | < 0.01 | |||
| Married/living with partner | 64.19 | 65.45 | 48.66 | |
| Never married | 17.57 | 17.30 | 20.90 | |
| Widowed | 5.42 | 5.25 | 7.44 | |
| Divorced/Separated | 12.82 | 12.00 | 23.00 | |
| Education, % | < 0.01 | |||
| Some college or AA degree | 32.00 | 31.81 | 34.42 | |
| College graduate or above | 30.27 | 31.58 | 14.17 | |
| High school grad/GED or equivalent | 23.04 | 22.70 | 27.18 | |
| Less than high school | 14.68 | 13.91 | 24.23 | |
| Hypertension, % | < 0.01 | |||
| Yes | 37.53 | 36.76 | 46.97 | |
| No | 62.47 | 63.24 | 53.03 | |
| Diabetes, % | < 0.01 | |||
| Yes | 13.89 | 13.37 | 20.25 | |
| No | 86.11 | 86.63 | 79.75 | |
| Smoke, % | < 0.01 | |||
| Never | 54.80 | 56.15 | 38.26 | |
| Former | 24.99 | 25.18 | 22.74 | |
| Now | 20.21 | 18.68 | 39.00 | |
| Alcohol user, % | < 0.01 | |||
| Never | 10.35 | 10.46 | 8.97 | |
| Former | 13.20 | 12.68 | 19.50 | |
| Now | 76.45 | 76.86 | 71.53 | |
| CVD, % | < 0.01 | |||
| Yes | 7.12 | 6.56 | 13.97 | |
| No | 92.88 | 93.44 | 86.03 |
Association of non-HDL-C with depression
The association between non-HDL-C and depression is presented in Table 2. Our findings indicate that elevated levels of non-HDL-C are linked to an increased risk of depression. The unadjusted model, which only accounted for non-HDL-C, revealed a statistically significant positive association between non-HDL-C and depression, with an odds ratio (OR) of 1.30 (95% confidence interval [CI]: 1.14–1.50) for the highest versus lowest quartile. Model 1, adjusted for age, sex, and race, showed an OR of 1.40 (95% CI: 1.22–1.62) for depression in the highest versus lowest quintile. Model 2, further adjusted for socioeconomic factors, lifestyle factors, and body mass index (BMI) based on model 1, resulted in an OR of 1.13 (95% CI: 0.96–1.33). Model 3, additionally adjusted for cardiovascular disease (CVD), diabetes, and hypertension based on model 2, revealed an OR of 1.22 (95% CI: 1.03–1.45). No significant positive trend was observed between increasing levels of non-HDL-C and the risk of depression (p for trend>0.05).
Table 2.
Association between non-HDL-C and depression.
| Unadjusted model | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| non-HDL-C | ||||
| Q1 | Ref | Ref | Ref | Ref |
| Q2 | 1.13 (0.99,1.30) | 1.16 (1.01,1.32) | 1.12 (0.97,1.29) | 1.16 (0.99,1.35) |
| Q3 | 0.99 (0.85,1.14) | 1.03 (0.89,1.20) | 0.95 (0.80,1.12) | 1.01 (0.85,1.21) |
| Q4 | 1.30 (1.14,1.50) | 1.40 (1.22,1.62) | 1.13 (0.96,1.33) | 1.22 (1.03,1.45) |
| p for trend | <0.01 | <0.01 | 0.15 | 0.07 |
Model 1: adjusted for age, sex and race; Model 2: adjusted for age, sex, race, marital status, PIR, education level, BMI, smoking status, alcohol consumption; Model 3: further adjusted for CVD, diabete, HDL and hypertension based on Model 2.
Subgroup analyses
Table 3 presents the findings of the subgroup analysis. Non-HDL-C was significantly associated with depression among male participants (OR, 1.31; 95% CI, 1.01–1.70), and those without hypertension (OR: 1.30; 95% CI: 1.01–1.68) in the highest quintiles. Non-HDL-C was significantly associated with depression among normal BMI (OR: 0.93; 95% CI: 0.66–1.32) in the higher quintiles. No association was observed among women or individuals with hypertension, in the obese, overweight and underweight as well as among those with and without diabetes.
Table 3.
Subgroup analyses stratified by sex, BMI and Hypertension, diabetes.
| non-HDL-C | Q1 | Q2 | p | Q3 | p | Q4 | p | p for trend | p for interaction |
|---|---|---|---|---|---|---|---|---|---|
| Sex | 0.88 | ||||||||
| Female | Ref | 1.11 (0.92,1.34) | 0.29 | 1.02 (0.80,1.31) | 0.86 | 1.23 (0.98,1.56) | 0.08 | 0.13 | |
| Male | Ref | 1.29 (0.97,1.71) | 0.08 | 1.06 (0.77,1.44) | 0.73 | 1.31 (1.01,1.70) | 0.04 | 0.11 | |
| BMI*(kg/m2) | 0.81 | ||||||||
| Underweight | Ref | 0.80 (0.27,2.36) | 0.68 | 2.00 (0.55,7.21) | 0.28 | 0.56 (0.08,3.73) | 0.54 | 0.91 | |
| Normal | Ref | 1.38 (1.07,1.78) | 0.01 | 1.00 (0.70,1.42) | 0.99 | 1.39 (0.92,2.11) | 0.12 | 0.24 | |
| Overweight | Ref | 0.94 (0.66,1.32) | 0.70 | 0.91 (0.62,1.32) | 0.61 | 1.11 (0.79,1.57) | 0.53 | 0.47 | |
| Obese | Ref | 1.12 (0.85,1.48) | 0.40 | 1.00 (0.76,1.32) | 1.00 | 1.17 (0.90,1.52) | 0.25 | 0.34 | |
| Hypertension | 0.33 | ||||||||
| Yes | Ref | 0.95 (0.72,1.25) | 0.72 | 0.92 (0.68,1.25) | 0.60 | 1.05 (0.82,1.34) | 0.70 | 0.14 | |
| No | Ref | 1.31 (1.06,1.62) | 0.01 | 1.04 (0.82,1.32) | 0.73 | 1.30 (1.01,1.68) | 0.04 | 0.63 | |
| Diabetes | 0.68 | ||||||||
| Yes | Ref | 0.93 (0.61,1.43) | 0.75 | 0.91 (0.59,1.41) | 0.68 | 1.22 (0.83,1.80) | 0.30 | 0.17 | |
| No | Ref | 1.21 (1.01,1.46) | 0.04 | 1.04 (0.85,1.28) | 0.70 | 1.22 (0.99,1.51) | 0.06 | 0.28 |
Adjusted for age, sex, race, marital status, PIR, education level, BMI, smoking status, alcohol consumption, CVD, HDL, diabetes and hypertension except the stratification factor itself. BMI*: BMI was classified as underweight (BMI < 18.5), normal (18.5 ≤ BMI < 25), overweight (25 ≤ BMI < 30), and obese (BMI ≥ 30) (33).
Sensitivity analyses
The results of the sensitivity analysis are displayed in Table 4. After IPTW, the OR for the highest versus lowest quartile was 1.18 (95% CI: 1.04–1.33). The trend test yielded consistent results as the previous analysis (p for trend <0.05). When participants using antidepressants were excluded, the OR for the highest versus lowest quartiles was 1.27 (95% CI: 1.03–1.57). However, the trend test did not yield significant results.
Table 4.
Sensitivity analyses.
| non-HDL-C | OR (95CI) | p for trend |
|---|---|---|
| Inverse probability treatment weighted analyses | ||
| Q1 | Ref | |
| Q2 | 1.08 (0.94,1.24) | |
| Q3 | 1.11 (0.96,1.29) | |
| Q4 | 1.18 (1.04,1.35) | <0.01 |
| Excluding participants take antidepressants | ||
| Q1 | Ref | |
| Q2 | 1.28 (1.07,1.54) | |
| Q3 | 0.98 (0.80,1.20) | |
| Q4 | 1.28 (1.03,1.58) | 0.12 |
Adjusted for age, sex, race, marital status, PIR, education level, BMI, smoking status, alcohol consumption, CVD, HDL, diabetes and hypertension.
Discussion
In this cross-sectional study, we found a positive and significant association between non-HDL-C and the odds of having depression. To our knowledge, this is the first study to demonstrate that non-HDL-C is associated with depression. Subgroup analysis revealed a higher odds ratio for the relationship between non-HDL-C and depression in men and individuals without hypertension. All sensitivity analyses were similar, indicating the robustness of our findings.
Cholesterol is a vital component of mammalian cell membranes and plays a crucial role in regulating cell membrane function and anabolic steroid hormones (34). Imbalances in cholesterol have been linked to cardiovascular disease, neurodegenerative disease, and psychiatric disorders (19, 35, 36). Previous studies have suggested that abnormal cholesterol metabolism contributes to the pathological mechanisms of depression (19, 37). However, due to variations in the focus on cholesterol types and research methodologies, prior investigations on cholesterol and depression have yielded conflicting conclusions (38–40). Non-HDL-C encompasses total cholesterol minus HDL and includes lipoprotein particles such as LDL, medium-density lipoprotein, lipoprotein (a), and very low-density lipoprotein remnants (41). Non-HDL is the sum of all cholesterol carried by apolipoprotein b – lipoprotein particles that can cause atherosclerosis (42). Endothelial dysfunction in the early stages of atherosclerosis can lead to depression (43, 44). Depressed patients may be less active, have an unhealthy diet (45), and are prone to dyslipidemia and higher non-HDL-C.
An increasing number of studies have demonstrated the association between non-HDL-C and various diseases (26, 46, 47). Elevated non-HDL-C levels in representative samples from the United States have been linked to a higher risk of depression, suggesting that non-HDL-C may play a significant role in depression. Consequently, non-HDL-C measurement could serve as a screening tool for depression and a guide for its treatment.
To provide a more comprehensive understanding of our dataset, we conducted subgroup analyses. Our study revealed that men and patients without hypertension with higher non-HDL-C levels were at a greater risk of depression compared to those with lower non-HDL-C levels. To gain further insight into the association between non-HDL-C and depression in terms of gender and hypertension, prospective studies with large sample sizes are warranted.
Despite the unclear pathophysiological mechanisms underlying the relationship between non-HDL-C and depression, multiple studies have explored their biological mechanisms. Depression has been closely associated with the hypothalamic–pituitary–adrenal (HPA) axis (48, 49). First, non-HDL-C may be involved in the pathophysiological processes of depression through the HPA system. Excessive serum non-HDL-C concentrations can be captured by macrophages and form foam cells (50), leading to the secretion of interleukin-6, which impacts the HPA axis (51–53). Second, non-HDL-C may influence depression by affecting immune cells. Interleukin-18 (IL-18) secreted by microglia is crucial in post-stroke depression in mice. The injection of exogenous IL-18 into the amygdala has been shown to induce severe depressive behavior in mice. An increase in IL-18 in the brain contributes to depressive-like behavior by promoting the IL-18 receptor signaling pathway (54). IL-10 alleviates depressive-like behavior in mice induced by astrocytes (55). A meta-analysis of longitudinal population studies has indicated that elevated CRP and IL-6 are significantly associated with the development of depressive symptoms (56). Fluoxetine regulates L-6 and TNF-α cytokines to improve depressive symptoms (57). These findings further support the role of inflammatory factors in the pathogenesis and treatment of depression.
In subgroup analyses, we found that people with higher non-HDL-C in normal BMI had a higher risk of depression, but this was not found in underweight, overweight and obese people. While this is often contradicted by the metabolic abnormalities associated with higher BMI (58), there may be a complex relationship between depression and BMI that requires further study in the future (45, 59).
Limitations and advantages
Our study had several advantages. First, it was the first study to examine the relationship between non-HDL-C and depression in a large sample of studies and to account for multiple potential confounders in our models. Second, we used a representative population, including different ethnicities, to make our results more generalizable. Third, non-HDL-C monitoring is convenient and can be performed in a non-fasting state. However, it is crucial to acknowledge the limitations of our study. First, depression was not clinically diagnosed by psychiatrists in our study, which may have led to misclassification of depression from the normal population and biased the results. However, in some studies, the use of the PHQ-9 to diagnose major depression has a high specificity and sensitivity, which reduces the impact of assessment errors on the results (60, 61). Second, we used only the results of a routine blood test to calculate non-HDL-C, and it is necessary to improve the reliability of the study with multiple measurements in the future. Finally, cross-sectional studies cannot infer causality, and prospective studies are needed to further determine validation.
Firstly, depression was not clinically diagnosed by psychiatrists, potentially resulting in misclassification of depression within the normal population and introducing bias to the results. Nonetheless, the use of the PHQ-9 in epidemiological survey studies for diagnosing major depression has demonstrated high specificity and sensitivity, mitigating the impact of assessment errors on the findings. Secondly, Second, we used only the results of a routine blood test to calculate non-HDL-C, and it is necessary to improve the reliability of the study with multiple measurements in the future. Lastly, the cross-sectional design of our study cannot establish causality, necessitating prospective investigations to validate our findings.
Conclusion
Our study showed that non-HDL-C was significantly associated with depression after adjustment for multiple confounders. Future prospective studies are needed to verify the results and to investigate the efficacy of non-HDL-C lowering therapy in patients with depression.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by NCHS Research Ethics Review Board (ERB). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
XZ: Conceptualization, Data curation, Investigation, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. YZ: Writing – review & editing. LL: Writing – review & editing. JL: Writing – review & editing. QH: Writing – review & editing. YS: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. SW: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Science and Technology Program Project of Guizhou Provincial Health Commission in 2021 (gzwkj2021-150), the Science and Technology Program Project of Guizhou Province (Basic of Guizhou Science and Technology – ZK[2023] General 195), Jiangsu Science and Technology Fifth 333 Project (BRA201711), Jiangsu Provincial Maternal and Child Health Research Project (F202060), and Changzhou City Health and Health High Level Talents Training Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Ferrari AJ, Charlson FJ, Norman RE, Flaxman AD, Patten SB, Vos T, et al. The epidemiological modelling of major depressive disorder: application for the global burden of disease study 2010. PLoS One. (2013) 8:e69637. doi: 10.1371/journal.pone.0069637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gold SM, Kohler-Forsberg O, Moss-Morris R, Mehnert A, Miranda JJ, Bullinger M, et al. Comorbid depression in medical diseases. Nat Rev Dis Primers. (2020) 6:69. doi: 10.1038/s41572-020-0200-2 [DOI] [PubMed] [Google Scholar]
- 3.Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry. (2014) 171:453–62. doi: 10.1176/appi.ajp.2013.13030325 [DOI] [PubMed] [Google Scholar]
- 4.Tanner JA, Hensel J, Davies PE, Brown LC, Dechairo BM, Mulsant BH. Economic burden of depression and associated resource use in Manitoba, Canada. Can J Psychiatr. (2020) 65:338–46. doi: 10.1177/0706743719895342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chodavadia P, Teo I, Poremski D, Fung DSS, Finkelstein EA. Prevalence and economic burden of depression and anxiety symptoms among Singaporean adults: results from a 2022 web panel. BMC Psychiatry. (2023):23. doi: 10.1186/s12888-023-04581-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vyas A, Desai R, Patel V, Bansal P, Jain A, Gupta T, et al. Rising burden of cardiovascular disease risk factors and acute cardiac events in young adults with comorbid depression: a comparison nationwide US cohorts hospitalized 10-years apart. Curr Prob Cardiol. (2023) 48:101755. doi: 10.1016/j.cpcardiol.2023.101755 [DOI] [PubMed] [Google Scholar]
- 7.Lepine JP, Briley M. The increasing burden of depression. Neuropsychiatr Dis Treat. (2011) 7:3–7. doi: 10.2147/NDT.S19617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho H, Lee K, Choi E, Cho HN, Park B, Suh M, et al. Association between social support and postpartum depression. Sci Rep. (2022):12. doi: 10.1038/s41598-022-07248-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barberio B, Zamani M, Black CJ, Savarino EV, Ford AC. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2021) 6:359–70. doi: 10.1016/S2468-1253(21)00014-5 [DOI] [PubMed] [Google Scholar]
- 10.Farooqi A, Gillies C, Sathanapally H, Abner S, Seidu S, Davies MJ, et al. A systematic review and meta-analysis to compare the prevalence of depression between people with and without type 1 and type 2 diabetes. Prim Care Diabetes. (2022) 16:1–10. doi: 10.1016/j.pcd.2021.11.001 [DOI] [PubMed] [Google Scholar]
- 11.Gu Y, Zhang W, Hu Y, Chen Y, Shi J. Association between nonalcoholic fatty liver disease and depression: a systematic review and meta-analysis of observational studies. J Affect Dis. (2022) 301:8–13. doi: 10.1016/j.jad.2021.12.128 [DOI] [PubMed] [Google Scholar]
- 12.Fonseca-Rodrigues D, Rodrigues A, Martins T, Pinto J, Amorim D, Almeida A, et al. Correlation between pain severity and levels of anxiety and depression in osteoarthritis patients: a systematic review and meta-analysis. Rheumatology (Oxford). (2021) 61:53–75. doi: 10.1093/rheumatology/keab512 [DOI] [PubMed] [Google Scholar]
- 13.Ren Q, Luo F, Ge S, Chen P. Major depression disorder may causally associate with the increased breast cancer risk: evidence from two-sample mendelian randomization analyses. Cancer Med. (2023) 12:1984–96. doi: 10.1002/cam4.5043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grossberg A, Rice T. Depression and suicidal behavior in adolescents. Med Clin North Am. (2023) 107:169–82. doi: 10.1016/j.mcna.2022.04.005 [DOI] [PubMed] [Google Scholar]
- 15.Liang Y, Gong YH, Wen XP, Guan CP, Li MC, Yin P, et al. Social determinants of health and depression: a preliminary investigation from rural China. PLoS One. (2012) 7:e30553. doi: 10.1371/journal.pone.0030553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torquati L, Mielke GI, Brown WJ, Burton NW, Kolbe-Alexander TL. Shift work and poor mental health: a Meta-analysis of longitudinal studies. Am J Public Health. (2019) 109:e13–20. doi: 10.2105/AJPH.2019.305278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou L, Tian Y, Wang Y, Chen D, Lu X, Zeng Z, et al. High-cholesterol diet promotes depression- and anxiety-like behaviors in mice by impact gut microbe and neuroinflammation. J Affect Dis. (2023) 327:425–38. doi: 10.1016/j.jad.2023.01.122 [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Chen J, Yin Z, Wang L, Peng L. The association between depression and metabolic syndrome and its components: a bidirectional two-sample Mendelian randomization study. Transl Psychiatry. (2021) 11:633. doi: 10.1038/s41398-021-01759-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheon SY. Impaired cholesterol metabolism, neurons, and neuropsychiatric disorders. Exp Neurobiol. (2023) 32:57–67. doi: 10.5607/en23010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu H, Lv W, Pan Q, Kalavagunta PK, Liu Q, Qin G, et al. Simvastatin therapy in adolescent mice attenuates HFD-induced depression-like behavior by reducing hippocampal neuroinflammation. J Affect Dis. (2019) 243:83–95. doi: 10.1016/j.jad.2018.09.022 [DOI] [PubMed] [Google Scholar]
- 21.Hocaoglu C, Kural B, Aliyazıcıoglu R, Deger O, Cengiz S. IL-1β, IL-6, IL-8, IL-10,IFN-γ,TNF-α and its relationship with lipid parameters in patients with major depression. Metab Brain Dis. (2012) 27:425–30. doi: 10.1007/s11011-012-9323-9 [DOI] [PubMed] [Google Scholar]
- 22.Feingold KR, Grunfeld C. Role of cytokines in inducing hyperlipidemia. Diabetes. (1992) 41:97–101. doi: 10.2337/diab.41.2.s97 [DOI] [PubMed] [Google Scholar]
- 23.Enkhmaa B, Prakash N, Berglund L. Non-HDL-C levels and residual cardiovascular risk: do population-specific precision approaches offer any advantages? Atherosclerosis. (2018) 274:230–1. doi: 10.1016/j.atherosclerosis.2018.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levinson SS. Non-high-density lipoprotein cholesterol and guidelines for cholesterol lowering in recent history. Lab Med. (2020) 51:14–23. doi: 10.1093/labmed/lmz032 [DOI] [PubMed] [Google Scholar]
- 25.Araki A, Iimuro S, Sakurai T, Umegaki H, Iijima K, Nakano H, et al. Non-high-density lipoprotein cholesterol: an important predictor of stroke and diabetes-related mortality in Japanese elderly diabetic patients. Geriatr Gerontol Int. (2012) 12:18–28. doi: 10.1111/j.1447-0594.2011.00809.x [DOI] [PubMed] [Google Scholar]
- 26.Wang G, Jing J, Wang A, Zhang X, Zhao X, Li Z, et al. Non–high-density lipoprotein cholesterol predicts adverse outcomes in acute ischemic stroke. Stroke. (2021) 52:2035–42. doi: 10.1161/STROKEAHA.120.030783 [DOI] [PubMed] [Google Scholar]
- 27.de Oliveira AR, Mourao CA, Magalhães GL, de Oliveira CM, Krieger JE, Mill JG, et al. Non-HDL cholesterol is a good predictor of the risk of increased arterial stiffness in postmenopausal women in an urban Brazilian population. Clinics. (2017) 72:106–10. doi: 10.6061/clinics/2017(02)07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen T, Li Y, Wang Y, Li X, Wan Y, Xiao X. ApoB, non-HDL-C, and LDL-C are more prominent in retinal artery occlusion compared to retinal vein occlusion. Ocul Immunol Inflamm. (2023) 141:1–7. doi: 10.1080/09273948.2023.2173245 [DOI] [PubMed] [Google Scholar]
- 29.Hassen CB, Machado Fragua MD, Landré B, Fayosse A, Dumurgier J, Kivimaki M, et al. Change in lipids before onset of dementia, coronary heart disease, and mortality: a 28-year follow-up Whitehall II prospective cohort study. Alzheimers Dement. (2023) 1–13. doi: 10.1002/alz.13140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Correll CU, Robinson DG, Schooler NR, Brunette MF, Mueser KT, Rosenheck RA, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders. Jama Psychiatry. (2014) 71:1350. doi: 10.1001/jamapsychiatry.2014.1314 [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention . NCHS research ethics review board (ERB) approval. (2023) Available at: https://www.cdc.gov/nchs/nhanes/irba98.htm (Accessed April 5, 2023) (Ref list).
- 32.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akram D, Astrup A, Atinmo T, Boissin J, Bray GA, Carroll KK, et al. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. (2000) 894:1–253. doi: 10.1002/jps.3080150106 [DOI] [PubMed] [Google Scholar]
- 34.Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. (2020) 21:225–45. doi: 10.1038/s41580-019-0190-7 [DOI] [PubMed] [Google Scholar]
- 35.Wang N, Woodward M, Huffman MD, Rodgers A. Compounding benefits of cholesterol-lowering therapy for the reduction of major cardiovascular events: systematic review and Meta-analysis. Circ Cardiovasc Qual Outcomes. (2022):15. doi: 10.1161/CIRCOUTCOMES.121.008552 [DOI] [PubMed] [Google Scholar]
- 36.Zhou Z, Liang Y, Zhang X, Xu J, Lin J, Zhang R, et al. Low-density lipoprotein cholesterol and alzheimer's disease: a systematic review and Meta-analysis. Front Aging Neurosci. (2020):12. doi: 10.3389/fnagi.2020.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papakostas GI, Petersen T, Mischoulon D, Hughes ME, Alpert JE, Nierenberg AA, et al. Serum cholesterol and serotonergic function in major depressive disorder. Psychiatry Res. (2003) 118:137–45. doi: 10.1016/S0165-1781(03)00066-0 [DOI] [PubMed] [Google Scholar]
- 38.Wagner CJ, Musenbichler C, Böhm L, Färber K, Fischer A, von Nippold F, et al. LDL cholesterol relates to depression, its severity, and the prospective course. Prog Neuro-Psychopharmacol Biol Psychiatry. (2019) 92:405–11. doi: 10.1016/j.pnpbp.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 39.Cepeda MS, Kern DM, Blacketer C, Drevets WC. Low levels of cholesterol and the cholesterol type are not associated with depression: results of a cross-sectional NHANES study. J Clin Lipidol. (2020) 14:515–21. doi: 10.1016/j.jacl.2020.06.001 [DOI] [PubMed] [Google Scholar]
- 40.Zhang C, Yang Y, Zhu D, Zhao W, Zhang Y, Zhang B, et al. Neural correlates of the association between depression and high density lipoprotein cholesterol change. J Psychiatr Res. (2020) 130:9–18. doi: 10.1016/j.jpsychires.2020.07.012 [DOI] [PubMed] [Google Scholar]
- 41.Su X, Kong Y, Peng D. Evidence for changing lipid management strategy to focus on non-high density lipoprotein cholesterol. Lipids Health Dis. (2019) 18:134. doi: 10.1186/s12944-019-1080-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobson TAM, Ito MKP, Maki KCP, Orringer CEM, Bays HEM, Jones PHM, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1—full report. J Clin Lipidol. (2015) 9:129–69. doi: 10.1016/j.jacl.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 43.Chrysohoou C, Kollia N, Tousoulis D. The link between depression and atherosclerosis through the pathways of inflammation and endothelium dysfunction. Maturitas. (2018) 109:1–5. doi: 10.1016/j.maturitas.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 44.Moran LJ, Wilson CJ, Buckley JD, Noakes M, Clifton PM, Brinkworth GD. Changes in endothelial function and depression scores are associated following long-term dietary intervention: a secondary analysis. Nutrition. (2013) 29:1271–4. doi: 10.1016/j.nut.2013.03.023 [DOI] [PubMed] [Google Scholar]
- 45.Hidese S, Asano S, Saito K, Sasayama D, Kunugi H. Association of depression with body mass index classification, metabolic disease, and lifestyle: a web-based survey involving 11,876 japanese people. J Psychiatr Res. (2018) 102:23–8. doi: 10.1016/j.jpsychires.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 46.Hodkinson A, Tsimpida D, Kontopantelis E, Rutter MK, Mamas MA, Panagioti M. Comparative effectiveness of statins on non-high density lipoprotein cholesterol in people with diabetes and at risk of cardiovascular disease: systematic review and network meta-analysis. BMJ. (2022):e67731. doi: 10.1136/bmj-2021-067731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aggarwal DJ, Kathariya MG, Verma DPK. LDL-C, NON-HDL-C and APO-B for cardiovascular risk assessment: looking for the ideal marker. Indian Heart J. (2021) 73:544–8. doi: 10.1016/j.ihj.2021.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marković VM, Čupić Ž, Maćešić S, Stanojević A, Vukojević V, Kolar-Anić L. Modelling cholesterol effects on the dynamics of the hypothalamic–pituitary–adrenal (HPA) axis. Math Med Biol. (2016) 33:1–28. doi: 10.1093/imammb/dqu020 [DOI] [PubMed] [Google Scholar]
- 49.Vinkers CH, Joëls M, Milaneschi Y, Gerritsen L, Kahn RS, Penninx BWJH, et al. Mineralocorticoid receptor haplotypes sex-dependently moderate depression. Susceptibility following childhood maltreatment. Psychoneuroendocrinology. (2015) 54:90–102. doi: 10.1016/j.psyneuen.2015.01.018 [DOI] [PubMed] [Google Scholar]
- 50.Miller YI, Choi SH, Fang L, Tsimikas S. Lipoprotein modification and macrophage uptake: role of pathologic cholesterol transport in atherogenesis. Subcell Biochem. (2010) 51:229–51. doi: 10.1007/978-90-481-8622-8_8 [DOI] [PubMed] [Google Scholar]
- 51.Gu H, Tang C, Yang Y. Psychological stress, immune response, and atherosclerosis. Atherosclerosis. (2012) 223:69–77. doi: 10.1016/j.atherosclerosis.2012.01.021 [DOI] [PubMed] [Google Scholar]
- 52.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. (2000) 148:209–14. doi: 10.1016/S0021-9150(99)00463-3 [DOI] [PubMed] [Google Scholar]
- 53.Ting EY, Yang AC, Tsai S. Role of interleukin-6 in depressive disorder. Int J Mol Sci. (2020) 21:2194. doi: 10.3390/ijms21062194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu D, Zhang G, Zhao C, Yang Y, Miao Z, Xu X. Interleukin-18 from neurons and microglia mediates depressive behaviors in mice with post-stroke depression. Brain Behav Immun. (2020) 88:411–20. doi: 10.1016/j.bbi.2020.04.004 [DOI] [PubMed] [Google Scholar]
- 55.Zhang HY, Wang Y, He Y, Wang T, Huang XH, Zhao CM, et al. A1 astrocytes contribute to murine depression-like behavior and cognitive dysfunction, which can be alleviated by IL-10 or fluorocitrate treatment. J Neuroinflammation. (2020) 17:200. doi: 10.1186/s12974-020-01871-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Dis. (2013) 150:736–44. doi: 10.1016/j.jad.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 57.García-García ML, Tovilla-Zárate CA, Villar-Soto M, Juárez-Rojop IE, González-Castro TB, Genis-Mendoza AD, et al. Fluoxetine modulates the pro-inflammatory process of IL-6, IL-1β and TNF-α levels in individuals with depression: a systematic review and meta-analysis. Psychiatry Res. (2022) 307:114317. doi: 10.1016/j.psychres.2021.114317 [DOI] [PubMed] [Google Scholar]
- 58.Jung SJ, Woo H, Cho S, Park K, Jeong S, Lee YJ, et al. Association between body size, weight change and depression: systematic review and meta-analysis. Br J Psychiatry. (2017) 211:14–21. doi: 10.1192/bjp.bp.116.186726 [DOI] [PubMed] [Google Scholar]
- 59.He K, Pang T, Huang H. The relationship between depressive symptoms and BMI: 2005–2018 NHANES data. J Affect Disord. (2022) 313:151–7. doi: 10.1016/j.jad.2022.06.046 [DOI] [PubMed] [Google Scholar]
- 60.Maske UE, Busch MA, Jacobi F, Beesdo-Baum K, Seiffert I, Wittchen H, et al. Current major depressive syndrome measured with the patient health Questionnaire-9 (PHQ-9) and the composite international diagnostic interview (CIDI): results from a cross-sectional population-based study of adults in Germany. BMC Psychiatry. (2015):15. doi: 10.1186/s12888-015-0463-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carey M, Jones KA, Yoong SL, D'Este C, Boyes AW, Paul C, et al. Comparison of a single self-assessment item with the PHQ-9 for detecting depression in general practice. Fam Pract. (2014) 31:483–9. doi: 10.1093/fampra/cmu018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.