Abstract
Introduction
The aim of this study was to explore the differences in status epilepticus (SE) management among pediatric neurology, emergency medicine, and intensive care specialists in Turkey.
Methods
A 22-item questionnaire regarding first-, second-, and third-line management strategies of SE including demographic characteristics and common etiologies according to the specialty of participants was mailed to 370 specialists working in Turkey.
Results
A total of 334 participants (response rate 90%) comprising 136 pediatric neurologists, 102 pediatric emergency medicine specialists, and 96 pediatric intensive care specialists completed the survey. While intensive care specialists frequently managed SE due to metabolic and autoimmune reasons, the most common etiologies encountered by emergency medicine specialists were epilepsy and infections. More than half of the intensive care specialists (64.6%) reported using non-BZD antiseizure medications in the 5th minute of the seizure. Most of the neurologists (76.4%) preferred to administer intravenous (IV) levetiracetam infusion as a second-line agent. About half of intensive care specialists and neurologists tried immunomodulatory therapies in super-refractory SE. Intensive care and emergency medicine specialists were less likely to favor ketogenic diet and pyridoxine therapy for the treatment of super-refractory SE. The rate of requesting EEG monitoring to recognize nonconvulsive SE (NCSE) was found to be very low except for neurologists.
Conclusion
There was no consensus among neurologists, intensive care specialists, and emergency medicine specialists in the management of SE in Turkey. Familiarity with particular antiseizure medications and the etiologies they manage seem to be the most important factors influencing the attitudes.
Keywords: Status epilepticus management, Pediatric neurologist, Intensive care, Emergency medicine
Introduction
Status epilepticus (SE) is defined as “a condition resulting either from the failure of mechanisms responsible for seizure termination or from the initiation of mechanisms, which lead to abnormally prolonged seizures (after time point t1) and which can have long-term consequences (after time point t2), including neuronal death, neuronal injury, and alteration of neural networks” by the International League Against Epilepsy (ILAE) [1]. It is known that long-term outcomes are known to be better in series in which the management of SE in childhood is carried out correctly with timely interventions [2, 3]. In a study comparing medically treated and untreated seizure episodes, differences were found in terms of age and etiology, and the relationship between treatment delay and response became significant after 30 min [4]. Although recent findings improve treatment strategies for pediatric SE [5–7], optimal first-, second-, and third-line steps for SE, refractory SE, and super-refractory SE are unclear [8–11]. In our country, decisions in SE management are largely based on expert opinion. In addition, there is no study investigating the differences in SE management strategies among experts. Therefore, we aimed to reveal the differences between the management of SE by pediatric neurologists, emergency medicine specialists, and intensive care specialists with a survey.
Materials and Methods
Preparation of the Questionnaire
A questionnaire of 22 items was used for data collection which consisted of four sections. In the first section, demographic and characteristic information of participants including specialty, experience in pediatrics, current position, number of patient with SE followed per month, common etiologies they encounter, and the opinion about the optimal management of SE (questions 1, 2, 3, 4, 5, 6) were questioned. In the second section, the knowledge of the definitions of SE (questions 7, 8, 9, 16, 18) was evaluated. In the third section, they were asked about the first-, second-, and third-line antiseizure medications and also preferences of neuroimaging and EEG monitoring (questions 10, 11, 12, 13, 14, 15, 17, 19, 20, 21). In the fourth section of the questionnaire, specialists were asked about their opinions regarding SE prognosis (Question 22). The statements were prepared by Dedeoğlu Ö., Akça H., and Emeksiz S. after a comprehensive discussion on guidelines and recent articles [9, 10, 12, 13]. After the authors arrived at consensus, relevance of the statements was judged independently by two competent authors in this field (Kartal A. and Kurt N.Ç.). Participants were expected to respond to statements 2, 7, 8, 9, 16, and 18 according to (a) agree, (b) disagree, and (c) neither agree nor disagree. Other statements were designed in the form of a multiple-choice questionnaire. To ensure that all questions were answered, the participants were not allowed to move on to the next question before answering the current one. The statements on the questionnaire are given in the online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000533191).
Study Design
A cross-sectional questionnaire-based study was conducted among pediatric neurologists, emergency medicine specialists, and intensive care specialists from Turkey between May 7, 2022, and June 7, 2022, in accordance with the Helsinki guidelines with the approval of the Institutional Ethics Committee from the Turkish Ministry of Health, Ankara City Hospital Ethics Committee (E2-22-1681). The staff members surveyed were consultant physicians practicing pediatric emergency medicine specialists, pediatric neurologists, and intensive care specialists in Turkey. Residents in these fields also participated in the survey. The questionnaire was submitted via Google Forms and emailed to all 370 registered specialists in the Turkey Database. Written informed consent was obtained by adding an “informed consent form” to the questionnaire prepared with Google Forms. After two mailings, the questionnaire was returned in 1 month. Responses to the questionnaire were anonymous, but respondents were asked about their specialty and affiliations.
Statistical Analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 25.0 software (IBM Corp., Armonk, NY, USA). The statistical analysis was based on descriptive statistics. The variables were investigated using analytical methods (Kolomogorov-Smirnov/Shapiro-Wilk test) to determine whether or not they are normally distributed. A p value <0.05 was considered to indicate statistical significance. The one-way ANOVA test was used to compare more than two independent groups of numerical variables with normal distribution. For the analyses in which nonparametric tests were used, the differences between the groups were evaluated with the Tukey test. Pearson χ2 and Fisher’s exact tests were used to compare the differences between categorical variables.
Results
A total of 334 people (response rate 90%), including 136 (41%) pediatric neurologists, 102 (30%) pediatric emergency medicine specialists, and 96 (29%) pediatric intensive care specialists, responded completely to all statements. Most of the participants (67.9%) had experience in pediatrics for more than 10 years. There was no significant difference between groups according to their experience in pediatrics and also their current position (p = 0.33; p = 0.07). Nearly one-third of emergency medicine specialists indicated that they encounter more than 10 SE patients per month (p = 0.014). While intensive care specialists frequently encounter patients with SE due to metabolic and autoimmune reasons, the most common etiologies encountered by emergency medicine specialists were epilepsy and infections (p = 0.001) (Table 1).
Table 1.
Demographic characteristics (study group, n = 334) and common etiologies according to the specialty of responders
| Statements | Specialty of responders | ||
|---|---|---|---|
| intensive care (n = 96; 28.7%) | emergency (n = 102; 30.5%) | neurology (n = 136; 40.7%) | |
| How many years of experience do you have in paediatrics? | |||
| 1–5 | – | – | – |
| 5–10 | 57 (59.4) | 34 (33.3) | 34 (25) |
| ≥10 | 39 (40.6) | 68 (66.7) | 102 (75) |
| Which of the following is your current position/hospital? | |||
| State | 59 (61.5) | 27 (26.5) | 109 (80.1) |
| University | 28 (29.2) | 26 (25.5) | 24 (17.6) |
| Private | 9 (9.4) | 49 (48) | 3 (2.2) |
| How many patients with SE do you follow per month? | |||
| ≤5 | 59 (61.5) | 44 (43.1) | 75 (55.1) |
| 5–10 | 31 (32.3) | 31 (30.4) | 57 (41.9) |
| ≥10 | 6 (6.3) | 27 (26.5) | 4 (2.9) |
| Which of the following etiologies of SE do you encounter more common? | |||
| Epilepsy | 11 (11.4) | 51 (50) | 104 (76) |
| Infections | 9 (9.3) | 42 (41) | 7 (5) |
| Metabolic reasons | 42 (43) | – | 2 (1.4) |
| Trauma | 9 (9.3) | 7 (6) | 1 (0.7) |
| Autoimmune encephalitis | 21 (21.8) | – | 19 (14.1) |
| Intoxication | 4 (4) | 2 (2) | – |
| Intracranial mass | – | – | 3 (2.2) |
Precisely, 92% of the neurologists (n = 125) remarked that they had self-confidence in managing SE appropriately until it ended. Twenty-seven participants, including 19 emergency medicine specialists, stated that they could not manage SE appropriately. When the participants’ knowledge of the definition of SE was questioned, almost the whole group (99%) correctly defined refractory SE and super-refractory SE. Most of them (93%) agreed that tonic-clonic seizures lasting longer than 5 min and unilateral seizures longer than 10 min in which consciousness is affected are considered SE. In addition, one-fifth of the participants (n = 66) did not agree with the statement about the absence of SE.
The majority of participants administered the antiseizure medications after providing adequate airway support and preferred intravenous (IV)/intramuscular (IM) or nasal benzodiazepine (BZD) as the first choice. More than half of the intensive care specialists (64.6%) reported using non-BZD antiseizure medications in the 5th minute of the seizure. Experience has been specified as the most important factor in the choice of second-line antiseizure medication. Additionally, nearly half of the participants working in public hospitals (45%) considered that the availability of the drug as the most important factor affecting the second-line medication. Approximately 46% of the emergency medicine specialists consulted the neurology department to determine antiseizure medication if the seizure persisted at the 10th minute. Most of the neurologists (76.4%) preferred loading IV levetiracetam infusion as a second-line agent. Half of the emergency medicine specialists (51%) followed the patients in intensive care conditions after SE. Intensive care specialists preferred to administer barbiturates infusion in refractory SE intervention more than other specialists (p = 0.001). All those who attempted to maintain the patient’s previous drug levels in refractory SE were neurologists. About half of the intensive care specialists and neurologists tried immunomodulatory therapies in super-refractory SE. Neurologists preferred starting ketogenic diet or pyridoxine administration more frequently than others (p = 0.001). Table 2 lists the statements and responses related to first-, second-, and third-line medication options.
Table 2.
Statements and responses related to first-, second-, and third-line treatment options according to groups
| Statements | Specialty | |||
|---|---|---|---|---|
| intensive care | emergency | neurology | p value | |
| When would you administer antiseizure medication to a patient with SE? | <0.001 | |||
| After providing adequate airway support | 41 (42.7%) | 70 (68.6%) | 101 (74.3%) | |
| When the patient’s intravenous line is provided | 31 (32.3%) | – | – | |
| After the 5th minute of the seizure | 16 (16.7%) | 28 (27.5%) | 35 (25.7%) | |
| By consulting neurology | 8 (8.3%) | 4 (3.9%) | – | |
| What would be your first choice when you decide on anticonvulsant treatment? | 0.077 | |||
| Rectal BZD | 8 (8.3%) | 20 (19.6%) | 21 (15.4%) | |
| Intravenous/intramuscular/nasal benzodiazepine | 88 (91.7%) | 82 (80.4%) | 115 (84.6%) | |
| IV phenytoin | – | – | – | |
| IV levetiracetam | – | – | – | |
| If the patient’s seizures still continue in the 5th minute despite the appropriate dose of BZD iv/rectal/nasal administration, which one of the following would you prefer? | <0.001 | |||
| IV BZD | 34 (35.4%) | 84 (82.4%) | 89 (65.4%) | |
| IV phenytoin | 17 (17.7%) | – | 13 (9.6%) | |
| IV levetiracetam | 22 (22.9%) | 10 (9.8%) | 24 (17.6%) | |
| If the patient is known to have epilepsy, I load the i.v. form of the drug he used | 23 (24%) | 8 (7.8%) | 10 (7.4%) | |
| If the seizure still continues in the 10th minute despite the appropriate dose of antiseizure administration, which of the following would you prefer? | <0.001 | |||
| Loading IV phenytoin | 30 (31.3%) | 28 (27.5%) | 25 (18.4%) | |
| Starting IV midazolam infusion | 48 (50%) | 9 (8.8%) | 12 (8.8%) | |
| Starting IV levetiracetam infusion | 14 (14.6%) | 23 (22.5%) | 104 (76.4%) | |
| Loading IV valproate infusion | – | – | – | |
| By consulting neurology | 4 (4.2%) | 42 (41.2%) | – | |
| What is the most important factor affecting your choice of second-line medication in a patient with a SE? | <0.001 | |||
| Availability | 27 (28.1%) | 30 (29.4%) | 24 (17.6%) | |
| Effectiveness | 37 (38.5%) | – | 12 (8.8%) | |
| Experiences | 14 (14.6%) | 61 (59.8%) | 53 (39%) | |
| Side-effects of the drug | – | 11 (10.8%) | 32 (23.5%) | |
| Additional disease of the patient | 18 (18.8%) | – | 15 (11%) | |
| How long would you follow the patient after SE if his level of consciousness returned to normal? | <0.001 | |||
| 24 h in the emergency room/service | 58 (60.4%) | 24 (23.5%) | 40 (29.4%) | |
| Until the etiology is determined | 23 (24%) | 15 (14.7%) | 36 (26.5%) | |
| Until he stabilizes in intensive care conditions | 13 (13.5%) | 52 (51%) | 60 (44.1%) | |
| Until oral intake starts at a sufficient level | 2 (2.1%) | 11 (10.8%) | – | |
| Which one would you prefer in the management of the resistant SE? | <0.001 | |||
| Starting infusion after the midazolam bolus, and increasing the post bolus infusion rate | 45 (46.9%) | 78 (76.5%) | 80 (58.8%) | |
| Providing blood levels of previous drugs | – | 1 (1%) | 35 (25.7%) | |
| Starting ketamine infusion | 4 (4.2%) | 20 (19.6%) | 1 (0.7%) | |
| Starting barbiturates infusion and increasing until the burst suppression pattern is established on the EEG | 47 (49%) | 3 (2.9%) | 20 (14.7%) | |
| Which of the following would you prefer in the treatment of super-resistant SE? | <0.001 | |||
| I decide by consulting neurology | 26 (27.1%) | 51 (50%) | – | |
| I would administer pyridoxine | – | 1 (1%) | 15 (11%) | |
| Considering IV immunoglobulin/methylprednisolone therapy as an immunomodulatory treatment option | 42 (43.8%) | 16 (15.7%) | 55 (40.4%) | |
| I would start ketogenic enteral nutrition | 13 (13.5%) | 3 (2%) | 81 (59.6%) | |
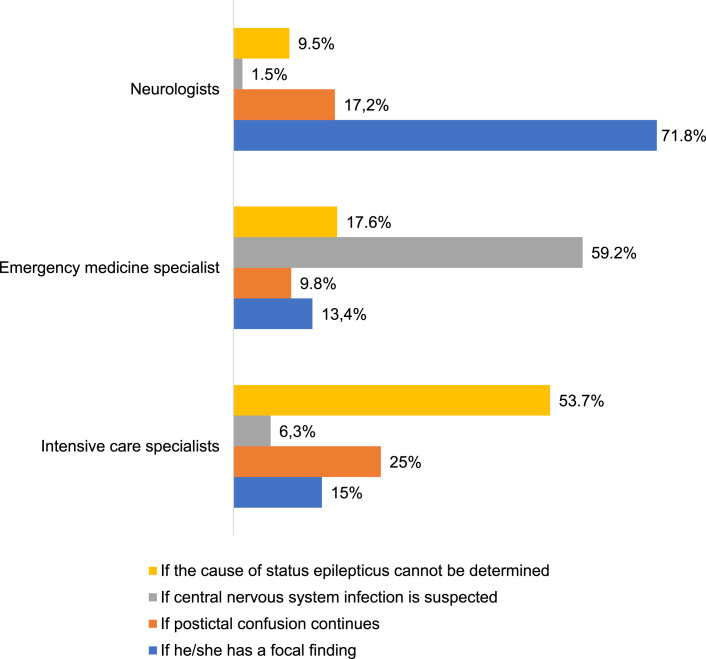
Based on the statement regarding situations in which they would request neuroimaging, while majority of neurologists prefer neuroimaging in the presence of focal findings, intensive care specialists frequently prefer to check if the cause of SE cannot be determined (p = 0.001) (Fig. 1). No relationship was found according to average number of SE patients they follow per month, experience in pediatrics, and also occupation (p = 0.74; p = 0.86; p = 0.29). Almost 25% of neurologists and only 4% of intensive care specialists considered checking EEG monitoring to recognize nonconvulsive SE (NCSE) (Fig. 2). When the participants were asked about the prognosis of SE, nearly three-quarters (72%) of emergency medicine specialists revealed that most patients can recover from SE without any sequelae. Statements concerning the prognosis of SE are summarized in Figure 3.
Fig. 1.
Responses to the statement regarding the situations in which they would request neuroimaging.
Fig. 2.
Responses to the statement regarding the situations in which they would request EEG monitoring.
Fig. 3.
Responses to the statement concerning prognosis of SE.
Discussion
Current guidelines recommended giving the first dose of BZD within 5–20 min after the onset of the seizure, a dose of non-BZD medication at 20–40 min, and a second dose of non-BZD medication at 40–60 min [12, 13]. A recent meta-analysis comparing midazolam, lorazepam, or diazepam with each other or with other non-BZD agents in pediatric patients with SE revealed that midazolam had the highest probability of achieving seizure cessation [14]. Non-intramuscular midazolam was also shown to be equally safe and effective as IV and rectal midazolam [15, 16]. Historically, phenytoin has been commonly used when BZDs have not been able to terminate the episode of imminent SE [17]. However, two randomized controlled trials, the Concept study and the Eclipse study, both published in 2019, examined the equally effective and safe role of levetiracetam as a second-line agent [18, 19]. According to responses from an international survey, for second-line therapy, the most common agents chosen were phenytoin/fosphenytoin, valproate sodium, and levetiracetam [20]. In a double-blind, responsive-adaptive, randomized controlled trial which investigated the efficacy of levetiracetam, fosphenytoin, and valproate for Established Status Epilepticus Treatment Trial (ESETT) by age group, it was found out that children, adults, and older adults with established SE respond similarly to levetiracetam, fosphenytoin, and valproate, with treatment success in approximately half of the patients [21]. In our survey, it was determined that rectal benzodiazepine was no longer used even in the emergency department. Following BZD, there was a tendency to administer levetiracetam infusion among neurologists. Another notable finding is that, although valproic acid infusion is a second-line treatment, none of the participants chose it. In children younger than 2 years of age and especially when there is no immediately identified cause of SE and in some conditions such as POLG-related disorders, many algorithms are known to exclude sodium valproate due to its side effects [22, 23]. This is supported by the fact that a third of the participants considered side effects of drugs in our cohort. Moreover, the use of specific antiseizure medications in the treatment of SE can vary depending on our country. For example, intravenous midazolam is used in Turkey because lorazepam is not available and there is a very limited amount of intravenous sodium valproate. Nearby facilities in public hospitals in our country may be insufficient, so that half of specialists working in public hospitals emphasized the availability of antiseizure medications over other factors. As a result, the choice of second-line antiseizure medication seems to be influenced by the health policies in our country.
Regarding the third-line antiseizure medication selection from the previous study of Kravljanac et al. [24], midazolam was the primary infusion agent used in most refractory SE patients. For the treatment of established convulsive SE, levetiracetam, valproic acid, and fosphenytoin are probably equally effective, but it is not known whether this also applies to refractory SE [25]. Clinicians typically use barbiturates as a second agent if midazolam is unsuccessful in achieving electrographic/clinical seizure arrest or burst suppression [8, 11, 20]. According to a survey among neurologists, about half indicated “burst suppression” (56%) and half indicated “elimination of seizures” (41%) as the titration goal [21]. In our survey, most of the emergency medicine specialists and neurologists preferred to start midazolam infusion in refractory SE, whereas intensive care specialists preferred to administer barbiturates infusion and increase until the burst suppression pattern is established on the EEG. The differences of management strategy at this stage of SE may be a consequence of intensive care specialists’ experience with these interventions. Furthermore, nonadherence to antiseizure medication is known to be the most common precipitating factor for SE in developing countries, and low antiseizure medication levels are a potentially modifiable situation in epilepsy patients, and only neurologists attempted to maintain the patient’s previous drug levels in refractory SE [26].
The study by Cobo et al. [27] supported the early initiation of enteral ketogenic nutrition as a safe and well-tolerated management of super-refractory SE in children. Nevertheless, IV pyridoxine therapy has been proven to be effective in super-refractory SE in the absence of a significant deficiency in pyridoxine and is now routinely recommended in cases of super-refractory SE in young children [28]. The very low preference for starting a ketogenic diet or pyridoxine administration in super-refractory SE may again be a consequence of the lack of experience with these therapies in our cohort.
Early elucidation of the etiology of SE provides guidance for treatment steps; however, recognition of more unusual reasons may become challenging in selected cases [9, 10]. For example, autoimmune encephalitis was previously thought to be an extremely rare disease; a recent study in Minnesota highlighted the prevalence and incidence of autoimmune encephalitis being similar to infectious encephalitis [29]. Immunotherapy has also been put forward as a standard treatment option for most refractory SE cases [30]. The most surprising finding of our survey was the relatively high proportion of trials of immunomodulatory therapies in super-refractory SE. It suggests that intensive care specialists and neurologists are aware of the rare causes of SE and want to determine whether refractory seizures are responsive to immunotherapy.
The diagnostic work-up needs to be individualized according to the initial clinical situation. Neuroimaging may be considered if the etiology remains unknown and can improve the clinical assessment of individual patients with SE by identifying hemodynamic patterns [31, 32]. Overall, it appears that the majority of participants knew basic information about the indications for neuroimaging; we revealed that etiology influences neuroimaging preferences. This is supported by the fact that emergency medicine specialists indicated that they prefer neuroimaging in suspected central nervous system infection, which is the most common etiology they encounter with.
Unfortunately, due to the lack of randomized clinical trials, the duration and cut-off parameters of pharmacologically induced coma remain unclear [12, 13]. Clinical manifestations of ongoing seizures often become more subtle over time, and the risk of nonconvulsive seizures after convulsive seizures is high [33]. Several studies have shown that pediatric nonconvulsive SE also has prognostic implications and continuous EEG monitoring is crucial in critically ill patients: in diagnosis, in detecting relapse, and in adjusting medications [34, 35]. On the other hand, about half of neurologists (42%) reported that they would add a new continuous antiseizure medication, and the other half (41%) would not add another agent to treat electrographic SE refractory to four antiseizure medications [21]. In our survey, the rate of preferring to perform EEG monitoring to recognize NCSE was very low except for neurologists, although the participants were given the opportunity to choose more than one option to this question. Emergency medicine specialists are generally involved in first-line treatment of SE in Turkey, and they might not know the indications for EEG. On the other hand, the fact that intensive care specialists did not request EEG monitoring despite using continuous infusions of highly sedating drugs suggests that they do not have sufficient knowledge on this subject. There is a great effort for the widespread availability of continuous EEG monitoring in our country. However, it is related to both labor problems and technical availability due to insufficient resources, especially in public hospitals. In addition, there is still no specific training center for EEG expertise in our country.
When the opinions of the specialists about the prognosis of SE were investigated, emergency medicine specialists may have thought that the prognosis was better in the etiologies they frequently encountered, in accordance with the literature that determined that cognitive outcome depends on treatment response on EEG and seizures and on the underlying etiology of SE [36]. Indeed pathophysiology, treatment, and the complications of SE are included in all of the participants’ education and training; neurologists are more likely to decide on the continuation of antiseizure medication after SE.
This is the first study among Turkish specialists from three different departments focusing on the decision-making processes during SE management. Although the study findings are not generalizable, it was observed that there was no consensus among neurologists, intensive care, and emergency medicine specialists. Also, this study has identified the lack of awareness of the importance of EEG and other therapeutic options in critical stages. As a recommendation, SE conferences such as the Advanced Pediatric Life Support course organized in our country can contribute to the discussion of SE diagnostic gaps.
Our study had some limitations. First, the majority of participants have been working in public hospitals which may lead to erroneously influence drug preferences. Second, it was not known whether there were other departments in the hospital where the participants worked. Approach strategies of emergency medicine specialists may differ according to the presence of pediatric intensive care and pediatric neurology departments. Also, a clear definition of resistant and super-resistant SE was not given in order to not affect the answers of the participants. It was tried to investigate which drugs and approach they use in daily practice and it was aimed at revealing the differences in practice.
Furthermore, the other limitation of our study is the question of which drug to administer first. Although guidelines recommend early treatment, clinical practice does not always comply with national and international guidelines, and logistic challenges include rapid seizure recognition, BDZ availability, convenience, expertise in BDZ administration, and timely arrival of emergency personnel. There is a need for innovations on practical considerations for opportunities, especially for public hospitals for improvement in the initial treatment of SE in our country.
Conclusion
This study provides a good insight into how SE management differs from pediatric intensive care and emergency medicine specialists to neurologists. Apparently, experience and the etiologies seem to play important roles in the preferences. As a result of the study, the necessity of trying to establish a standard algorithm of pediatric SE in Turkey was revealed.
Acknowledgments
We thank all the pediatricians for their participation in this study. This research did not receive any specific grants from funding agencies in the public, commercial, or non-profit sectors.
Statement of Ethics
This study protocol was reviewed and approved by the Institutional Ethics Committee from the Turkish Ministry of Health, Ankara City Hospital Ethics Committee (E2-22-1681). Written informed consent was obtained for participation in this study also.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There is no funding of any research relevant to the study and also no sponsor in the preparation of data or the manuscript.
Author Contributions
Özge Dedeoglu (corresponding author) contributed to conception, design, acquisition, and analysis; drafted the manuscript; and agrees to be accountable for all aspects of work ensuring integrity and accuracy and that there is no potential conflict of interest. Halise Akça contributed to conception, design, acquisition, and analysis; drafted the manuscript; and agrees to be accountable for all aspects of work ensuring integrity and accuracy and that there is no potential conflict of interest. Serhat Emeksiz contributed to preparation of the manuscript, critically reviewed the manuscript, and agrees to be accountable for all aspects of work ensuring integrity and accuracy and that there is no potential conflict of interest. Ayşe Kartal and Neşe Çıtak Kurt contributed to conception, design, acquisition, and analysis and agree to be accountable for all aspects of work ensuring integrity and accuracy and that there is no potential conflict of interest. All authors commented on previous versions of the manuscript, approved the final manuscript, and agree to be accountable for all aspects of work ensuring integrity and accuracy and that there is no potential conflict of interest.
Funding Statement
There is no funding of any research relevant to the study and also no sponsor in the preparation of data or the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus: report of the ILAE task force on classification of status epilepticus. Epilepsia. 2015;56(10):1515–23. 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 2. Gurcharran K, Grinspan ZM. The burden of pediatric status epilepticus: epidemiology, morbidity, mortality, and costs. Seizure. 2019 May;68:3–8. 10.1016/j.seizure.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 3. Novorol CL, Chin RF, Scott RC. Outcome of convulsive status epilepticus: a review. Arch Dis Child. 2007;92(11):948–51. 10.1136/adc.2006.107516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eriksson K, Metsaranta P, Huhtala H, Auvinen A, Kuusela AL, Koivikko M. Treatment delay and the risk of prolonged status epilepticus. Neurology. 2005;65(8):1316–8. 10.1212/01.wnl.0000180959.31355.92. [DOI] [PubMed] [Google Scholar]
- 5. Messahel S, Bracken L, Appleton R. Optimal management of status epilepticus in children in the emergency setting: a review of recent advances. Open Access Emerg Med. 2022;14:491–506. 10.2147/OAEM.S293258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zimmern V, Korff C. Status epilepticus in children. J Clin Neurophysiol. 2020;37(5):429–33. 10.1097/WNP.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 7. Abend NS, Loddenkemper T. Pediatric status epilepticus management. Curr Opin Pediatr. 2014;26(6):668–74. 10.1097/MOP.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vasquez A, Farias-Moeller R, Tatum W. Pediatric refractory and super-refractory status epilepticus. Seizure. 2019;68:62–71. 10.1016/j.seizure.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 9. Lawton B, Davis T, Goldstein H, Tagg A. An update in the initial management of paediatric status epilepticus. Curr Opin Pediatr. 2018;30(3):359–63. 10.1097/MOP.0000000000000616. [DOI] [PubMed] [Google Scholar]
- 10. Barcia Aguilar C, Sánchez Fernández I, Loddenkemper T. Status epilepticus-work-up and management in children. Semin Neurol. 2020;40(6):661–74. 10.1055/s-0040-1719076. [DOI] [PubMed] [Google Scholar]
- 11. Crawshaw AA, Cock HR. Medical management of status epilepticus: emergency room to intensive care unit. Seizure. 2020;75:145–52. 10.1016/j.seizure.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 12. Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the guideline committee of the American epilepsy society. Epilepsy Curr. 2016;16(1):48–61. 10.5698/1535-7597-16.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Capovilla G, Beccaria F, Beghi E, Minicucci F, Sartori S, Vecchi M. Treatment of convulsive status epilepticus in childhood: recommendations of the Italian League against Epilepsy. Epilepsia. 2013;54(Suppl 7):23–34. 10.1111/epi.12307. [DOI] [PubMed] [Google Scholar]
- 14. Zhao ZY, Wang HY, Wen B, Yang ZB, Feng K, Fan JC. A comparison of midazolam, lorazepam, and diazepam for the treatment of status epilepticus in children: a network meta-analysis. J Child Neurol. 2016;31(9):1093–107. 10.1177/0883073816638757. [DOI] [PubMed] [Google Scholar]
- 15. Brigo F, Nardone R, Tezzon F, Trinka E. Nonintravenous midazolam versus intravenous or rectal diazepam for the treatment of early status epilepticus: a systematic review with meta-analysis. Epilepsy Behav. 2015;49:325–36. 10.1016/j.yebeh.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 16. Cohen NT, Chamberlain JM, Gaillard WD. Timing and selection of first antiseizure medication in patients with pediatric status epilepticus. Epilepsy Res. 2019;149:21–5. 10.1016/j.eplepsyres.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 17. Lewena S, Pennington V, Acworth J, Thornton S, Ngo P, McIntyre S, et al. Emergency management of pediatric convulsive status epilepticus: a multicenter study of 542 patients. Pediatr Emerg Care. 2009;25(2):83–7. 10.1097/PEC.0b013e318196ea6e. [DOI] [PubMed] [Google Scholar]
- 18. Dalziel SR, Borland ML, Furyk J, Bonisch M, Neutze J, Donath S, et al. Levetiracetam versus phenytoin for second-line treatment of convulsive status epilepticus in children (ConSEPT): an open-label, multicentre, randomised controlled trial. Lancet. 2019;393(10186):2135–45. 10.1016/S0140-6736(19)30722-6. [DOI] [PubMed] [Google Scholar]
- 19. Lyttle MD, Rainford NEA, Gamble C, Messahel S, Humphreys A, Hickey H, et al. Levetiracetam versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (EcLiPSE): a multicentre, open-label, randomised trial. Lancet. 2019;393(10186):2125–34. 10.1016/S0140-6736(19)30724-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Riviello JJ Jr, Claassen J, LaRoche S, Sperling MR, Alldredge B, Bleck TP, et al. Treatment of status epilepticus: an international survey of experts. Neurocrit Care. 2013;18(2):193–200. 10.1007/s12028-012-9790-1. [DOI] [PubMed] [Google Scholar]
- 21. Chamberlain JM, Kapur J, Shinnar S, Elm J, Holsti M, Babcock L, et al. Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial [published correction appears in Lancet. Lancet. 2020;395(10231):1217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Min L, Chunyan W, Biaoxue R. Effects of valproic acid on skeletal metabolism in children with epilepsy: a systematic evaluation and meta-analysis based on 14 studies. BMC Pediatr. 2020;20(1):97. 10.1186/s12887-020-1984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Star K, Edwards IR, Choonara I. Valproic acid and fatalities in children: a review of individual case safety reports in VigiBase. PLoS One. 2014;9(10):e108970. 10.1371/journal.pone.0108970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kravljanac R, Djuric M, Jankovic B, Pekmezovic T. Etiology, clinical course and response to the treatment of status epilepticus in children: a 16-year single-center experience based on 602 episodes of status epilepticus. Eur J Paediatr Neurol. 2015;19(5):584–90. 10.1016/j.ejpn.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 25. Vossler DG, Bainbridge JL, Boggs JG, Novotny EJ, Loddenkemper T, Faught E, et al. Treatment of refractory convulsive status epilepticus: a comprehensive review by the American epilepsy society treatments committee. Epilepsy Curr. 2020;20(5):245–64. 10.1177/1535759720928269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qadir W, Wani KA, Bhat BA. Sociodemographic profile, semiology, and etiology of patients with status epilepticus: a study from a tertiary care hospital in north India. J Neurosci Rural Pract. 2018;9(4):487–91. 10.4103/jnrp.jnrp_102_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cobo NH, Sankar R, Murata KK, Sewak SL, Kezele MA, Matsumoto JH. The ketogenic diet as broad-spectrum treatment for super-refractory pediatric status epilepticus: challenges in implementation in the pediatric and neonatal intensive care units. J Child Neurol. 2015;30(2):259–66. 10.1177/0883073813516192. [DOI] [PubMed] [Google Scholar]
- 28. Ferlisi M, Shorvon S. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain. 2012;135(Pt 8):2314–28. 10.1093/brain/aws091. [DOI] [PubMed] [Google Scholar]
- 29. Dubey D, Pittock SJ, Kelly CR, McKeon A, Lopez-Chiriboga AS, Lennon VA, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83(1):166–77. 10.1002/ana.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arayakarnkul P, Chomtho K. Treatment options in pediatric super-refractory status epilepticus. Brain Dev. 2019;41(4):359–66. 10.1016/j.braindev.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 31. Meletti S, Monti G, Mirandola L, Vaudano AE, Giovannini G. Neuroimaging of status epilepticus. Epilepsia. 2018;59(Suppl 2):113–9. 10.1111/epi.14499. [DOI] [PubMed] [Google Scholar]
- 32. Mendes A, Sampaio L. Brain magnetic resonance in status epilepticus: a focused review. Seizure. 2016;38:63–7. 10.1016/j.seizure.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 33. Payne ET, Zhao XY, Frndova H, McBain K, Sharma R, Hutchison JS, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014;137(Pt 5):1429–38. 10.1093/brain/awu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abend NS, Arndt DH, Carpenter JL, Chapman KE, Cornett KM, Gallentine WB, et al. Electrographic seizures in pediatric ICU patients: cohort study of risk factors and mortality. Neurology. 2013;81(4):383–91. 10.1212/WNL.0b013e31829c5cfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sansevere AJ, Hahn CD, Abend NS. Conventional and quantitative EEG in status epilepticus. Seizure. 2019;68:38–45. 10.1016/j.seizure.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 36. Liukkonen E, Kantola-Sorsa E, Paetau R, Gaily E, Peltola M, Granström ML. Long-term outcome of 32 children with encephalopathy with status epilepticus during sleep, or ESES syndrome. Epilepsia. 2010;51(10):2023–32. 10.1111/j.1528-1167.2010.02578.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.