Abstract
Muscle wasting (sarcopenia) is one of the hallmarks of critical illness. Patients admitted to intensive care unit develop sarcopenia through increased protein catabolism, a decrease in protein syntheses, or both. Among the factors known to promote wasting are chronic inflammation and cytokine imbalance, insulin resistance, hypermetabolism, and malnutrition. Moreover, muscle wasting, known to develop in chronic kidney disease patients, is a harmful consequence of numerous complications associated with deteriorated renal function. Plenty of published data suggest that serum creatinine (SCr) reflects increased kidney damage and is also related to body weight. Based on the concept that urea and creatinine are nitrogenous end products of metabolism, the urea:creatinine ratio (UCR) could be applied but with limited clinical usability in case of kidney damage, hypovolemia, excessive, or protein intake, where UCR can be high and independent of catabolism. Recent data suggest that the sarcopenia index should be considered an alternative to serum creatinine. It is more reliable in estimating muscle mass than SCr. However, the optimal biomarker of catabolism is still an unresolved issue. The SCr is not a promising biomarker for renal function and muscle mass based on the influence of several factors. The present review highlights recent findings on the limits of SCr as a surrogate marker of renal function and the assessment modalities of nutritional status and muscle mass measurements.
Keywords: Creatinine, Intensive care, Metabolism, Nutrition, Skeletal muscle wasting
Key Points
-
•
Current data suggest that the optimal biomarker with acceptable sensitivity and specificity able to monitor catabolism is still an unresolved issue.
-
•
Although several conditions could lead to muscle wasting and subsequently affect the SCr concentration, SCr levels at the steady state could be used as a surrogate of muscle mass measurements.
-
•
New stratified approaches integrating clinical judgment, nutritional, and muscle assessment should be considered an alternative to the current “one-size-fits-all.”
-
•
Until such new biomarkers are identified, SCr should be better investigated according to the nutritional, muscle, and hydration status.
Introduction
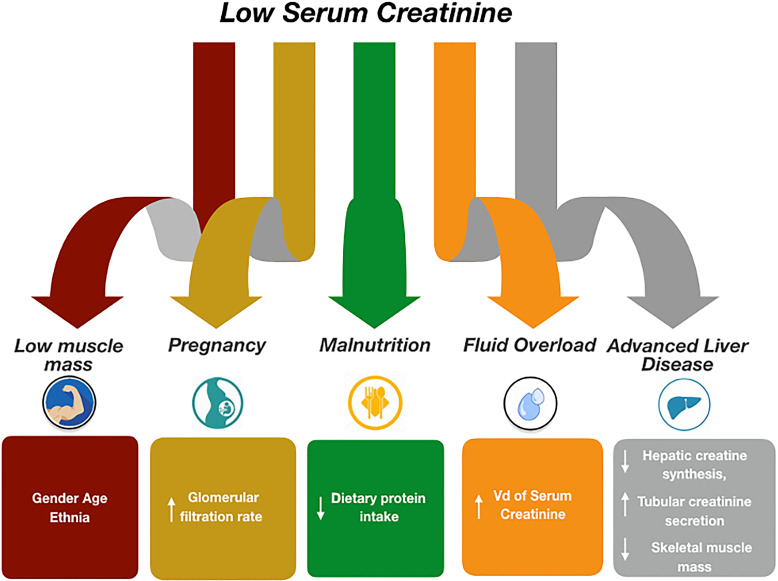
In recent years, particular emphasis has been placed on high resting energy expenditure, hyperglycaemia, abnormal utilization of substrates, and increased oxygen consumption that characterize this critical state [1–4]. On the contrary, less research was conducted on hypermetabolism and muscle biomarkers for a rapid assessment and progression of muscle damage in intensive care. The absence of an agreement in the literature about diagnosing hypermetabolism makes it difficult to compare studies [5, 6]. Indeed, even if critically ill patients are adequately nourished before the intensive care unit (ICU) admission, nutritional disorders can result from increased metabolic demands, fluid shifts, and loss of vitamins. Remarkably, this has led to the fact that in the absence of an appropriate nutritional and muscle mass assessment for adequate nutritional support, the catabolic process is associated with dramatic adverse events [7] and severe impairment of skeletal muscles [8]. Serum creatinine (SCr), used for the diagnosis of AKI, is also used as an indicator of muscle mass and could be significantly affected by the altered nutritional status and the wasting of skeletal muscle mass [9] (Fig. 1). The present review explores the literature that explicitly documents the current knowledge on SCr levels in critical care as muscle surrogates. We resume this review to highlight a reasoned path on the evidence in the literature relating to SCr as a muscle marker and its limitations as a renal marker. In addition, we will highlight the assessment modalities of nutritional status and muscle mass measurements. The present review was conducted by searching relevant articles on PubMed using the following research terms: Intensive Care, Serum Creatinine, Muscle Mass, Muscle Wasting, and Nutritional Status. The choice of the article was based on scientific rigor, sample size, mesh with inclusion terms, and recency of publication.
Fig. 1.
Factors associated with low serum creatinine levels.
“The Bad”: Acute Critical Illness, Catabolism, and Sarcopenia
Once the organism has lost its homeostasis, derangement leads to life-threatening organ dysfunction, requiring appropriate organ support techniques. Function capacity and deterioration of a pre-existing or new condition are definitions of critical illness characterized by high morbidity and mortality [10]. The disease’s unpredictable course is further associated with the development of multi-organ failure (MOF), in conjunction with other unavoidable aspects ICU-related, mechanical ventilation, bed rest, and sepsis [11]. The resulting condition, i.e., acute critical illness, is characterized by a neuro-endocrine and inflammatory response leading to amino acids mobilization from the skeletal muscle to synthesize acute phase protein and glucose. This catabolic response usually resolves when clinical conditions improve. Few patients, however, never enter the anabolic phase and remain catabolic for weeks [12–14]. Unfortunately, nutrition cannot suppress inflammatory changes associated with the catabolic state complicated by prolonged immobilization [15, 16]. As a result of catabolism, the resulting atrophy and weakness impair the outcome [15–17]. Moreover, muscle wasting starts early in the first week of critical illness (an amount of nearly 2% of skeletal muscle per day) [18], and patients with MOF lose more muscle mass than other patients [19]. Patients who develop muscle wasting have higher risk of ICU-acquired weakness [20], increased length of ICU stay [21], and loss of muscle mass during the first week of ICU stay, is associated with increased 60-day mortality [22].
“The Good”: Assess and Monitor Muscle Mass, Nutrition, and Physical Activity
Assessment of Muscle Mass and Nutritional Status
In the ICU, metabolic assessment of all critically ill patients upon admission and during hospitalization should be performed to assess muscle mass. Unfortunately, not only the clinician’s poor sensitivity to nutritional and metabolic problems but also the assessment tools are few and underused. In addition, sarcopenic obesity (excess fat and fluid retention of 10–20%) can hide muscle wasting [23]. Furthermore, edema and measured weight, BMI, and anthropometric measurements (mid-upper arm circumference and triceps skinfold thickness) may not represent the effective body muscle mass with limiting results [24, 25]. In addition, albumin is not a good marker of nutritional status due to changes in intravascular volume, infection, inflammation, and alteration in hepatic function [26].
Moreover, metabolism assessment tools [27] are not easy to perform, limiting the identification of patients at risk of malnutrition. Bioelectrical impedance vector analysis (BIA) allows the detection of tissue hydration and muscle mass variations [28, 29]. Therefore, a baseline muscle mass assessment in critically ill patients is limited (see Table 1). The BIA estimates hydration status about to fat-free mass (muscle mass in the limbs) [30], but, since patients in the ICU are often fluid overloaded and BIA indirectly measures muscle mass using electrical resistance, which is affected by fluid status, eventually BIA could fall in accuracy of monitoring and thus not be feasible [31]. Muscle ultrasound can offer qualitative analysis [32], with lower costs and excellent availability at the bedside. Puthucheary et al. [19] proposed using the rectus femoris cross-sectional area (RFcsa) as a replacement for muscle thickness measurements. In contrast, to measures of muscle atrophy based on muscle thickness, changes in RFcsa are directly correlated with changes in muscle strength in critically ill septic patients. A decrease of RFcsa ≥10% is considered significant and sufficient to affect muscle function. Some other authors demonstrated ultrasound to be accurate in muscle mass evaluation, and in the study by Nakanishi et al. [30], they found that muscle mass assessment by ultrasound was strongly correlated with computed tomography (r = 0.76–0.84, p ≤ 0.03). However, there has to be an international consensus on the methodology used yet [32]. Furthermore, although computed tomography scans are indicatively reliable for muscle mass, they are often not performed on all critically ill patients due to cost and radiation exposure and the need for them to be transferred to the examination room with some risks and the availability of dedicated personnel [33].
Table 1.
Summary of the most used tools for muscle mass assessment
| Tool | Assessment | Strength | Limitation |
|---|---|---|---|
| Hand grip strength | On the dominant hand measured using a handgrip dynamometer | • Qualitative assessment of strength in ICU patients | • Requires patient to be cooperative |
| • Strong correlation with BIA | |||
| Body mass index (BMI, kg/m2) | LBM estimation | • Low values of BMI may indicate protein-energy malnutrition | • Does not discriminate between the lean (muscle mass) and fat or water body mass |
| • Decreased sensitivity and specificity as a marker of poor nutritional status | |||
| • Direct correlation with mortality | |||
| Muscle biopsy | Provides histological measurement of the muscle fibers, cellular infiltrates | • Gold standard for diagnosis | • Invasive |
| Bio impedence analysis (BIA) | Indirectly estimates muscle mass using electrical resistance | • Non invasive | • Resistance is affected by fluid status, thus BIA could not be accurate in monitor the change of muscle mass |
| • Could be used for whole-body muscle mass assessment at one point | |||
| • Difference among available devices | |||
| Ultrasound (US) | Measurement of the rectus femoris quadriceps muscle, and biceps brachii taken for cross-sectional area (CSA) or thickness | • Easy to use | • Variability in the measurement technique to quantify muscle mass: CSA versus thickness |
| • Risk of underestimation of muscle mass | |||
| • Reliable | • Need of measurement skill to avoid misinterpretation | ||
| • Lack of standard approach | |||
| • Available at bedside | |||
| Computed tomography (CT) | Measurement of skeletal muscle cross-sectional area at the third vertebrae (L3) level and the cross-sectional area of the femoral muscle volume using sagittal direction integration | • Can separate muscle, fat, and other tissues | • Requires transferring patients in the CT scan room and dedicated trained staff |
| • Radiation exposure | |||
| • CT is considered as an accurate and precise method for muscle mass assessment | • Time consuming | ||
| Anthropometry | Body weight in kilograms (kg) trunk length (cm) measured as distance between the right shoulder and a point 5 cm above the right greater trochanter | • Easy to measure | • Underestimation of muscle mass |
| Mid-thigh, knee, and above-ankle circumferences measured on the right side of the body | |||
| The body cell mass (BCM) in kg calculated using the formula: (0.266*height [cm]) + (0.287*leg circumference [cm]) +(0.305 *Dweight [kg]) – (0.406* truck length [cm]) −13.52. Dweight is the difference between ICU and post-ICU | • Non invasive | • Not done routinely | |
| Triceps skinfold thickness measured (in mm) using callipers with the right elbow flex and right hand touching the left shoulder, at the midpoint between the acromion and olecranon process in the posterior surface of the right arm |
Effects of Physical Activity, Nutrition (Arginine, Glycine, Creatine) on Serum Creatinine
Physical activity (PA) is generally considered important in regulating health. Considering the general population, muscle mass is variable among the elderly [34] and children [35, 36] and can be modified by physical exercise. Baxmann et al. [37] recruited 170 healthy volunteers classified into two groups based on PA. The authors found a significant correlation between SCr and urinary creatinine and body weight, but the level of correlation with lean mass was even more remarkable. No significant correlation was found between body weight, fat-free mass, or body cell mass and serum cystatin [38]. The group with moderate/intense PA presented a significantly higher mean SCr and higher urinary creatinine. However, Bharakhada et al. [39] showed that low PA is associated with a higher risk for higher SCr.
Nutrition can influence the urinary excretion of creatinine by:
• Proteins with arginine and glycine that are precursors of creatine and guanidinoacetate production.
• Creatine (Cr) causes an increase in urinary excretion of creatinine; short frame times of supplementation with higher doses of Cr (20–30 g/day over 5–7 days) can improve work performance.
Biomarkers
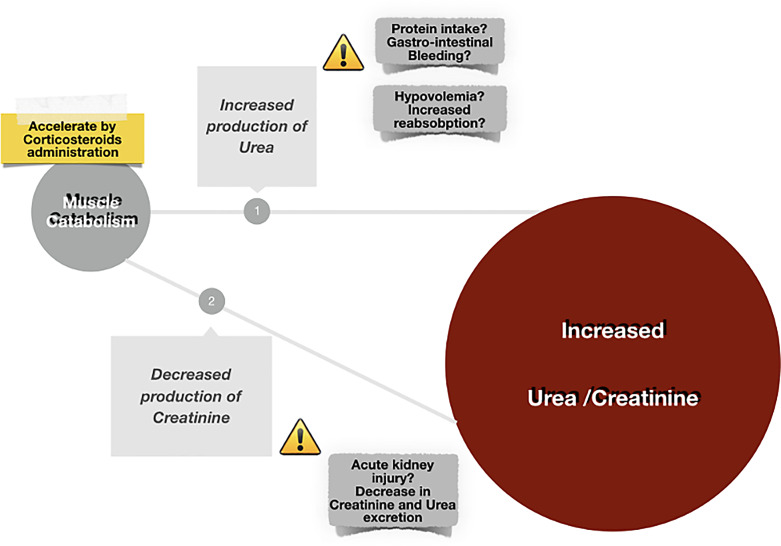
Currently, there is no specific and appropriate biomarker for catabolism monitoring. Although assessing nitrogen losses is complex, critically ill patients present muscle loss and weakness as an expression of catabolism severity. Skeletal muscle plays a crucial role in generating SCr [39], a metabolite of creatine phosphate, which is generated at a constant rate in healthy subjects [40, 41]. A reduction in muscle mass could determine an increase in urea generation [37, 42–44] and a decrease in SCr levels. Based on the direct correlation between muscle mass and SCr levels, the latter has been used to indicate muscle mass [45]. Additionally, individuals with lower muscle mass have a much lower SCr generation that may be constitutional or disease-related [16]. In critically ill patients, while the SCr levels decrease, the urea progressively rises from three to 4 days after admission to ICU, with a high peak and duration in patients with longer ICU length of stay. A persistent urea level may indicate an increased muscle catabolism and metabolism [46]. Elevated urea:creatinine ratio is an expression of constant muscle wasting and could be considered a metabolic alert [47–49]. Unfortunately, hypovolemia, protein intake, and AKI are limited by clinical usability based on the increased urea:creatinine ratio independent of catabolism (Fig. 2). Recently, Kashani et al. [50] proposed the sarcopenia index, defined as (SCr value/serum cystatin C) ×100, to find patients with decreased muscle mass. This index correlates well with imaging and superior performance compared to only SCr [50, 51]. More extensive prospective multicentre studies are needed to evaluate the associations between sarcopenia index levels and clinical outcomes.
Fig. 2.
The UCR in critical illness.
Patients with chronic kidney disease (CKD), including end-stage kidney disease, experience an increased wasting, malnutrition, and inflammation associated to decreased body stores of protein and energy availability, so called “protein-energy wasting” [52]. Metabolic acidosis, together with uremia, proinflammatory cytokines, endocrine disorders, and the dialysis procedure, accelerates protein catabolism induced by metabolic acidosis that may lead to lean mass degradation and sarcopenia in patients with CKD or ESRD [53]. The need for additional biomarkers capable of detecting the sarcopenia process early and precisely in chronic patients is evident to avoid delays in diagnosis recognition, and more specifically, in treatments. Among the most promising biomarkers involved it must be counted.
Brain-derived neurotrophic factor (BDNF) has been investigated among neuromuscular junction and neuro-inflammation biochemical markers. Miyazaki et al. [54] showed that plasma BDNF level was significantly lower in patients with severe sarcopenia or frailty and that a lower BDNF level was correlated with deteriorated physical functions. Lower BDNF was also correlated with more extended dialysis periods.
C-terminal agrin fragment (CAF) has been investigated as a neuromuscular junction – a related biomarker of muscle dysfunction [55–58]. Interestingly, also renal function has been linked to raised levels of circulating CAF since this biomarker has been reported to be higher in patients developing acute kidney injury and undergoing kidney transplantation [55]. Reports showed CAF to have a high predictive power in determining rapid kidney function decline in patients affected by CKD, and – above that – CAF was cross-sectionally correlated to appendicular lean mass, handgrip, and gait speed from estimated glomerular filtration rate (GFR) [56].
Moreover, CAF increases with age and in sarcopenic individuals when compared with peers’ age, non-sarcopenic patients [57]. In addition, CAF was higher than controls under other muscle wasting conditions, such as diabetes, COPD, chronic heart failure, and stroke, and in pancreatic and colorectal cancer cachectic patients [58].
Liver-type fatty acid-binding protein (L-FABP), a 14-kDa protein, participates in intracellular fatty acid homeostasis in several tissues and organs, including the liver, kidney, muscle, and other tissues. Urinary liver-type fatty acid-binding protein (L-FABP) has been shown accurate in detecting the degree of tubulointerstitial damage. Indeed, many studies have focused on the involvement of CKD in the onset of sarcopenia which is characterized by decreased skeletal muscle mass and strength (i.e., decreases in both muscle weight and cross-sectional areas of type I and IIb fibers) [59]. Type 2 diabetes (T2D) with insulin resistance represents a common risk factor for diabetic kidney disease and sarcopenia. Sarcopenia progression in T2D patients has been associated with lower renal function. As a marker of the severity of kidney disease, urinary L-FABP might be helpful for the detection of renal hemodynamics dysfunction, aggravated by sarcopenia, and glomerular sclerosis, leading to renal hypoxia derived from reduction of postglomerular blood flow [59]. Despite the emerging data on the biomarkers mentioned above, including the biomarkers above in clinical practice and their application to humans, they need further elucidation.
“The SCr” Adequacy of Serum Creatinine as a Marker of Renal Injury
Although an optimal biomarker able to timely assess structural kidney damage does not exist in critical care, the most used to evaluate functional impairment is SCr. The SCr is an endogenous substance generated by the non-enzymatic conversion of creatine and phosphate [60]. In addition, SCr is an uncharged, small molecular weight unfilled substance (113 Da) unrelated to whey protein and is filtered freely by the glomerulus without tubular resorption. SCr is also secreted by the kidney tubules, although only in small quantities. Accurate renal function evaluation is necessary to diagnose and treat kidney diseases, adjust drug dosages, and decide when to initiate renal replacement therapy (RRT) [61]. During the inflammatory state, acute loss of kidney homeostatic function plays a central role in worsening the critical illness’s dysmetabolic state. Low levels of SCr could be considered an indicator of protein-energy use in specific situations and could be influenced by diet [62]. Notably, low dietary protein intake can limit the generation of SCr. However, factors related to low SCr levels are female gender, advanced age, ethnic background, protein malnutrition, diet, pregnancy (high GFR), advanced liver disease (decreased creatinine production), fluid overload (increased volume of distribution of SCr), and augmented renal clearance (Fig. 1), [63]. On the other hand, an elevation in SCr usually reflects a decrease in GFR and is associated with a concomitant increase in urea and blood urea nitrogen. However, SCr may also increase acutely independently of a decrease in GFR and, therefore, no real change in overall kidney function including decreased creatinine secretion, interference with serum assay, or increased creatinine production [64].
Many critically ill patients undergoing RRT or pre-existing CKD are also treated in the ICU. In these patients, it is noteworthy to evaluate the issue of nutrition and anthropometrics other than the SCr marker [65]. Disorders like enteropathies and nephrotic syndrome impact muscle mass and creatinine production [9].
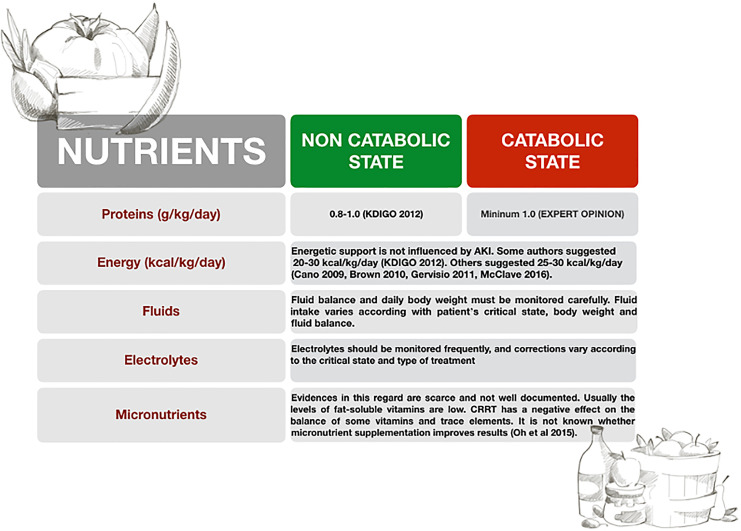
AKI commonly complicates and contributes to MOF. In addition, AKI patients’ metabolic and nutritional demands are affected by the uremic state, underlying pathology, and associated complications. Therefore, a specific and personalized approach for each patient is necessary to improve the outcome of these patients (Fig. 3).
Fig. 3.
Nutritional support in AKI patients.
Standardized definitions of AKI have enabled a better evaluation of the epidemiology of ICU‐acquired AKI [66]. However, there is still controversy in the literature regarding the diagnosis of AKI based on a joint approach to baseline serum creatinine (bSCr) concentration that influenced the creatinine‐based AKI definitions. Remarkably, this concept is also complicated by pre‐existing undiagnosed CKD in most of the population and the unknown baseline renal function status in patients affected by AKI. Muscle wasting can compromise a rapid recognition of AKI, affecting the actual value of the SCr in patients with unrecognized ICU‐acquired myopathy [37]. Theoretically, SCr may be falsely increased in individuals with higher muscle mass and normal renal function [67, 68]. Different equations used to predict GFR do not include parameters of body composition. Elevated GFR may not reflect the renal function in patients with atypically decreased muscle mass (elderly, amputees, and chronic muscle disease patients) [4]. The re-expressed four-variable MDRD [69] equation estimates GFR adjusted for body surface area based on serum creatinine, age, gender, and race. As for the Cockcroft-Gault formula, muscle mass is not considered. Therefore, in individuals with high creatinine production and higher muscle mass, an underestimation of MDRD is expected [69].
Considering what has been explained above, sCr is inaccurate at detecting mild renal impairment and can vary with muscle mass but little (aside from bodybuilders) with protein intake. Urea levels might change with protein intake and are also urine flow rate dependent [70]. Several studies have compared cystatin C (CysC) – and creatinine-based prediction equations for estimated GFR (eGFR) to a gold standard [71]. CysC is a non-glycosylated protein with cysteine proteinase inhibitor activity, with a constant production rate by all nucleated cells. GFR determines the CysC serum level, and the concentration is not influenced much by age, gender, muscle mass, infections, and inflammatory or liver diseases [71].
Particularly, studies reported that CysC-based prediction equations were more precise and could benefit the earlier detection of renal impairment in specific subpopulations. In patients with reduced muscle mass because of amputations or in those with degenerative muscle disease, also in both children and obese patients, CysC might assess renal function [72, 73].
Despite several studies demonstrated, the superiority of serum CysC in comparison with creatinine in the detection of minor GFR reduction data regarding the validity of CysC as a marker of renal function in ICU patients are often inconclusive and most studied populations were small too.
Adequacy of Serum Creatinine as a Marker of Muscle Mass
Human body mass is divided into fat mass and lean mass (fat-free), which includes body cell mass, bone mass, and extracellular water. Skeletal muscle is a storage depot of amino acids and the regulator of immune function, glucose disposal, protein synthesis, and mobility [4, 68, 69, 74] that can decrease due to the requirements of other tissues. Significant muscle wasting can decrease protein storage in the first 7–20 days of ICU stay, predisposing to a relative glutamine deficiency [4, 68, 69, 75], despite adequate caloric and protein intake [76].
Loss of muscle mass is associated with a decrease in ventilator-free days and an increase in ICU length of stay and mortality [77, 78]. In addition, high mortality has been found to be associated with low admission SCr values and low muscle mass in ICU patients [79, 80].
However, frequent critical ill increase after discharge from the ICU, as fat mass but not functional lean body mass (LBM) [23]. The catabolic/hypermetabolic state injury-related persists at least 2 years after the discharge from the hospital, significantly hampering LBM recovery and function following an injury [45]. In clinical practice, as previously described, SCr is not only used to determine either AKI or CKD [6, 7] but also as an indicator of muscle mass. In healthy subjects, the contribution of LBM to the variation in serum creatinine is small [81].
Renal Replacement Therapies on Muscle Mass as a Catabolic State
RRT, whichever modality is used, also profoundly affects metabolism. Data on patients with CKD receiving RRT show that loss of LBM in patients on maintenance hemodialysis is strongly associated with increased mortality. Involuntary loss (5% of body weight) leads to significant morbidity and mortality. Nutritional recommendations for this population provide abundant protein (1.0–1.2 g/kg/day) and calories (30–35 g/kg/day) to maintain or build muscle mass [82].
However, in the acute care setting, patients undergoing CRRT are subjected to extreme metabolic stress and are in a catabolic state. Macronutrients (small proteins and peptides) and micronutrients (amino acids, glucose, trace elements, water-soluble vitamins, etc.) are cleared from the patient’s blood into the blood effluent [83]. During this catabolic state, amino acid losses peak at 1.3–1.8 g/kg/day, while in severe AKI in RRT, amino acid losses through RRT can be even higher, up to 15 g/day [84, 85]. An increased supply of protein and amino acids during the nutritional intervention can counterbalance and even match the nitrogen losses. However, it can neither reverse the catabolic process nor enhance it. Although in critical illness, the combination of both consumption and degradation of proteins leads to a negative nitrogen balance, when RRT starts, the high rate of amino acid loss causes some non-essential amino acids to become essential [85]. Critically ill patients with AKI on CRRT who achieved a positive nitrogen balance had better hospital outcomes [86].
Conclusion
Based on the current data from relevant studies, several conditions could lead to muscle wasting and subsequently affect the SCr concentration; as a result, SCr may appear to be low or average despite renal damage. Consequently, the sensitivity of SCr for staging AKI is compromised. Our practical consideration/suggestion on the use of SCr in clinical practice is resumed in Figure 4. We expect that, in the coming years, more attention will be focused on the need for an effective strategy that will permit to identify the effect of nutrition and muscle wasting on accurate SCr concentration, in association to adequate and inexpensive tools for assessing muscle mass, able to confirm or disprove SCr meaning for each ICU patients. This can be used to optimize the management of the insane, alongside using 6D’s Framework of Nutritional Stewardship in critical care, as recent evidence suggests [87].
Fig. 4.
Practical consideration on the use of serum creatinine.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research received no external funding.
Author Contributions
Conceptualization, S.D.R; writing—review and editing, S.D.R., M.G., M.R., and M.G.A. All authors have read and agreed to the published version of the manuscript.
Funding Statement
This research received no external funding.
References
- 1. Fontaine E, Müller MJ. Adaptive alterations in metabolism: practical consequences on energy requirements in the severely ill patient. Curr Opin Clin Nutr Metab Care. 2011;14(2):171–5. 10.1097/MCO.0b013e328342bad4. [DOI] [PubMed] [Google Scholar]
- 2. Schulman RC, Mechanick JI. Metabolic and nutrition support in the chronic critical illness syndrome. Respir Care. 2012;57(6):958–77; discussion 977–8. 10.4187/respcare.01620. [DOI] [PubMed] [Google Scholar]
- 3. Terao Y, Miura K, Saito M, Sekino M, Fukusaki M, Sumikawa K. Quantitative analysis of the relationship between sedation and resting energy expenditure in postoperative patients. Crit Care Med. 2003;31(3):830–3. 10.1097/01.CCM.0000054868.93459.E1. [DOI] [PubMed] [Google Scholar]
- 4. Griffiths RD. Muscle mass, survival, and the elderly ICU patient. Nutrition. 1996;12(6):456–8. 10.1016/s0899-9007(96)00141-4. [DOI] [PubMed] [Google Scholar]
- 5. Frankenfield DC, Smith JS Jr, Cooney RN, Blosser SA, Sarson GY. Relative association of fever and injury with hypermetabolism in critically ill patients. Injury. 1997 Nov–Dec;28(9–10):617–21. 10.1016/s0020-1383(97)00117-4. [DOI] [PubMed] [Google Scholar]
- 6. Dev R, Hui D, Chisholm G, Delgado-Guay M, Dalal S, Del Fabbro E, et al. Hypermetabolism and symptom burden in advanced cancer patients evaluated in a cachexia clinic. J Cachexia Sarcopenia Muscle. 2015 Mar;6(1):95–8. 10.1002/jcsm.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barr J, Hecht M, Flavin KE, Khorana A, Gould MK. Outcomes in critically ill patients before and after the implementation of an evidence-based nutritional management protocol. Chest. 2004;125(4):1446–57. 10.1378/chest.125.4.1446. [DOI] [PubMed] [Google Scholar]
- 8. Ziegler TR. Parenteral Nutrition in the critically ill patient. N Engl J Med. 2009;361(11):1088–97. 10.1056/NEJMct0806956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thomas ME, Blaine C, Dawnay A, Devonald MA, Ftouh S, Laing C, et al. The definition of acute kidney injury and its Use in practice. Kidney Int. 2015 Jan;87(1):62–73. 10.1038/ki.2014.328. [DOI] [PubMed] [Google Scholar]
- 10. Fan E, Cheek F, Chlan L, Gosselink R, Hart N, Herridge MS, et al. An official American Thoracic Society Clinical Practice guideline: the diagnosis of intensive care unit-acquired weakness in adults. Am J Respir Crit Care Med. 2014;190(12):1437–46. https://doi. org/10. 1164/rccm.201411- 2011ST. [DOI] [PubMed] [Google Scholar]
- 11. Weijs PJ, Looijaard WG, Dekker IM, Stapel SN, Girbes AR, Oudemans-van Straaten HM, et al. Low skeletal muscle area is a risk factor for mortality inmechanically ventilated critically ill patients. Crit Care. 2014;18(2):R12. 10.1186/cc13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iwashyna TJ, Hodgson CL, Pilcher D, Bailey M, van Lint A, Chavan S, et al. Timing of onset and burden of persistent critical illness in Australia and New Zealand: a retrospective, population-based, observational study. Lancet Respir Med. 2016;4(7):566–73. 10.1016/S2213-2600(16)30098-4. [DOI] [PubMed] [Google Scholar]
- 13. Bagshaw SM, Stelfox HT, Iwashyna TJ, Bellomo R, Zuege D, Wang X. Timing of onset of persistent critical illness: a multicentre retrospective cohort study. Intensive Care Med. 2018;44(12):2134–44. 10.1007/s00134-018-5440-1. [DOI] [PubMed] [Google Scholar]
- 14. Iwashyna TJ, Viglianti EM. Patient and population-level approaches to persistent critical illness and prolonged intensive care unit stays. Crit Care Clin. 2018 Oct;34(4):493–500. 10.1016/j.ccc.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014 Apr 24;370(17):1626–35. 10.1056/nejmra1209390. [DOI] [PubMed] [Google Scholar]
- 16. Van den Berghe G. On the neuroendocrinopathy of critical illness. Perspectives for feeding and novel treatments. Am J Respir Crit Care Med. 2016 Dec 1;194(11):1337–48. 10.1164/rccm.201607-1516CI. [DOI] [PubMed] [Google Scholar]
- 17. Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care. 2015 Aug 5;19(1):274. 10.1186/s13054-015-0993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fazzini B, Märkl T, Costas C, Blobner M, Schaller SJ, Prowle J, et al. The rate and assessment of muscle wasting during critical illness: a systematic review and meta-analysis. Crit Care. 2023;27(1):2. 10.1186/s13054-022-04253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–600. 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 20. Mayer KP, Thompson Bastin ML, Montgomery-Yates AA, Pastva AM, Dupont-Versteegden EE, Parry SM, et al. Acute skeletal muscle wasting and dysfunction predict physical disability at hospital discharge in patients with critical illness. Crit Care. 2020;24:637–12. 10.1186/s13054-020-03355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Biolo G, Fleming RYD, Maggi SP, Nguyen TT, Herndon DN, Wolfe RR. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J Clin Endocrinol Metab. 2002;87(7):3378–84. 10.1210/jcem.87.7.8699. [DOI] [PubMed] [Google Scholar]
- 22. Lee ZY, Ong SP, Ng CC, Yap CSL, Engkasan JP, Barakatun-Nisak MY, et al. Association between ultrasound quadriceps muscle status with premorbid functional status and 60-day mortality in mechanically ventilated critically ill patient: a single-center prospective observational study. Clin Nutr. 2021;40(3):1338–47. 10.1016/j.clnu.2020.08.022. [DOI] [PubMed] [Google Scholar]
- 23. Reid CL, Campbell IT, Little RA. Muscle wasting and energy balance in critical illness. Clin Nutr. 2004;23:273–80. 10.1016/S0261-5614(03)00129-8. [DOI] [PubMed] [Google Scholar]
- 24. Manning EM, Shenkin A. Nutritional assessment in the critically ill. Crit Care Clin. 1995;11(3):603–34. 10.1016/s0749-0704(18)30055-1. [DOI] [PubMed] [Google Scholar]
- 25. Kuzuya M, Izawa S, Enoki H, Okada K, Iguchi A. Is serum albumin a good marker for malnutrition in the physically impaired elderly? ClinNutr. 2007;26(1):84–90. 10.1016/j.clnu.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 26. Kalaiselvan MS, Renuka MK, Arunkumar AS. Use of nutrition risk in critically ill (NUTRIC) score to assess nutritional risk in mechanically ventilated patients: a prospective observational study. Indian J Crit Care Med. 2017;21(5):253–6. 10.4103/ijccm.IJCCM_24_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Foster KR, Lukaski HC. Whole-body impedance: what does it measure? Am J Clin Nutr. 1996;64(3 Suppl l):388s–96s. 10.1093/ajcn/64.3.388S. [DOI] [PubMed] [Google Scholar]
- 28. Naranjo-Hernandéz D, Reina-Tosina J, Min M. Fundamentals, recent advances, and future challenges in bioimpedance devices for healthcare applications. J Sensors. 2019;2019:1–42. 10.1155/2019/9210258. [DOI] [Google Scholar]
- 29. Mulasi U, Kuchnia AJ, Cole AJ, Earthman CP. Bioimpedance at the bedside: current applications, limitations, and opportunities. Nutr Clin Pract. 2015;30(2):180–93. 10.1177/0884533614568155. [DOI] [PubMed] [Google Scholar]
- 30. Puthucheary ZA, Phadke R, Rawal J, McPhail MJ, Sidhu PS, Rowlerson A, et al. Qualitative ultrasound in acute critical illness muscle wasting. Crit Care Med. 2015 Aug;43(8):1603–11. 10.1097/CCM.0000000000001016. [DOI] [PubMed] [Google Scholar]
- 31. Nakanishi N, Tsutsumi R, Okayama Y, Takashima T, Ueno Y, Itagaki T, et al. Monitoring of muscle mass in critically ill patients: comparison of ultrasound and two bioelectrical impedance analysis devices. J Intensive Care. 2019;7:61. 10.1186/s40560-019-0416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohkawa S, Odamaki M, Yoneyama T, Hibi I, Miyaji K, Kumagai H. Standardized thigh muscle area measured by computed axial tomography as an alternate muscle mass index for nutritional assessment of hemodialysis patients. Am J Clin Nutr. 2000;71(2):485–90. 10.1093/ajcn/71.2.485. [DOI] [PubMed] [Google Scholar]
- 33. Goldberg TH, Finkelstein MS. Difficulties in estimating glomerular filtration rate in the elderly. Arch Intern Med. 1987 Aug;147(8):1430–3. 10.1001/archinte.1987.00370080066014. [DOI] [PubMed] [Google Scholar]
- 34. Brion LP, Boeck MA, Gauthier B, Nussbaum MP, Schwartz GJ. Estimation of glomerular filtration rate in anorectic adolescents. Pediatr Nephrol. 1989 Jan;3(1):16–21. 10.1007/BF00859618. [DOI] [PubMed] [Google Scholar]
- 35. Seikaly MG, Browne R, Bajaj G, Arant BS Jr. Limitations to body length/serum creatinine ratio as an estimate of glomerular filtration in children. Pediatr Nephrol. 1996 Dec;10(6):709–11. 10.1007/s004670050195. [DOI] [PubMed] [Google Scholar]
- 36. Slentz CA, Duscha BD, Johnson JL, Ketchum K, Aiken LB, Samsa GP, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE–a randomized controlled study. Arch Intern Med. 2004 Jan 12;164(1):31–9. 10.1001/archinte.164.1.31. [DOI] [PubMed] [Google Scholar]
- 37. Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3(2):348–54. 10.2215/CJN.02870707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bharakhada N, Yates T, Davies MJ, Wilmot EG, Edwardson C, Henson J, et al. Association of sitting time and physical activity with CKD: a cross-sectional study in family practices. Am J Kidney Dis. 2012;60(4):583–90. 10.1053/j.ajkd.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 39. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000 Jul;80(3):1107–213. 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 40. Convertino VA. Effects of exercise and inactivity on intravascular volume and cardiovascular control mechanisms. Acta Astronaut. 1992 Jul;27:123–9. 10.1016/0094-5765(92)90188-o. [DOI] [PubMed] [Google Scholar]
- 41. Coker RH, Wolfe RR. Bedrest and sarcopenia. Curr Opin Clin Nutr Metab Care. 2012 Jan;15(1):7–11. 10.1097/MCO.0b013e32834da629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bankir L. Urea and the kidney. In: Brenner BM, editor. The kidney. 1996. Philadelphia: WB Saunders. p. 571–606. [Google Scholar]
- 43. Scrimshaw NS. Effect of infection on nutrient requirements. Am J Clin Nutr. 1977 Sep;30(9):1536–44. 10.1093/ajcn/30.9.1536. [DOI] [PubMed] [Google Scholar]
- 44. Weitzman RE, Kleeman CR. The clinical physiology of water metabolism– Part III: the water depletion (hyperosmolar) and water excess (hyperosmolar) syndromes. West J Med. 1980;132:16–8. [PMC free article] [PubMed] [Google Scholar]
- 45. Schutte JE, Longhurst JC, Gaffney FA, Bastian BC, Blomqvist CG. Total plasma creatinine: an accurate measure of total striated muscle mass. J Appl Physiol Respir Environ Exerc Physiol. 1981;51(3):762–6. 10.1152/jappl.1981.51.3.762. [DOI] [PubMed] [Google Scholar]
- 46. Puthucheary ZA, Astin R, McPhail MJW, Saeed S, Pasha Y, Bear DE, et al. Metabolic phenotype of skeletal muscle in early critical illness. Thorax. 2018;73(10):926–35. 10.1136/thoraxjnl-2017-211073. [DOI] [PubMed] [Google Scholar]
- 47. Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–93. 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 48. Kyhse-Andersen J, Schmidt C, Nordin G, Andersson B, Nilsson-Ehle P, Lindström V, et al. Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin Chem. 1994;40(10):1921–6. 10.1093/clinchem/40.10.1921. [DOI] [PubMed] [Google Scholar]
- 49. Shlipak MG, Mattes MD, Peralta CA. Update on cystatin C: incorporation into clinical practice. Am J Kidney Dis. 2013;62(3):595–603. 10.1053/j.ajkd.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kashani KB, Frazee EN, Kukrálová L, Sarvottam K, Herasevich V, Young PM, et al. Evaluating muscle mass by using markers of kidney function: development of the sarcopenia index. Crit Care Med. 2017;45(1):e23–9. 10.1097/CCM.0000000000002013. [DOI] [PubMed] [Google Scholar]
- 51. Andrews R, Greenhaff P, Curtis S, Perry A, Cowley AJ. The effect of dietary creatine supplementation on skeletal muscle metabolism in congestive heart failure. Eur Heart J. 1998;19(4):617–22. 10.1053/euhj.1997.0767. [DOI] [PubMed] [Google Scholar]
- 52. Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–8. 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 53. Kittiskulnam P, Chertow GM, Carrero JJ, Delgado C, Kaysen GA, Johansen KL. Sarcopenia and its individual criteria are associated, in part, with mortality among patients on hemodialysis. Kidney Int. 2017;92(1):238–47. 10.1016/j.kint.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Miyazaki S, Iino N, Koda R, Narita I, Kaneko Y. Brain-derived neurotrophic factor is associated with sarcopenia and frailty in Japanese hemodialysis patients. Geriatr Gerontol Int. 2021;21(1):27–33. 10.1111/ggi.14089. [DOI] [PubMed] [Google Scholar]
- 55. Arampatzis S, Chalikias G, Devetzis V, Konstantinides S, Huynh-Do U, Tziakas D. C-terminal fragment of agrin (CAF) levels predict acute kidney injury after acute myocardial infarction. BMC Nephrol. 2017;18(1):202. 10.1186/s12882-017-0611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lorenz G, Hettwer S, McCallum W, Angermann S, Wen M, Schmaderer C, et al. Plasma C-terminal agrin fragment and rapid kidney function decline in chronic kidney disease patients. Medicine. 2019;98(19):e15597. 10.1097/MD.0000000000015597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Drey M, Sieber CC, Bauer JM, Uter W, Dahinden P, Fariello RG, et al. C-terminal Agrin Fragment as a potential marker for sarcopenia caused by degeneration of the neuromuscular junction. Exp Gerontol. 2013;48(1):76–80. 10.1016/j.exger.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 58. Steinbeck L, Ebner N, Valentova M, Bekfani T, Elsner S, Dahinden P, et al. Detection of muscle wasting in patients with chronic heart failure using C-terminal agrin fragment: results from the studies investigating comorbidities aggravating heart failure (SICA-HF). Eur J Heart Fail. 2015;17(12):1283–93. 10.1002/ejhf.400. [DOI] [PubMed] [Google Scholar]
- 59. Tanabe J, Ogura Y, Kosaki K, Nagai Y, Sugaya T, Ohata K, et al. Relationship between urinary liver-type fatty acid-binding protein (L-FABP) and sarcopenia in spontaneously diabetic torii fatty rats. J Diabetes Res. 2020 Jan 13;2020:7614035. 10.1155/2020/7614035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lawson N, Lang T, Broughton A, Prinsloo P, Turner C, Marenah C. Creatinine assays: time for action? Ann Clin Biochem. 2002 Nov;39(Pt 6):599–602. 10.1177/000456320203900609. [DOI] [PubMed] [Google Scholar]
- 61. Park J, Mehrotra R, Rhee CM, Molnar MZ, Lukowsky LR, Patel SS, et al. Serum creatinine level, a surrogate of muscle mass, predicts mortality in peritoneal dialysis patients. Nephrol Dial Transplant. 2013;28(8):2146–55. 10.1093/ndt/gft213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Assy N, Kayal M, Mejirisky Y, Gorenberg M, Hussein O, Schlesinger S. The changes in renal function after a single dose of intravenous furosemide in patients with compensated liver cirrhosis. BMC Gastroenterol. 2006 Nov 29;6:39. 10.1186/1471-230X-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. De Waele JJ, Dumoulin A, Janssen A, Hoste EA. Epidemiology of augmented renal clearance in mixed ICU patients. Minerva Anestesiol. 2015 Oct;81(10):1079–85. [PubMed] [Google Scholar]
- 64. Demirjian S, Bashour CA, Shaw A, Schold JD, Simon J, Anthony D, et al. Predictive accuracy of a perioperative laboratory test-based prediction model for moderate to severe acute kidney injury after cardiac surgery. JAMA. 2022 Mar 8;327(10):956–64. 10.1001/jama.2022.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero JJ, Chan W, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. 2020 Sep;76(3 Suppl 1):S1–S107. 10.1053/j.ajkd.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 66. Koyner JL. Assessment and diagnosis of renal dysfunction in the ICU. Chest. 2012 Jun;141(6):1584–94. 10.1378/chest.11-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Odden MC, Shlipak MG, Tager IB. Serum creatinine and functional limitation in elderly persons. J Gerontol A Biol Sci Med Sci. 2009 Mar;64(3):370–6. 10.1093/gerona/gln037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wischmeyer PE. Are we creating survivors, or victims in critical care? Delivering targeted Nutrition to improve outcomes. CurrOpinCrit Care. 2016 Aug;22(4):279–84. 10.1097/MCC.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 69. Levey AS, Coresh J, Greene T. Expressing the MDRD study equation for estimating GFR with IDMS traceable (Gold Standard) serum creatinine values [Abstract]. J Am Soc Nephrol. 2005;16:69A. [Google Scholar]
- 70. Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008 Mar;3(2):348–54. 10.2215/CJN.02870707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Poventud-Fuentes I, Garnett E, Akcan-Arikan A, Devaraj S. Comparison of cystatin C and creatinine-based equations with measured glomerular filtration rate in a diverse pediatric population. J Appl Lab Med. 2022 Sep 1;7(5):1016–24. 10.1093/jalm/jfac043. [DOI] [PubMed] [Google Scholar]
- 72. Lin YL, Chen SY, Lai YH, Wang CH, Kuo CH, Liou HH, et al. Serum creatinine to cystatin C ratio predicts skeletal muscle mass and strength in patients with non-dialysis chronic kidney disease. Clin Nutr. 2020 Aug;39(8):2435–41. 10.1016/j.clnu.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 73. Nateghi Haredasht F, Viaene L, Vens C, Callewaert N, De Corte W, Pottel H. Comparison between cystatin C- and creatinine-based estimated glomerular filtration rate in the follow-up of patients recovering from a stage-3 AKI in ICU. J Clin Med. 2022 Dec 7;11(24):7264. 10.3390/jcm11247264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lightfoot A, McArdle A, Griffiths RD. Muscle in defense. Crit Care Med. 2009;37(10 Suppl l):S384–90. 10.1097/CCM.0b013e3181b6f8a5. [DOI] [PubMed] [Google Scholar]
- 75. Annetta MG, Pittiruti M, Silvestri D, Grieco DL, Maccaglia A, La Torre MF, et al. Ultrasound assessment of rectus femoris and anterior tibialis muscles in young trauma patients. Ann Intensive Care. 2017 Oct;7(1):104. 10.1186/s13613-017-0326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mogensen KM, Robinson MK, Casey JD, Gunasekera NS, Moromizato T, Rawn JD, et al. Nutritional status and mortality in the critically ill. Crit Care Med. 2015 Dec;43(12):2605–15. 10.1097/CCM.0000000000001306. [DOI] [PubMed] [Google Scholar]
- 77. Moisey LL, Mourtzakis M, Cotton BA, Premji T, Heyland DK, Wade CE, et al. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. 2013 Sep 19;17(5):R206. 10.1186/cc12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Stanojcic M, Finnerty CC, Jeschke MG. Anabolic and anticatabolic agents in critical care. Curr Opin Crit Care. 2016 Aug;22(4):325–31. 10.1097/MCC.0000000000000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Thongprayoon C, Cheungpasitporn W, Kashani K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J Thorac Dis. 2016;8(5):E305–11. 10.21037/jtd.2016.03.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cartin-Ceba R, Afessa B, Gajic O. Low baseline serum creatinine concentration predicts mortality in critically ill patients independent of body mass index. Crit Care Med. 2007 Oct;35(10):2420–3. 10.1097/01.ccm.0000281856.78526.f4. [DOI] [PubMed] [Google Scholar]
- 81. Cerra FB, Benitez MR, Blackburn GL, Irwin RS, Jeejeebhoy K, Katz DP, et al. Applied nutrition in ICU patients. A consensus statement of the American college of chest physicians. Chest. 1997;111(3):769–78. 10.1378/chest.111.3.769. [DOI] [PubMed] [Google Scholar]
- 82. Franch HA. Nutrition and muscle catabolism in maintenance hemodialysis: does feeding make muscle cells selective self-eaters? J Ren Nutr. 2009 Jan;19(1):86–90. 10.1053/j.jrn.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fiaccadori E, Sabatino A, Barazzoni R, Carrero JJ, Cupisti A, De Waele E, et al. ESPEN guideline on clinical nutrition in hospitalized patients with acute or chronic kidney disease. Clin Nutr. 2021;40(4):1644–68. 10.1016/j.clnu.2021.01.028. [DOI] [PubMed] [Google Scholar]
- 84. Bellomo R, Tan HK, Bhonagiri S, Gopal I, Seacombe J, Daskalakis M, et al. High protein intake during continuous hemodiafiltration: impact on amino acids and nitrogen balance. Int J Artif Organs. 2002;25(4):261–8. 10.1177/039139880202500403. [DOI] [PubMed] [Google Scholar]
- 85. Onichimowski D, Goraj R, Jalali R, Grabala J, Mayzner-Zawadzka E, Czuczwar M. Practical issues of nutrition during continuous renal replacement therapy. Ther. 2017;49(4):309–16. 10.5603/AIT.a2017.0052. [DOI] [PubMed] [Google Scholar]
- 86. Scheinkestel CD, Adams F, Mahony L, Bailey M, Davies AR, Nyulasi I, et al. Impact of increasing parenteral protein loads on amino acid levels and balance in critically ill anuric patients on continuous renal replacement therapy. Nutrition. 2003;19(9):733–40. 10.1016/s0899-9007(03)00107-2. [DOI] [PubMed] [Google Scholar]
- 87. Pisani D, Navalesi P, De Rosa S. Do we need a 6D’s Framework of Nutritional Stewardship in critical care? J Anesth Analg Crit Care. 2021;1:5. 10.1186/s44158-021-00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]