Abstract
Introduction
Owing to their low incidence, no reliable statistics about prognostication derived from large sample sizes have been reported of malignant ovarian germ cell tumors (MOGCTs) and sex cord-stromal tumors (SCSTs). The present study aimed to investigate the clinicopathological prognostic factors and the survival trends of MOGCTs and SCSTs.
Materials and Methods
Patients with MOGCTs and SCSTs were recorded in the Surveillance, Epidemiology, and End Results (SEER) database diagnosed between 2000 and 2019. Clinical, demographic, and treatment characteristics were compared between groups of MOGCTs and SCSTs. Cox risk regression analysis and Kaplan-Meier survival curves were used to compare overall survival (OS) and cancer-specific survival (CSS) and to assess the prognostic factors.
Results
Information about 2,506 patients with MOGCTs and 1,556 patients with SCSTs was extracted from the SEER database, respectively. Aged <40 years and single were more common in patients with MOGCTs than in those with SCSTs. The vast majority of patients with MOGCTs and SCSTs underwent surgery (98.1% vs. 94.5%; p < 0.001), and women with MOGCTs were more likely to receive chemotherapy than women with SCSTs (56.1% vs. 32.2%; p < 0.001). For both patients before and after propensity-score matching, the 5-year OS rates of patients with SCSTs were lower than those of patients with MOGCTs (p < 0.05). In multivariate Cox regression analysis, both age and surgery were independent predictors of OS in patients with MOGCTs and SCSTs. FIGO staging was an independent predictor of CSS in MOGCT patients. Tumor size and chemotherapy were also independent predictors of CSS in patients with SCSTs.
Conclusion
Compared to patients with SCSTs, those with MOGCTs tended to be younger and had a higher OS and CSS. Adjuvant chemotherapy after surgery did not prolong OS and CSS in patients with SCSTs.
Keywords: Non-epithelial ovarian cancer; Malignant ovarian germ cell tumors; Sex cord-stromal tumors; Surveillance, Epidemiology, and End Results (SEER); Prognosis
Introduction
Ovarian cancer is a common gynecological disease among women and is divided into epithelial and non-epithelial types, of which epithelial cancers constitute by far the majority. Ovarian non-epithelial tumors arising from the ovary are rare and account for 3–8% of ovarian cancers [1–3]. Non-epithelial ovarian cancers are broadly classified into 2 major groups: malignant ovarian germ cell tumors (MOGCTs) and sex cord-stromal tumors (SCSTs), and the yearly-adjusted incidence rate is 3.7/1,000,000 and 2.1/1,000,000, respectively [4]. Although MOGCTs and SCSTs are rare, they are usually detected at an early stage and have a more favorable prognosis than ovarian epithelial tumors. The first choice of treatment is surgery. Most MOGCTs and SCSTs can be treated surgically at an early stage of tumor progression and recurrence [5]. Concerning the prognosis of these two types of non-epithelial ovarian cancer, to date, there have been a few small population-based studies. In a Netherlands study, the 5-year OS of MOGCTs was 82% and of SCSTs was 78% [3]. Unfortunately, to the present date there still has been the absence of established practical guidelines for clinicians to deal with such cases as the current lack of data from large samples of a population-based study. In the current study, we obtained information on 4,062 patients from the Surveillance, Epidemiology, and End Results (SEER) database to compare clinical features and the results between patients with MOGCTs and those with SCSTs to provide a benchmark for clinical therapy and therapeutic activities.
Materials and Methods
Data Sources and Extraction
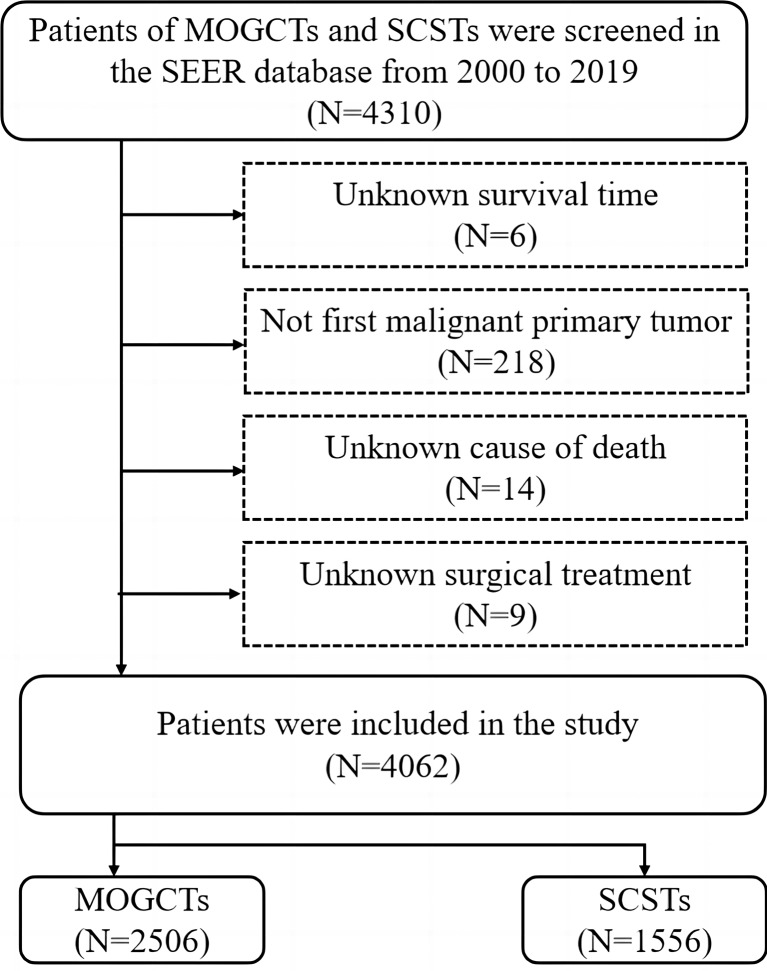
These data were taken from the National Cancer Institute program SEER, which gathered data from 17 population-based cancer registries that encompassed approximately 30% of the entire US population. Cases of MOGCTs and SCSTs were screened in the SEER database from 2000 to 2019. As the data used were extracted from SEER dataset (public), ethics approval and consent to participate were retrieved from SEER. The cases were coded using ICD‐O‐3. Inclusion criteria were as follows: histology code: dysgerminoma (9060/3), embryonal carcinoma (9070/3), yolk sac tumor (9071/3), malignant teratoma (9080/3), mixed germ cell tumor (9085/3), granulosa cell tumor (8620/3), Sertoli-Leydig cell tumor (8631/3, 8634/3, 8640/3, 8650/3). The exclusion criteria included (1) unknown survival time, (2) not the first malignant primary tumor, (3) unknown cause of death, and (4) unknown surgical treatment. A patient selection criteria flowchart is shown in Figure 1.
Fig. 1.
Flow diagram of patient selection criteria. MOGCTs, malignant ovarian germ cell tumor; SCSTs, sex cord-stromal tumor; SEER, Surveillance, Epidemiology, and End Results.
Clinical and Demographic Characteristics
We analyzed patient demographic data, clinical characteristics, and treatment patterns, including the age at the time of diagnosis (grouped into <40, 40–59, and >59 years), marital status (married, widowed/separated/divorced, single, and unknown), race (black, white, other, and unknown), laterality (bilateral, only one side, and unknown), tumor size (grouped into <93, 93–219, >219 mm, and unknown), SEER stage (distant, regional, localized, and unknown), grade (grade I-II, grade III-IV, and unknown), International Federation of Gynecology and Obstetrics (FIGO) stage (I, II, III, IV, and unknown), cancer antigen 125 (CA125) status (elevated, normal, and unknown), lymph nodes status (positive, negative, no examined, and unknown),surgery (yes and no), and chemotherapy (yes and no). CA125, although not as specific as inhibin (A or B) and AFP for non-epithelial tumors, was not able to obtain additional tumor markers from the SEER database. In the SEER database, the overall survival (OS) is defined as the length of time between the diagnosis of cancer and the date of death of the patient. Cancer-specific survival (CSS) will be calculated from the last day of treatment until death from MOGCTs and SCSTs. To assess OS and CSS, we extracted follow-up data on time since diagnosis, the vital status of the patient, and the cause of death.
Statistical Analysis
X-tile software was used to determine the optimal cut-off values for age of diagnosis and tumor size [6]. For age, the best minimum and maximum cut-off values were assigned at 40 and 59 years, respectively. For tumor size, the best minimum and maximum cut-off values were assigned at 93 mm and 219 mm, respectively (shown in Fig. 2). Comparisons of demographic and clinical features between patients with MOGCTs and patients with SCSTs were performed using Fisher’s exact test or χ2 test. To balance confounding factors, we conducted propensity-score matching to create a 1:1 matched set. For the MOGCTs and SCSTs, the Kaplan-Meier survival curve was used for comparing the rates of survival before and after propensity-score matching [7]. Cox proportional hazard regression analysis was used to identify independent predictors of survival. This study employed R version 4.1.1 to conduct all statistical analyses. Hazard ratios and 95% confidence intervals were calculated. Statistical significance was set at p < 0.05.
Fig. 2.
The thresholds for age and tumor sizes were established by X-tile analysis. a, b The thresholds for age were 40 and 59 years. c, d The cut-off values for sizes of tumor were 93 mm and 219 mm.
Results
Basic Clinical Information of the Patients
The study collected data for 4,062 patients with a diagnosis of MOGCTs or SCSTs from 2000 to 2019 from the SEER database, which consisted of 2,506 patients with MOGCTs and 1,556 patients with SCSTs. According to the histological type documented patient demographics, which are shown in Table 1. Compared with patients with MOGCTs, those with SCSTs were more probable between the ages of forty and fifty-nine (48.3% vs. 5.3%; p < 0.001). Additionally, individuals with SCSTs were more likely to be black (23.3% vs. 11.6%; p < 0.001) as well as married (46.9% vs. 23.1%) or separated/widowed/divorced (18.4% vs. 3.7%; p < 0.0001) compared with those with MOGCTs. Furthermore, MOGCTs and SCSTs occurred more in the single-sided (95.1% vs. 91.1%; p < 0.0001), and the tumor size of MOGCTs was bigger at the time of diagnosis than the tumor size of SCSTs (93–219 mm, 44.9% vs. 25.4% and >219 mm, 12.1% vs. 8.7%; p < 0.001), respectively. Most MOGCTs and SCSTs patients had a diagnosis of FIGO stage I disease (57.5% vs. 53.9%; p < 0.001) and SEER localization stage (51.6% vs. 48.4%; p < 0.001), respectively. Surgery was performed in the vast majority of patients with both MOGCTs and SCSTs (98.1% vs. 94.5%; p < 0.001), meanwhile patients in the MOGCTs group received more chemotherapy than those in the SCSTs group (56.1% vs. 32.2%; p < 0.001), respectively. Higher levels of CA125 (20.5% vs. 12.0%; p < 0.001) and more common lymph node-positive (8.4% vs. 1.9%; p < 0.001) were seen in patients with MOGCTs compared with SCSTs.
Table 1.
Demographic and clinical characteristics comparing MOGCTs and SCSTs
| Variables | MOGCT, N (%) | SCST, N (%) | Overall, N (%) | p value |
|---|---|---|---|---|
| Total | 2,506 (61.7) | 1,556 (38.3) | 4,062 (100.0) | |
| Age of diagnosis | <0.001 | |||
| <40 years | 2,320 (92.6) | 428 (27.5) | 2,748 (67.7) | |
| 40–59 years | 132 (5.3) | 752 (48.3) | 884 (21.8) | |
| >59 years | 54 (2.2) | 376 (24.2) | 430 (10.6) | |
| Race | <0.001 | |||
| Black | 299 (11.9) | 362 (23.3) | 661 (16.3) | |
| White | 1,815 (72.4) | 1,056 (67.9) | 2,871 (70.7) | |
| Other | 344 (13.7) | 119 (7.6) | 463 (11.4) | |
| Unknown | 48 (1.9) | 19 (1.2) | 67 (1.6) | |
| Marital status | <0.001 | |||
| Married | 578 (23.1) | 730 (46.9) | 1,308 (32.2) | |
| Separated/widowed/divorced | 92 (3.7) | 287 (18.4) | 379 (9.3) | |
| Single | 1,753 (70.0) | 449 (28.9) | 2,202 (54.2) | |
| Unknown | 83 (3.3) | 90 (5.8) | 173 (4.3) | |
| Laterality | <0.001 | |||
| Bilateral | 96 (3.8) | 52 (3.3) | 148 (3.6) | |
| Only one side | 2,382 (95.1) | 1,418 (91.1) | 3,800 (93.6) | |
| Unknown | 28 (1.1) | 86 (5.5) | 114 (2.8) | |
| Tumor size, mm | <0.001 | |||
| <93 | 330 (13.2) | 478 (30.7) | 808 (19.9) | |
| 93–219 | 1,126 (44.9) | 395 (25.4) | 1,521 (37.4) | |
| >219 | 302 (12.1) | 136 (8.7) | 438 (10.8) | |
| Unknown | 748 (29.8) | 547 (35.2) | 1,295 (31.9) | |
| SEER stage | <0.001 | |||
| Distant | 465 (18.6) | 255 (16.4) | 720 (17.7) | |
| Regional | 696 (27.8) | 451 (29.0) | 1,147 (28.2) | |
| Localized | 1,293 (51.6) | 753 (48.4) | 2,046 (50.4) | |
| Unknown | 52 (2.1) | 97 (6.2) | 149 (3.7) | |
| Grade | <0.001 | |||
| Grade I-II | 548 (21.9) | 221 (14.2) | 769 (18.9) | |
| Grade III-IV | 460 (18.4) | 219 (14.1) | 679 (16.7) | |
| Unknown | 1,498 (59.8) | 1,116 (71.7) | 2,614 (64.4) | |
| FIGO stage | <0.001 | |||
| I | 1,440 (57.5) | 839 (53.9) | 2,279 (56.1) | |
| II | 166 (6.6) | 136 (8.7) | 302 (7.4) | |
| III | 404 (16.1) | 162 (10.4) | 566 (13.9) | |
| IV | 120 (4.8) | 70 (4.5) | 190 (4.7) | |
| Unknown | 376 (15.0) | 349 (22.4) | 725 (17.8) | |
| CA125 | <0.001 | |||
| Elevated | 513 (20.5) | 186 (12.0) | 699 (17.2) | |
| Normal | 216 (8.6) | 243 (15.6) | 459 (11.3) | |
| Unknown | 1,777 (70.9) | 1,127 (72.4) | 2,904 (71.5) | |
| Surgery | <0.001 | |||
| Yes | 2,458 (98.1) | 1,470 (94.5) | 3,928 (96.7) | |
| No | 48 (1.9) | 86 (5.5) | 134 (3.3) | |
| Chemotherapy | <0.001 | |||
| Yes | 1,406 (56.1) | 501 (32.2) | 1,907 (46.9) | |
| No | 1,100 (43.9) | 1,055 (67.8) | 2,155 (53.1) | |
| Lymph nodes status | <0.001 | |||
| Positive | 210 (8.4) | 29 (1.9) | 239 (5.9) | |
| Negative | 981 (39.1) | 660 (42.4) | 1,641 (40.4) | |
| No examined | 1,276 (50.9) | 784 (50.4) | 2,060 (50.7) | |
| Unknown | 39 (1.6) | 83 (5.3) | 122 (3.0) |
MOGCT, malignant ovarian germ cell tumor; SCST, sex cord-stromal tumor; SEER, Surveillance, Epidemiology, and End Results; FIGO, International Federation of Gynecology and Obstetrics; CA125, cancer antigen 125.
Survival Analysis of the Study Population in MOGCTs and SCSTs
Kaplan-Meier survival curves were used to describe the survival difference between patients with MOGCTs and those with SCSTs. The 1-, 3-, and 5-year OS rates of MOGCTs were 96.9%, 95.2%, and 94.3%, respectively, while those of SCSTs were 95.3%, 89.8% and 86.2%, respectively. In contrast, as far as CSS is concerned, SCSTs at 1, 3, and 5 years had worse rates of survival than MOGCTs (all p < 0.0001; shown in Fig. 3). Age at the time of diagnosis (years), laterality, SEER stage, grade, FIGO stage, CA125, surgery, chemotherapy, and lymph node status are prognostic factors independent of OS and CSS in univariate Cox regression models that were limited to women who underwent MOGCTs (Table 2). Cox multivariable regression analysis revealed that age at diagnosis, grade of differentiation (III-IV), FIGO III-IV, and surgery were independent risk factors for OS and CSS with MOGCTs (shown in Fig. 4, 5). The results of the univariate and multivariate Cox regression analyses for SCSTs to identify independent risk factors are shown in Table 3. In multivariable Cox regression analysis for SCSTs, laterality, tumor size, SEER stage, grade of differentiation (III-IV), and CA125 were independent risk factors for CSS and OS, in contrast to MOGCTs. Surgery was identified as an independent predictor of OS, while chemotherapy was identified as an independent predictor of CSS (shown in Fig. 6, 7). Furthermore, the results of the SEER database indicate that positive lymph node status was a worse prognosis factor for SCSTs. There was no difference in OS and CSS between patients with FIGO stage I-IV SCSTs who did and did not receive any type of chemotherapy (shown in Fig. 8). In patients with MOGCTs, chemotherapy led to improvements in OS and CSS in both II and III stages of FIGO (shown in Fig. 9).
Fig. 3.
Survival outcomes before propensity-score matching. Overall survival (a) and cancer-specific survival (b) based on tumor types. Log-rank tests were used to generate p values. MOGCT, malignant ovarian germ cell tumor; SCST, sex cord-stromal tumor.
Table 2.
Univariate analysis of overall survival and cancer-specific survival in MOGCTs
| Variables | Overall survival (OS) | Cancer-specific survival (CSS) | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age of diagnosis | ||||
| <40 years | Reference | Reference | ||
| 40–59 years | 5.215 (3.383–8.038) | <0.001 | 5.635 (3.422–9.278) | <0.001 |
| >59 years | 22.521 (14.968–33.885) | <0.001 | 20.717 (12.773–33.602) | <0.001 |
| Race | ||||
| Black | Reference | Reference | ||
| White | 0.490 (0.335–0.718) | <0.001 | 0.503 (0.318–0.797) | 0.003 |
| Other | 0.295 (0.153–0.569) | <0.001 | 0.358 (0.171–0.748) | 0.006 |
| Unknown | NA | NA | NA | NA |
| Marital status | ||||
| Married | Reference | Reference | ||
| Separated/widowed/divorced | 2.819 (1.698–4.680) | <0.001 | 2.885 (1.609–5.175) | <0.001 |
| Single | 0.500 (0.353–0.709) | <0.001 | 0.460 (0.304–0.697) | <0.001 |
| Unknown | NA | NA | NA | NA |
| Laterality | ||||
| Bilateral | Reference | Reference | ||
| Only one side | 0.491 (0.265–0.908) | 0.023 | 0.394 (0.198–0.783) | 0.008 |
| Unknown | NA | NA | NA | NA |
| Tumor size, mm | ||||
| <93 | Reference | Reference | ||
| 93–219 | 1.475 (0.813–2.678) | 0.201 | 0.882 (0.787–3.263) | 0.193 |
| >219 | 1.325 (0.630–2.785) | 0.458 | 1.603 (0.329–2.369) | 0.804 |
| Unknown | NA | NA | NA | NA |
| SEER stage | ||||
| Distant | Reference | Reference | ||
| Regional | 0.227 (0.150–0.342) | <0.001 | 0.175 (0.105–0.290) | <0.001 |
| Localized | 0.134 (0.090–0.199) | <0.001 | 0.094 (0.057–0.157) | <0.001 |
| Unknown | NA | NA | NA | NA |
| Grade | ||||
| Grade I-II | Reference | Reference | ||
| Grade III-IV | 3.264 (1.807–5.896) | <0.001 | 5.273 (2.322–11.975) | <0.001 |
| Unknown | NA | NA | NA | NA |
| FIGO stage | ||||
| I | Reference | Reference | ||
| II | 2.382 (1.187–4.781) | 0.015 | 3.017 (1.198–7.601) | 0.019 |
| III | 5.006 (3.289–7.620) | <0.001 | 7.670 (4.367–13.472) | <0.001 |
| IV | 13.641 (8.585–21.674) | <0.001 | 24.277 (13.477–43.730) | <0.001 |
| Unknown | NA | NA | NA | NA |
| CA125 | ||||
| Elevated | Reference | Reference | ||
| Normal | 0.215 (0.077–0.599) | 0.003 | 0.253 (0.090–0.713) | 0.009 |
| Unknown | NA | NA | ||
| Surgery | ||||
| No | Reference | Reference | ||
| Yes | 0.081 (0.051–0.128) | <0.001 | 0.058 (0.035–0.094) | <0.001 |
| Chemotherapy | ||||
| No | Reference | Reference | ||
| Yes | 1.584 (1.133–2.215) | 0.007 | 1.583 (1.063–2.357) | 0.024 |
| Lymph nodes status | ||||
| Negative | Reference | Reference | ||
| Positive | 2.044 (1.159–3.605) | 0.014 | 2.122 (1.044–4.313) | 0.038 |
| No examined | 1.893 (1.307–2.740) | <0.001 | 2.243 (1.421–3.541) | <0.001 |
| Unknown | NA | NA | NA | NA |
SEER, Surveillance, Epidemiology, and End Results; FIGO, International Federation of Gynecology and Obstetrics; CA125, cancer antigen 125.
Fig. 4.
Forest plot of multivariable Cox regression of overall survival with MOGCTs. MOGCTs, malignant ovarian germ cell tumor; SEER, Surveillance, Epidemiology, and End Results; FIGO, International Federation of Gynecology and Obstetrics; CA125, cancer antigen 125.
Fig. 5.
Forest plot of multivariable Cox regression of cancer-specific survival with MOGCTs. MOGCTs, malignant ovarian germ cell tumor; SEER, Surveillance, Epidemiology, and End Results; FIGO, International Federation of Gynecology and Obstetrics; CA125, cancer antigen 125.
Table 3.
Univariate analysis of overall survival and cancer-specific survival in SCSTs
| Variables | Overall survival (OS) | Cancer-specific survival (CSS) | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age of diagnosis | ||||
| <40 years | Reference | Reference | ||
| 40–59 years | 1.129 (0.831–1.532) | 0.438 | 0.967 (0.684–1.367) | 0.850 |
| >59 years | 3.685 (2.741–4.954) | <0.001 | 2.289 (1.614–3.245) | <0.001 |
| Race | ||||
| Black | Reference | Reference | ||
| White | 0.792 (0.622–1.008) | 0.058 | 0.754 (0.561–1.014) | 0.062 |
| Other | 0.560 (0.338–0.927) | 0.024 | 0.595 (0.327–1.080) | 0.088 |
| Unknown | NA | NA | NA | NA |
| Marital status | ||||
| Married | Reference | Reference | ||
| Separated/widowed/divorced | 1.873 (1.438–2.440) | <0.001 | 1.509 (1.070–2.129) | 0.019 |
| Single | 1.265 (0.970–1.651) | 0.083 | 1.359 (0.990–1.865) | 0.058 |
| Unknown | NA | NA | NA | NA |
| Laterality | ||||
| Bilateral | Reference | Reference | ||
| Only one side | 0.317 (0.211–0.478) | <0.001 | 0.215 (0.139–0.334) | <0.001 |
| Unknown | NA | NA | NA | NA |
| Tumor size, mm | ||||
| <93 | Reference | Reference | ||
| 93–219 | 3.403 (2.287–5.062) | <0.001 | 4.054 (2.446–6.718) | <0.001 |
| >219 | 5.216 (3.318–8.201) | <0.001 | 6.171 (3.503–10.870) | <0.001 |
| Unknown | NA | NA | NA | NA |
| SEER stage | ||||
| Distant | Reference | Reference | ||
| Regional | 0.400 (0.305–0.524) | <0.001 | 0.337 (0.244–0.464) | <0.001 |
| Localized | 0.230 (0.175–0.300) | <0.001 | 0.140 (0.098–0.200) | <0.001 |
| Unknown | NA | NA | NA | NA |
| Grade | ||||
| Grade I-II | Reference | Reference | ||
| Grade III-IV | 2.221 (1.476–3.343) | <0.001 | 3.701 (2.121–6.459) | <0.001 |
| Unknown | NA | NA | NA | NA |
| FIGO stage | ||||
| I | Reference | Reference | ||
| II | 2.315 (1.618–3.314) | <0.001 | 2.955 (1.874–4.660) | <0.001 |
| III | 3.084 (2.293–4.148) | <0.001 | 4.513 (3.114–6.541) | <0.001 |
| IV | 6.967 (4.860–9.986) | <0.001 | 11.288 (7.439–17.128) | <0.001 |
| Unknown | NA | NA | NA | NA |
| CA125 | ||||
| Elevated | Reference | Reference | ||
| Normal | 0.187 (0.096–0.365) | <0.001 | 0.173 (0.080–0.375) | <0.001 |
| Unknown | NA | NA | ||
| Surgery | ||||
| No | Reference | Reference | ||
| Yes | 0.331 (0.237–0.462) | <0.001 | 0.287 (0.194–0.424) | <0.001 |
| Chemotherapy | ||||
| No | Reference | Reference | ||
| Yes | 1.491 (1.196–1.859) | <0.001 | 2.097 (1.609–2.734) | <0.001 |
| Lymph nodes status | ||||
| Negative | Reference | Reference | ||
| Positive | 3.151 (1.773–5.602) | <0.001 | 4.593 (2.486–8.486) | <0.001 |
| No examined | 1.725 (1.363–2.183) | <0.001 | 1.707 (1.268–2.299) | <0.001 |
| Unknown | NA | NA | NA | NA |
SEER, Surveillance, Epidemiology, and End Results; FIGO, International Federation of Gynecology and Obstetrics; CA125, cancer antigen 125.
Fig. 6.
Forest plot of multivariable Cox regression of overall survival with SCSTs. SCSTs, sex cord-stromal tumor; SEER, Surveillance, Epidemiology, and End Results; FIGO, International Federation of Gynecology and Obstetrics; CA125, cancer antigen 125.
Fig. 7.
Forest plot of multivariable Cox regression of cancer-specific survival with SCSTs. SCSTs, sex cord-stromal tumor; SEER, Surveillance, Epidemiology, and End Results; FIGO, International Federation of Gynecology and Obstetrics; CA125, cancer antigen 125.
Fig. 8.
Kaplan-Meier survival curve for with or without chemotherapy who had undergone surgery in patients with MOGCTs. a, b FIGO stage I. c, d FIGO stage II. e, f FIGO stage III. g, h FIGO stage IV. MOGCTs, malignant ovarian germ cell tumor; FIGO, International Federation of Gynecology and Obstetrics.
Fig. 9.
Kaplan-Meier survival curve for with or without chemotherapy who had undergone surgery in patients with SCSTs. a, b FIGO stage I. c, d FIGO stage II. e, f FIGO stage III. g, h FIGO stage IV. SCSTs, sex cord-stromal tumor; FIGO, International Federation of Gynecology and Obstetrics.
Comparison between MOGCTs and SCSTs after Matching
The study matched 563 MOGCT patients with 563 SCST patients, as shown in Table 4. p values were all greater than 0.05, as expected, demonstrating that the data were balanced. Continuing this line of investigation, the K-M method was used for survival analysis. The rate of 1‐year and 3‐year OS in patients with SCSTs was 96.1% and 90.2%, respectively, which was higher than that of MOGCTs (with 1-year OS rate of 92.9% and 3-year OS rate of 89.9%), which is in disagreement with the previous study that at 5 years, 87.6% of patients had OS rates that were poorer than those of MOGCTs (with 5-year OS rate of 89.2%) (p = 0.0038; shown in Fig. 10a). Patients with SCSTs had higher 1-year CSS rates (with 1-year CSS rate of 96.3%) but poorer 3-year and 5-year CSS rates (with 3-year CSS rate of 90.9% and 5-year CSS rate of 88.8%) compared to patients with MOGCTs (with 1-year CSS rate of 94.0%, 3-year CSS rate of 91.9%, and 5-year CSS rate of 91.5%) (p = 0.0022; shown in Fig. 10b).
Table 4.
Demographic and clinical characteristics comparing MOGCTs and SCSTs following propensity-score matching
| Variables | MOGCT, N (%) | SCST, N (%) | Overall, N (%) | p value |
|---|---|---|---|---|
| Total | 563 | 563 | 1,126 | |
| Age of diagnosis | 0.943 | |||
| <40 years | 386 (68.6) | 390 (69.3) | 776 (68.9) | |
| 40–59 years | 125 (22.2) | 115 (20.4) | 240 (21.3) | |
| >59 years | 52 (9.2) | 58 (10.3) | 110 (9.8) | |
| Race | 0.999 | |||
| Black | 107 (19.0) | 111 (19.7) | 218 (19.4) | |
| White | 409 (72.6) | 402 (71.4) | 811 (72.0) | |
| Other | 41 (7.3) | 42 (7.5) | 83 (7.4) | |
| Unknown | 6 (1.1) | 8 (1.4) | 14 (1.2) | |
| Marital status | 0.995 | |||
| Married | 225 (40.0) | 221 (39.3) | 446 (39.6) | |
| Separated/widowed/divorced | 54 (9.6) | 59 (10.5) | 113 (10.0) | |
| Single | 264 (46.9) | 259 (46.0) | 523 (46.4) | |
| Unknown | 20 (3.6) | 24 (4.3) | 44 (3.9) | |
| Laterality | 0.651 | |||
| Bilateral | 20 (3.6) | 17 (3.0) | 37 (3.3) | |
| Only one side | 529 (94.0) | 523 (92.9) | 1,052 (93.4) | |
| Unknown | 14 (2.5) | 23 (4.1) | 37 (3.3) | |
| Tumor size, mm | 0.986 | |||
| <93 | 145 (25.8) | 132 (23.4) | 277 (24.6) | |
| 93–219 | 174 (30.9) | 174 (30.9) | 348 (30.9) | |
| >219 | 56 (9.9) | 57 (10.1) | 113 (10.0) | |
| Unknown | 188 (33.4) | 200 (35.5) | 388 (34.5) | |
| SEER stage | 0.697 | |||
| Distant | 96 (17.1) | 102 (18.1) | 198 (17.6) | |
| Regional | 151 (26.8) | 174 (30.9) | 325 (28.9) | |
| Localized | 286 (50.8) | 254 (45.1) | 540 (48.0) | |
| Unknown | 31 (5.3) | 31 (5.9) | 63 (5.6) | |
| Grade | 0.997 | |||
| Grade I-II | 96 (17.1) | 91 (16.2) | 187 (16.6) | |
| Grade III-IV | 125 (22.2) | 125 (22.2) | 250 (22.2) | |
| Unknown | 342 (60.7) | 347 (61.6) | 689 (61.2) | |
| FIGO stage | 0.992 | |||
| I | 324 (57.5) | 306 (54.4) | 630 (56.0) | |
| II | 40 (7.1) | 41 (7.3) | 81 (7.2) | |
| III | 62 (11.0) | 63 (11.2) | 125 (11.1) | |
| IV | 28 (5.0) | 34 (6.0) | 62 (5.5) | |
| Unknown | 109 (19.4) | 119 (21.1) | 228 (20.2) | |
| CA125 | 0.971 | |||
| Elevated | 83 (14.7) | 82 (14.6) | 165 (14.7) | |
| Normal | 66 (11.7) | 74 (13.1) | 140 (12.4) | |
| Unknown | 414 (73.5) | 407 (72.3) | 821 (72.9) | |
| Surgery | 0.686 | |||
| Yes | 541 (96.1) | 535 (95.0) | 1,076 (95.6) | |
| No | 22 (3.9) | 28 (5.0) | 50 (4.4) | |
| Chemotherapy | 0.956 | |||
| Yes | 244 (43.3) | 249 (44.2) | 493 (43.8) | |
| No | 319 (56.7) | 314 (55.8) | 633 (56.2) | |
| Lymph nodes status | 0.965 | |||
| Positive | 16 (2.8) | 17 (3.0) | 33 (2.9) | |
| Negative | 222 (39.4) | 227 (40.3) | 449 (39.9) | |
| No examined | 305 (54.2) | 292 (51.9) | 597 (53.0) | |
| Unknown | 20 (3.6) | 27 (4.8) | 47 (4.2) |
MOGCT, malignant ovarian germ cell tumor; SCST, sex cord-stromal tumor; SEER, Surveillance, Epidemiology, and End Results; FIGO, International Federation of Gynecology and Obstetrics; CA125, cancer antigen 125.
Fig. 10.
Survival outcomes following propensity-score matching. Overall survival (a) and cancer-specific survival (b) based on tumor types. Log-rank tests were used to generate p values. MOGCTs, malignant ovarian germ cell tumor; SCST, sex cord-stromal tumor.
Discussion
Because of the scarcity of non-epithelial ovarian cancer, the collection of a vast amount of data to advance medical knowledge regarding non-epithelial ovarian cancer is challenging. As far as we know, this is the largest population-based study investigating the epidemiology and clinical outcomes of female patients with non-epithelial ovarian cancer. We enrolled 4,062 patients from the SEER database and assessed survival outcome trends in patients with MOGCTs and SCSTs from the SEER database between 2000 and 2019 using both Kaplan-Meier and Cox regression analyses.
There is variation in the percentage of ovarian tumors that are non-epithelial in origin in different research and there seems to be some geographic variation. A study based on the basis of a global population of ovarian tumors found that non-epithelial tumors account for approximately 5–6% of all North American cases in Oceania and Europe, and the ratio has tended to be more elevated in Central and South America as well as in Asia (approximately 9%) [2]. MOGCTs and SCSTs accounted for 2.3% and 1.4% of all ovarian cancers in the SEER database, respectively, in our study. Non-epithelial ovarian cancers tend to have a much better prognosis than the bulk of their counterparts in the epithelium [8]. However, the probability of survival of MOGCTs and SCSTs varies noticeably between studies, and there are only a small number of previous investigations that have looked at the time course of survival of these cancers, as well as the influence of stage at diagnosis [3, 8, 9]. Consistent with our findings, several researchers have suggested that patients with SCSTs have a poor survival rate when compared with MOGCTs [1, 3, 10]. In our research, PSM was used to adjust the confounders, increasing the robustness of the results [11]. Torre et al. [12] found that both MOGCTs and SCSTs have high 5-year survival rates of 99% for MOGCTs and 98% for SCSTs. Survival rates remain relatively high even for FIGO stage IV disease. A large multicenter study of global ovarian cancer survival comparisons reported wide worldwide variations in the 5-year survival of non-epithelial ovarian cancers, ranging from 59% in Japan to 100% in Korea for SCSTs and 42% in China to 76% in Australia for MOGCTs [8].
MOGCTs are derived from the primitive germ cell of the embryonic gonad, approximately 1–2% of all ovarian cancers [13–15]. 70% of patients develop FIGO stage I disease at the time of diagnosis [16]. In terms of clinical characteristics, MOGCTs mainly take place during the first 30 years of life [17]. Siegel et al. [18] found that 3% of cases occurred from birth to the age of 14 years and that 11% of cases in those occurred between the ages of 15 and 19. In our study, we found that MOGCTs had a younger age distribution (<40 years old) and more often had FIGO stage I disease (57%). Guidelines from the Gynecologic Cancer Inter Group (GCIG) recommend that the gold standard for early MOGCT patient management is fertility preservation [16]. In our study, we received surgical intervention that reduced the risk of death for patients with MOGCTs. A 5-day regimen of bleomycin/etoposide/platinum (BEP) is recommended by the guidelines of the European Society for Medical Oncology (ESMO) as the adjuvant chemotherapy of choice for MOGCTs [19]. In our study, we found that there was no statistical difference in the survival rate between patients with or without chemotherapy for FIGO stage I and IV patients with MOGCTs.
SCSTs originate from either stromal cells or the sex cord, or both, and comprise 1.3% of all ovarian cancers [1]. Over 70% of SCST patients are diagnosed at an early stage, which is usually indolent [20]. SCSTs occur across age groups, with granulosa cell tumors occurring mainly in peri- and postmenopausal women and Sertoli-Leydig cell tumors largely occurring in young females [19, 21]. SCSTs can be treated with surgery, chemotherapy, radiation, and targeted therapy. The standard management is reductive debulking surgery, which may be followed by platinum-based adjuvant chemotherapy. The prognosis for patients with stage I SCST is excellent; adjuvant therapy is not required in patients with FIGO stage I clinical disease who have received surgical treatment [22]. Although the use of chemotherapy remains a subject of debate in FIGO stage IC, a study demonstrated that adjuvant chemotherapy was not able to prolong disease-free survival in FIGO stage IC [23]. Adjuvant chemotherapy is recommended for patients with stage II or greater [24]. However, another study found that adjuvant chemotherapy did not lead to improved survival in patients with FIGO stage II-IV SCSTs [25]. In our study, there was no statistically significant difference in FIGO stage I-IV SCSTs response with or without chemotherapy, which is in agreement with the results of Oseledchyk’s study [26].
In clinical decision-making, based on findings on tumor biomarkers, ultrasonography, and physical examination, women undergo surgery to diagnose and treat adnexal mass. For example, more specific tumor biomarkers such as fetoprotein α, human chorionic gonadotropin β-subunit (β-hCG), and lactic dehydrogenase (LDH) were useful in diagnosing non-epithelial ovarian cancer [27]. Given the rarity of non-epithelial ovarian cancer, data on the role of CA125 in the preoperative diagnosis of these rare tumors are scarce. In earlier research, Pitta et al. [28] found that women with non-epithelial ovarian cancer did not express increased levels of CA125 as did women with epithelial ovarian cancer. As part of our research, we found elevated CA125 levels accounted for 20.5% of MOGCTs and 12% of SCSTs.
This study has several limitations that need to be acknowledged. The largest strength of the current study was the large sample size provided for studying non-epithelial ovarian cancer by the SEER database. The SEER database gives information about whether or not subjects received any form of chemotherapy. Nevertheless, there is no indication of the type of chemotherapy or the number of chemotherapy cycles received. In addition, details of the timing and location of tumor recurrence are not available in the database. Finally, our study focused solely on OS and CSS of patients, unaccounted for disease-free survival or cancer recurrence, which limits the applicability of our findings in clinical research. Further research with a larger sample population is needed to deal with these limitations.
Statement of Ethics
We had signed the SEER research data agreement. The data in this research were obtained from the SEER database following approved guidelines. The information on patients had been studied by the US Department of Health and Human Services. The data is publicly available and deidentified after permission. We confirm that the research was performed in accordance with the principles stated in the Declaration of Helsinki. No personally identifying information was used in the study, which eliminated the requirement for Institutional Review Board approval or informed patient consent.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There are no funding sources to declare.
Author Contributions
C.X.: conception, design of the study, acquisition of data, analysis and interpretation of data, and drafting the article; X.X.: critical revision for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding Statement
There are no funding sources to declare.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found here: https://seer.cancer.gov/data/. Further inquiries can be directed to the corresponding author.
References
- 1. Bennetsen AKK, Baandrup L, Aalborg GL, Kjaer SK. Non-epithelial ovarian cancer in Denmark: incidence and survival over nearly 40 years. Gynecol Oncol. 2020;157(3):693–9. 10.1016/j.ygyno.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 2. Matz M, Coleman MP, Sant M, Chirlaque MD, Visser O, Gore M, et al. The histology of ovarian cancer: worldwide distribution and implications for international survival comparisons (CONCORD-2). Gynecol Oncol. 2017;144(2):405–13. 10.1016/j.ygyno.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van der Hel OL, Timmermans M, van Altena AM, Kruitwagen RFPM, Slangen BFM, Sonke GS, et al. Overview of non-epithelial ovarian tumours: incidence and survival in The Netherlands, 1989-2015. Eur J Cancer. 2019;118:97–104. 10.1016/j.ejca.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 4. Gatta G, van der Zwan JM, Casali PG, Siesling S, Dei Tos AP, Kunkler I, et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. 2011;47(17):2493–511. 10.1016/j.ejca.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 5. El Helali A, Kwok GST, Tse KY. Adjuvant and post-surgical treatment in non-epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2022;78:74–85. 10.1016/j.bpobgyn.2021.06.001. [DOI] [PubMed] [Google Scholar]
- 6. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–9. 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 7. Abd ElHafeez S, Torino C, D’Arrigo G, Bolignano D, Provenzano F, Mattace-Raso F, et al. An overview on standard statistical methods for assessing exposure-outcome link in survival analysis (Part II): the Kaplan-Meier analysis and the Cox regression method. Aging Clin Exp Res. 2012;24(3):203–6. 10.1007/BF03325249. [DOI] [PubMed] [Google Scholar]
- 8. Matz M, Coleman MP, Carreira H, Salmerón D, Chirlaque MD, Allemani C, et al. Worldwide comparison of ovarian cancer survival: histological group and stage at diagnosis (CONCORD-2). Gynecol Oncol. 2017;144(2):396–404. 10.1016/j.ygyno.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hinchcliff E, Rauh-Hain JA, Clemmer JT, Diver E, Hall T, Stall J, et al. Racial disparities in survival in malignant germ cell tumors of the ovary. Gynecol Oncol. 2016;140(3):463–9. 10.1016/j.ygyno.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 10. Balkhy AL, Saleh ER, Jabali HI, Al-Jifree HM, Alwazzan AB. Demographic features, clinical characteristics, and prognostic factors of non-epithelial ovarian tumors at princess noorah Oncology center, national guard hospital, jeddah, Saudi arabia. Saudi Med J. 2022;43(2):208–12. 10.15537/smj.2022.43.2.20210433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim GH, Choi KD, Gong CS, Lee IS, Park YS, Han M, et al. Comparison of the treatment outcomes of endoscopic and surgical resection of GI stromal tumors in the stomach: a propensity score-matched case-control study. Gastrointest Endosc. 2020;91(3):527–36. 10.1016/j.gie.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 12. Torre, LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–96. 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Veneris JT, Mahajan P, Frazier AL. Contemporary management of ovarian germ cell tumors and remaining controversies. Gynecol Oncol. 2020;158(2):467–75. 10.1016/j.ygyno.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 14. Shaaban AM, Rezvani M, Elsayes KM, Baskin H Jr, Mourad A, Foster BR, et al. Ovarian malignant germ cell tumors: cellular classification and clinical and imaging features. Radiographics. 2014;34(3):777–801. 10.1148/rg.343130067. [DOI] [PubMed] [Google Scholar]
- 15. Euscher ED. Germ cell tumors of the female genital tract. Surg Pathol Clin. 2019;12(2):621–49. 10.1016/j.path.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 16. Nasioudis D, Mastroyannis SA, Latif NA, Ko EM. Trends in the surgical management of malignant ovarian germcell tumors. Gynecol Oncol. 2020;157(1):89–93. 10.1016/j.ygyno.2020.01.033. [DOI] [PubMed] [Google Scholar]
- 17. Cheung A, Shah S, Parker J, Soor P, Limbu A, Sheriff M, et al. Non-epithelial ovarian cancers: how much do we really know? Int J Environ Res Public Health. 2022;19(3):1106. 10.3390/ijerph19031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 19. Ray-Coquard I, Morice P, Lorusso D, Prat J, Oaknin A, Pautier P, et al. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv1–8. 10.1093/annonc/mdy001. [DOI] [PubMed] [Google Scholar]
- 20. Ray-Coquard I, Brown J, Harter P, Provencher DM, Fong PC, Maenpaa J, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian sex cord stromal tumors. Int J Gynecol Cancer. 2014;24(9 Suppl 3):S42–7. 10.1097/IGC.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 21. Boussios S, Moschetta M, Zarkavelis G, Papadaki A, Kefas A, Tatsi K. Ovarian sex-cord stromal tumours and small cell tumours: pathological, genetic and management aspects. Crit Rev Oncol Hematol. 2017;120:43–51. 10.1016/j.critrevonc.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 22. Sessa C, Schneider DT, Planchamp F, Baust K, Braicu EI, Concin N, et al. ESGO-SIOPE guidelines for the management of adolescents and young adults with non-epithelial ovarian cancers. Lancet Oncol. 2020;21(7):e360–8. 10.1016/S1470-2045(20)30091-7. [DOI] [PubMed] [Google Scholar]
- 23. Wang D, Xiang Y, Wu M, Shen K, Yang J, Huang H, et al. Is adjuvant chemotherapy beneficial for patients with FIGO stage IC adult granulosa cell tumor of the ovary? J Ovarian Res. 2018;11(1):25. 10.1186/s13048-018-0396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nasioudis D, Orfanelli T, Frey MK, Chapman-Davis E, Caputo TA, Witkin SS, et al. Role of adjuvant chemotherapy in the management of non-granulosa cell ovarian sex cord-stromal tumors. J Gynecol Oncol. 2019;30(2):e19. 10.3802/jgo.2019.30.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seagle BL, Ann P, Butler S, Shahabi S. Ovarian granulosa cell tumor: a National Cancer Database study. Gynecol Oncol. 2017;146(2):285–91. 10.1016/j.ygyno.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 26. Oseledchyk A, Gennarelli RL, Leitao MM Jr, Aghajanian CA, Iasonos A, Zivanovic O, et al. Adjuvant chemotherapy in patients with operable granulosa cell tumors of the ovary: a surveillance, epidemiology, and end results cohort study. Cancer Med. 2018;7(6):2280–7. 10.1002/cam4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology . Practice bulletin No. 174: evaluation and management of adnexal masses. Obstet Gynecol. 2016;128(5):e210–26. 174 10.1097/AOG.0000000000001768. [DOI] [PubMed] [Google Scholar]
- 28. Pitta DR, Sarian LO, Campos EA, Andrade LLDA, Sallum LF, Bragança JF, et al. HE4 can help discriminate women with malignant ovarian tumors only if CA125 levels are elevated. Int J Biol Markers. 2013;28(4):e377–86. 10.5301/jbm.5000029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found here: https://seer.cancer.gov/data/. Further inquiries can be directed to the corresponding author.