Abstract
Poultry isolates of Campylobacter jejuni derived from a survey of meat processing batches were genotyped by pulsed-field gel electrophoresis (PFGE) of chromosomal DNA to establish the clonal relationships between single-colony isolates. In the majority of batches studied, one or two genotype patterns predominated. However, in one batch (batch A), 21 single-colony isolates gave 14 different PFGE genotypes. The banding patterns obtained with SmaI were sufficiently different to distinguish between genotypes, although the patterns also produced many common bands. The question of whether these isolates represented different clones or had a common clonal ancestry was addressed by additional genotypic and phenotypic methods. Restriction length polymorphism of PCR products obtained from the flagellin genes showed an identical flagellin genotype for all of these isolates. In contrast, unrelated control isolates resulted in different flagellin genotypes. Moreover, all 14 different PFGE genotypes of batch A had identical Penner serotypes and identical or similar biotypes and phage types. It was concluded that the isolates were of clonal origin and that the diversity in the PFGE banding patterns had most likely originated from genomic rearrangements. However, the PFGE genotypes were shown to be stable upon subculturing in vitro and after in vivo passage in chickens, and natural transformation between isogenic mutants carrying antibiotic markers did not occur in vivo in a chick colonization model. The possible mechanisms for the hypothesized genomic recombinations and the conditions that allow, induce, or select for such events are discussed.
Campylobacter jejuni is a common cause of human acute bacterial enteritis. This bacterium can be isolated from the gastrointestinal tracts of most domestic animals but appears to be highly adapted to the avian gut. Epidemiological studies indicate that the handling and consumption of raw or undercooked chicken pose a significant risk for human infection. Several factors contribute to the high incidence of contamination of poultry. Firstly, chickens can be colonized in the gut and, more specifically, in the cecum at very high levels (maximally about 1010 organisms per g of cecal content) without symptoms. Secondly, once some birds in a flock have acquired Campylobacter, the whole flock usually becomes colonized. In this way, many flocks of chickens are infected on the day of slaughter; e.g., in the United Kingdom up to 90% of the flocks can be infected (7). Thirdly, during slaughter and processing, cross-contamination of previously Campylobacter-negative carcasses may occur.
Flock colonization is generally restricted to one strain or a limited number of strains (3, 11, 16), and typing techniques indicate that certain subtypes may predominate in poultry. This predominance may be a reflection of enhanced survival of such strains in different environments, hosts, and hostile conditions, and their relative absence in human infections suggests a lower level of virulence (12). In order to investigate potential relationships between survival mechanisms and infection, more epidemiological data are required, especially at the subspecies level. Therefore, a survey was initiated to determine the rate of contamination of poultry produced in different European countries with Campylobacter and Salmonella species and to investigate a possible link to locally occurring human infections (9). The original survey was then extended by typing the Campylobacter isolates at the subspecies level in order to identify epidemiological trends.
In the past, subtyping of Campylobacter has been confined to serotyping. However, phenotyping methods such as serotyping are increasingly being replaced by methods based on molecular genetic techniques. The method selected for genotyping in our survey was pulsed-field gel electrophoresis (PFGE) of chromosomal DNA digested with rare cutting enzymes (19, 28). This method proved suitable for the identification of individual strains and therefore for the recognition of epidemiological trends within chickens and poultry samples. The general finding was that PFGE genotypes of isolates within a batch were either identical or considerably different (9a).
In the course of this epidemiological survey, our attention was drawn to one particular batch in which the genotypes of the isolates were shown to be similar but not identical. The clonal relationship of these isolates was determined. Aside from PFGE genotyping, single-locus PCR-restriction fragment length polymorphism (RFLP) and several phenotypic typing methods, including serotyping, were applied to establish the genotypic and phenotypic similarities of the isolates. The evidence presented here suggests that these isolates were of clonal origin but had undergone genomic rearrangements. Attempts to reproduce such events experimentally were unsuccessful. The mechanisms by which such events could have occurred in vivo or in vitro are discussed.
MATERIALS AND METHODS
Bacterial strains.
Campylobacter species were isolated from processed poultry from meat processing plants in Germany, The Netherlands, and France. Meat batch A (The Netherlands) comprised 30 packets of poultry collected in numerical order directly after packaging. Swabs were taken from the outside of the meat, a fresh cut inside the meat, the packet wrapping, and the dripping fluid and cultured for Campylobacter according to the method described by Geilhausen et al. (9). Isolates of batch B (The Netherlands) and batches C and D (France) were included in this study as controls, since they represent the diversity in genotypes that is usually observed. Preliminary bacterial characterization was undertaken according to the method described by Geilhausen et al. (9). Of the 30 meat packages tested from batch A, 19 were found positive for Campylobacter but not necessarily by all swabs. From each positive swab (31 in total), pure colonies were isolated, cultivated, and stored frozen at −80°C before genetic analysis. In one case, colonies were recognized with different morphologies—smooth and rough colonies. Both colony types were included in the analysis.
PFGE analysis.
Chromosomal DNA was isolated from Campylobacter isolates cultivated on Mueller-Hinton agar. Lysis of harvested and washed bacteria (in phosphate-buffered saline [PBS]) was performed in 1.2% agarose blocks with proteinase K and sarcosyl according to the method described by Yan et al. (28). After two washing steps in Tris-EDTA, the lysed agarose blocks were equilibrated three times in restriction enzyme reaction buffer, and the consequent DNA digestion with SmaI was performed for 4 h. For PFGE, a Pharmacia-LKB apparatus was used with pulses increasing from 5 to 40 for 20 h at 200 V and 9°C.
Flagellin PCR-RFLP analysis.
PCR-RFLP of the flagellin A and B genes was performed according to the technique of Ayling et al. (3), except that digestion was done with DdeI and HinfI.
Phenotyping methods.
Serotyping was performed according to the technique described by Penner and Hennessy (14). Phage typing and biotyping were performed as previously described (4, 15). All phenotyping was kindly undertaken by D. Wareing, Preston Public Health Laboratory Service, Preston, United Kingdom.
Chicken colonization model.
Eggs derived from specific-pathogen-free hens were hatched, and chicks were maintained in groups of 10 in isolators, with free access to feed and water. The chicks were orally dosed (105 CFU per bird) at 1 day of age with a Campylobacter suspension in PBS as described previously (25). After 5 days, the birds were killed, their abdomens were aseptically opened, and the contents of one cecum were collected and diluted in PBS for enumeration. Dilutions were plated on sheep blood agar plates containing 30 μg of cephaperazone per ml to suppress normal cecal flora and microaerobically incubated at 42°C to cultivate Campylobacter.
Colonization experiments to determine whether natural transformation occurs in vivo were carried out with two isogenic mutants of C. jejuni 81116, T1 and R3, which carried tetracycline (Tcr) and kanamycin (Kmr) cassettes in their flagellin B genes, respectively, at different insertion sites (27). One-day-old birds were dosed concurrently with the two mutants with approximately equal doses of 5 × 105 bacteria. Deletion of the flaB gene of strain 81116 has no deleterious effect on colonization in this chick model (25). To prevent selection pressure, the birds did not receive antibiotics. After 6 days, the cecal contents were cultured for Campylobacter on four different agar plates: without antibiotic selection, with tetracycline present, with kanamycin present, and under double-selection pressure.
RESULTS
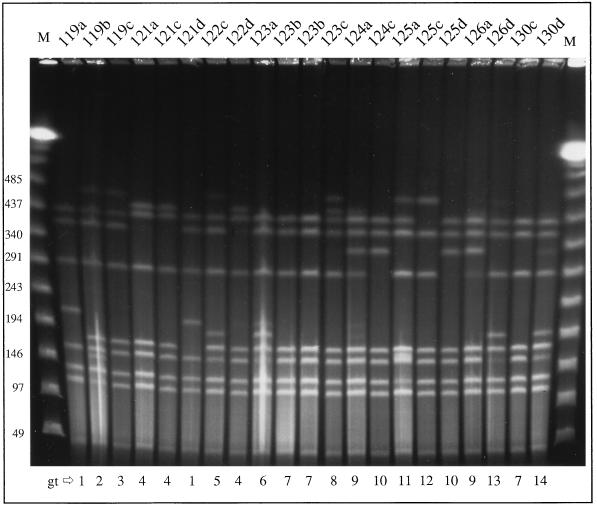
Genomic DNA of all batch A isolates was digested with SmaI and analyzed by PFGE. The resulting banding patterns looked similar in all isolates, but minor differences were observed between different swabs from one meat packet as well as between isolates from different packets. The similarity was greater than that of chicken isolates derived from unrelated meat batches, but the minor variations were significant enough and numerous enough to constitute strain differentiation according to accepted criteria (22). In total, 14 different but closely related genotype patterns, designated genotypes 1 to 14, were recognized. A subset of 21 of these isolates, comprising all 14 genotypes, was selected for further characterization and is summarized in Table 1. The SmaI-digested PFGE patterns obtained from these 21 isolates are shown in Fig. 1. In one instance identical genotypes were present in two swabs from one packet (genotype 4 in 121a and 121c), and in a few cases identical genotypes were present in swabs from different packets (e.g., genotype 1 in 121d and 119a; genotype 4 in 121a, 121c, and 122d; genotype 7 in 123b and 130c; and genotype 10 in 124c and 125d). The morphologically different isolates 123bs (smooth) and 123br (rough) had identical genotypes.
TABLE 1.
Sources and genotypic and phenotypic properties of the C. jejuni isolates investigated
| Batch | Meat package | Swaba | PFGE SmaI genotype | fla genotypeb | HS serotypec | Biotype | Phage type
|
|
|---|---|---|---|---|---|---|---|---|
| Type | Group | |||||||
| A | 119 | a | 1 | 6, 13 | 18 | 6404 | 0020 | 128 |
| 119 | b | 2 | 6, 13 | 18 | 6404 | 0020 | 128 | |
| 119 | c | 3 | 6, 13 | 18 | 6404 | 0020 | 128 | |
| 121 | a | 4 | 6, 13 | 18 | 6404 | 0020 | 128 | |
| 121 | c | 4 | 6, 13 | 18 | 6404 | 0020 | 128 | |
| 121 | d | 1 | 6, 13 | 18 | 6404 | 0020 | 128 | |
| 122 | c | 5 | 6, 13 | 18 | 6404 | 0020 | 128 | |
| 122 | d | 4 | 6, 13 | 18 | 6404 | 0020 | 128 | |
| 123 | a | 6 | 6, 13 | 18 | 6404 | 0020 | 128 | |
| 123 | bs | 7 | 6, 13 | 18 | 6400 | NT | NT | |
| 123 | br | 7 | 6, 13 | ND | ND | ND | ND | |
| 123 | c | 8 | 6, 13 | 18 | 6404 | NT | NT | |
| 124 | a | 9 | 6, 13 | 18 | 6404 | NT | NT | |
| 124 | c | 10 | 6, 13 | 18 | 6404 | 0020 | 128 | |
| 125 | a | 11 | 6, 13 | 18 | 6404 | NT | NT | |
| 125 | c | 12 | 6, 13 | 18 | 6404 | NT | NT | |
| 125 | d | 10 | 6, 13 | 18 | 6404 | NT | NT | |
| 126 | a | 9 | 6, 13 | 18 | 6004 | NT | NT | |
| 126 | d | 13 | 6, 13 | 18 | 6404 | 0020 | 128 | |
| 130 | c | 7 | 6, 13 | 18 | 6000 | 0020 | 128 | |
| 130 | d | 14 | 6, 13 | 18 | 6400 | 0020 | 128 | |
| B | 302 | a | 20 | 3, 4 | 27 | 6406 | 0800 | 117 |
| 303 | a | 20 | 1, 4 | 27 | 6406 | 0800 | 117 | |
| 307 | a | 21 | 8, 17 | 17 | 6004 | CEA9 | 52 | |
| 308 | a | 21 | 8, 17 | 17 | 6004 | EFEB | 1 | |
| C | 317 | a | 22 | 1, 1 | 4 complex | 6610 | 0001 | 146 |
| 318 | a | 22 | 1, 1 | 4 complex | 6710 | NT | NT | |
| D | 331 | a | 23 | 1, 12 | NT | 6610 | 2142 | 90 |
Isolates obtained from swabs taken from (a) fluid dripping from meat packet, (b) meat surface, (c) freshly cut meat, and (d) wrapping. Two different colony morphologies were recognized in isolate 123b, with bs representing smooth colonies and br representing rough colonies.
The fla genotype designation comprises the HinfI and the DdeI profile numbers, respectively.
HS, heat-stable antigen serotype. Serotyping was kindly performed by D. Wareing according to the technique of Penner and Hennessy (14). NT, not typeable; i.e., the strain is not sensitive to any of the tested bacteriophages. ND, not determined.
FIG. 1.
PFGE of chromosomal DNA of C. jejuni isolates from poultry batch A digested with SmaI. Isolates are designated above the lanes. The genotypes (gt) are given below the lanes. M, molecular mass marker (in kilobase pairs).
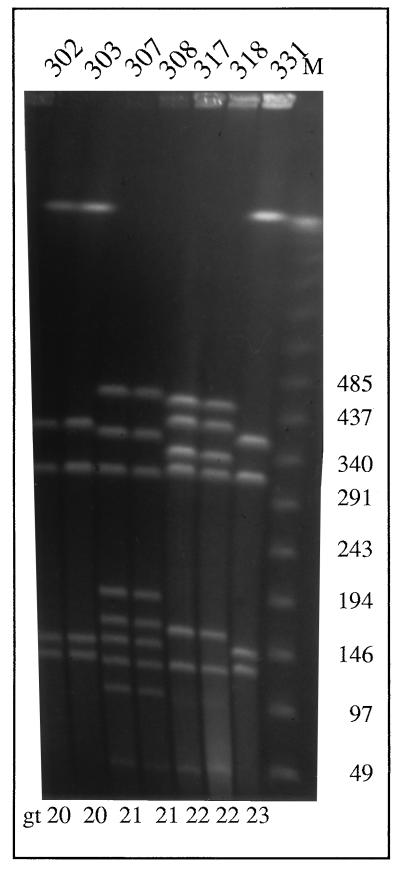
The 14 genotypes found within one poultry batch confirmed the high discriminatory power of the PFGE genotyping technique, but this high number was rather unexpected given that for all other poultry batches examined, the genotypic diversity was limited to one or two PFGE patterns (results not shown). However, isolates from unrelated batches had distinct genotypes. To illustrate, seven chicken strains isolated from three unrelated batches (B, C, and D) demonstrate the limited diversity that is normally observed (Fig. 2); two banding patterns within batch B were clearly different from each other but were observed in several isolates from the same batch (302 and 303 versus 307 and 308).
FIG. 2.
PFGE of SmaI digests of isolates from batch B (302, 303, 307, and 308), batch C (317 and 318), and batch D (331).
To determine if the diversity in genotypes of batch A isolates was specific for SmaI digestions, the PFGE analysis was repeated with several other enzymes. PFGE analysis of Campylobacter DNA has been described for KpnI and SalI, but in our experience, KpnI does not give good results and SalI gives too few bands to be discriminative. Another enzyme, BssHII, gave good banding patterns, and the results obtained confirmed the numerous differences in genotypes within batch A. The isolates with identical SmaI genotypes were also identical by BssHII digestion, and different genotypes corresponded to both enzymes (results not shown).
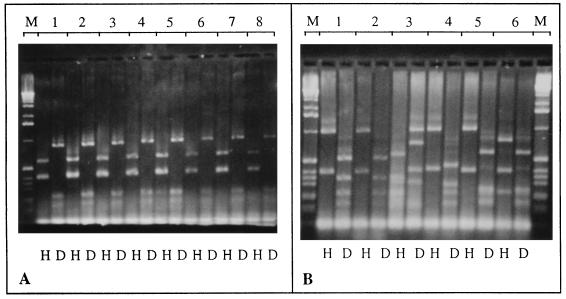
To determine the clonal relationship of these poultry isolates, the 21 batch A isolates and 7 unrelated control isolates were further characterized by a second molecular-typing method, flagellin PCR-RFLP genotyping (fla genotyping) as well as by phenotyping. All isolates of batch A had identical fla genotypes (Fig. 3A). In contrast, the fla genotype varied between batches and between the PFGE genotypes of batches B, C, and D (Fig. 3B). There was a strict correlation between the fla genotypes and the PFGE genotypes in the control isolates, with the exception of 302 and 303, which had identical PFGE genotypes but different fla genotypes (Table 1). All isolates with identical fla genotypes had identical Penner serotypes, whereas the unrelated isolates had different serotypes. Furthermore, the biotypes and phage types were identical or similar in batch A isolates (Table 1). Phage typing resulted in lysis with one phage in 13 of the isolates, while 7 isolates had lost this single-phage sensitivity and thus became nontypeable. The control strains showed a wide variation in biotype and phage type (Table 1).
FIG. 3.
PCR-RFLP profiles of amplified flagellin products digested with DdeI (D) and HinfI (H). (A) A subset of eight isolates (lanes 1 to 8: 123a, 123b, 123c, 124a, 124c, 125a, 125d, and 125c, respectively) from batch A is shown. All other isolates from this batch displayed identical profiles. (B) Isolates 318 (lane 1) and 317 (lane 2) from batch C; 307 (lane 3), 303 (lane 5), and 302 (lane 6) from batch B; and 331 (lane 4) from batch D. M, marker.
The stability of 14 isolates of batch A, representing all 14 different PFGE genotypes, was tested by repeated subculture for 10 passages. Care was taken to inoculate each subsequent plate from the beginning of the streak to avoid selection of single colonies. Only after the 10th generation was a single colony taken for further cultivation to isolate DNA. The PFGE genotypes of all 14 colonies remained stable during the in vitro passaging and after storage at −80°C (results not shown).
Genotypic stability was also tested during in vivo passage. One isolate, 130c (genotype 7), was selected because its genotype was the most common in the isolates investigated. Two 1-day-old chicks were orally dosed with 105 bacteria, and passaged organisms were recovered from the cecal contents after 5 days. Twenty single colonies per bird were isolated and subcultured for DNA isolation and PFGE genotyping. All 40 postcolonization isolates tested had the same genotype as the original infective strain (results not shown), indicating that the genotype was stable during colonization in chicks under the conditions applied.
To determine whether the observed genetic variations were a result of natural transformation, experiments were carried out to allow DNA exchange under in vivo conditions in which numerous bacteria are present, i.e., in chicken cecum. Isogenic mutants that contained antibiotic resistance markers were used to enable screening for recombinants. Birds were inoculated with two isogenic mutants of the laboratory-adapted strain C. jejuni 81116, R3 and T1, containing a Kmr and a Tcr cassette, respectively. These mutants have been shown to exchange DNA in vitro, resulting in double-resistant recombinants (27). After colonization for 6 days, the cecal content was cultured for the presence of both mutants and Tcr and Kmr recombinants. In three independent experiments, the birds had become colonized by the individual mutants at >106 CFU per g of cecal content, but double-resistant recombinants were not detected. In these experiments, a comparison of the number of CFU detected on agar plates with and without antibiotics suggested that no detectable proportion of the colonizing bacterial population had lost antibiotic resistance markers. Therefore, these results suggest that natural transformation does not occur during colonization in this model of 1-day-old chicks.
DISCUSSION
As part of a survey on poultry contamination, isolates of C. jejuni were genotyped by PFGE. In one instance, an interesting variation in PFGE genotypes was observed between isolates derived from a single batch of poultry. These isolates, which had similar but not identical PFGE genotypes, subsequently were shown to have identical fla genotypes as well as identical or similar Penner serotypes, biotypes, and phage types. These typing data suggest that these isolates, which were isolated from a common source, are of clonal origin. The variation in PFGE genotype may be attributable to genomic variation. Such variations may arise by several mechanisms. The most likely mechanisms are mosaic rearrangements due to genomic instability either occurring spontaneously or induced by mobile elements, programmed DNA inversion, or natural transformation with foreign DNA (23, 24). The number of variants observed (14 genotypes in 31 isolates) suggests that such events can occur with a high frequency, at least in this strain.
Programmed DNA inversion has been shown to be the mechanism for antigenic variation in the S-layer protein of Campylobacter fetus (5, 6, 8). However, there are two reasons that a similar mechanism is not likely to be responsible for the genomic variation observed here: (i) although the DNA fragment involved in the inversion of C. fetus is relatively large, it is shorter than 10 kb, and therefore such inversions would result in minute shifts in pulsed-field banding patterns and (ii) programmed DNA inversion is limited to one genomic locus, so changes in PFGE banding patterns would be limited to a few bands, with all other bands remaining constant.
The observed variation in PFGE patterns is significant enough to confound interpretation of PFGE genotyping. In contrast, single-locus PCR-RFLP, in this case based on the flagellin genes, was not influenced by this PFGE-detectable genomic variation, but PCR-RFLP may not be as discriminatory as PFGE (17). It should be noted, however, that in the flagellin, locus recombinations between flaA and flaB probably occur at an unknown rate. Such recombinations potentially reduce the value of a flagellin-genotyping scheme (2, 10, 27). The direct correlation between different flagellin genotypes and Penner serotypes is not always consistent (1, 3) and is a reflection of the small numbers of isolates investigated. Nevertheless, in this study, in which isolates were collected and compared batchwise, the flagellin-genotyping method was more consistent with accepted phenotypic techniques than the PFGE method. The results of this study indicate that a combination of at least two genotyping methods may be necessary to accurately answer questions of bacterial lineage.
Of the possible explanations for genotypic variation—spontaneous intramolecular genomic rearrangements, recombinations as a result of mobile elements, or recombinations between the genomes of two distinct strains as a consequence of natural transformation—the first two mechanisms require active DNA recombination and repair machinery, and a role for genes such as recA would be anticipated. Natural transformation and possibly phage or transposon activity, but not recombination, could result in partial duplication of genetic information. The presence of duplications in the genotypic variants of batch A could not be deduced from the PFGE banding patterns. Interestingly, similar genetic variations, also identified by PFGE during an epidemiological study of Campylobacter coli and C. jejuni from ostriches, were apparently associated with an increase in genome size and therefore indicated that natural transformation is the mechanism that explains genotypic variation (18).
Experiments were conducted to determine if spontaneous transformation is possible during naturally occurring conditions. Since the chicken gut can harbor an extremely high density of bacteria, and the rapid turnover suggests the ample presence of DNA, the possibility that natural transformation occurs during chicken colonization was investigated. To optimize the transformation conditions and ensure a sensitive detection system, isogenic mutants were used which had previously been shown to exchange DNA in vitro at a high frequency (27). Nevertheless, DNA exchange was not observed in vivo. This result, which can be explained by either a high amount of nucleases present in the gut or by the assumption that bacteria are not competent to take up DNA under in vivo conditions, makes it less likely that the genetic variation observed in batch A isolates resulted from natural transformation during chicken colonization. It cannot be excluded, however, that transformation can occur under stressful environmental conditions such as horizontal transmission throughout a flock or meat processing.
It cannot be excluded that genomic instability via intramolecular rearrangements is the mechanism for the observed genetic heterogeneity, although such events have not yet been reproduced in the laboratory. The predominant genotype was stable upon in vitro subculturing over 10 generations and during passage within the chicken gut. The observed loss of sensitivity to a single phage in some batch A isolates is particularly interesting and suggests that phage activity, possibly in combination with environmental pressure, could be involved. It seems likely that Campylobacter encounters bacteriophages in the chicken gut or in the environment.
Bacteria with relatively small genomes, such as C. jejuni and the related organism Helicobacter pylori, may undergo genetic variation to increase their potential to adapt to new environments (1, 13, 20, 21). Such genotypic variation could result in phenotypic changes. The observed minor variations in biotype and phage type of batch A isolates may reflect such changes. Although the differences in biotype 6404 (16 isolates), 6400 (2 isolates), 6006, and 6000 were not considered significant enough to justify the discrimination of more than one strain within batch A (24), they could be indicative of changes in a single genetic locus.
The phenotypes and adaptive abilities of the genotypic variants isolated in this study are now being investigated. In general, C. jejuni must be able to adapt to many different environments. The bacteria have been isolated from the intestines of a number of hosts and during the contamination route from chicken to man must survive several hostile environments. Genotypic variation that results in phenotypic changes could allow genotypic variants to be formed or selected during such environmental transitions.
ACKNOWLEDGMENTS
We thank D. Wareing (PHLS Preston) for phenotyping the strains and R. A. Ayling, S. Cawthraw, and K. Clow (CVL Weybridge) for undertaking the chicken studies and fla typing. J. Wagenaar and W. Jacobs-Reitsma are thanked for critical reading of the manuscript.
We thank the Ministry of Agriculture, Fisheries and Foods, GB, for funding for some of this work.
REFERENCES
- 1.Aarts H J, Van Lith L A, Jacobs-Reitsma W F. Discrepancy between Penner serotyping and polymerase chain reaction fingerprinting of Campylobacter isolated from poultry and other animal sources. Lett Appl Microbiol. 1995;20:371–374. doi: 10.1111/j.1472-765x.1995.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 2.Alm R A, Guerry P, Trust T J. Significance of duplicated flagellin genes in Campylobacter. J Mol Biol. 1993;230:359–363. doi: 10.1006/jmbi.1993.1151. [DOI] [PubMed] [Google Scholar]
- 3.Ayling R D, Woodward M J, Evans S, Newell D G. Restriction fragment length polymorphism of polymerase chain reaction products applied to the differentiation of poultry campylobacters for epidemiological investigations. Res Vet Sci. 1996;60:168–172. doi: 10.1016/s0034-5288(96)90013-2. [DOI] [PubMed] [Google Scholar]
- 4.Bolton F J, Wareing D R A, Skirrow M B, Hutchinson D N. SAB tech series no. 29. London, England: Academic Press; 1992. pp. 151–161. [Google Scholar]
- 5.Dworkin J, Blaser M J. Molecular mechanisms of Campylobacter fetus surface layer protein expression. Mol Microbiol. 1997;26:433–440. doi: 10.1046/j.1365-2958.1997.6151958.x. [DOI] [PubMed] [Google Scholar]
- 6.Dworkin J, Shedd O L, Blaser M J. Nested DNA inversion of Campylobacter fetus S-layer genes is recA dependent. J Bacteriol. 1997;179:7523–7539. doi: 10.1128/jb.179.23.7523-7529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans S J. Introduction and spread of thermophilic campylobacters in broiler flocks. Vet Rec. 1997;131:574–576. [PubMed] [Google Scholar]
- 8.Garcia M M, Lutze-Wallace C L, Denes A S, Eaglesome M D, Holst E, Blaser M J. Protein shift and antigenic variation in the S-layer of Campylobacter fetus subsp. venerealis during bovine infection accompanied by genomic rearrangement of sapA homologs. J Bacteriol. 1995;177:1976–1980. doi: 10.1128/jb.177.8.1976-1980.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geilhausen B, Schutt-Gerowitt H, Aleksic S, Koenen R, Mauff G, Pulverer G. Campylobacter and Salmonella contaminating fresh chicken meat. Zentralbl Bakteriol. 1996;284:241–245. doi: 10.1016/s0934-8840(96)80099-5. [DOI] [PubMed] [Google Scholar]
- 9a.Geilhausen, B. Unpublished data.
- 10.Harrington C S, Thomson-Carter F M, Carter P E. Evidence for recombination in the flagellin locus of Campylobacter jejuni: implications for the flagellin gene typing scheme. J Clin Microbiol. 1997;35:2386–2392. doi: 10.1128/jcm.35.9.2386-2392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs-Reitsma W F, Van de Giessen A W, Bolder N M, Mulder R W A W. Epidemiology of Campylobacter spp. at two Dutch broiler farms. Epidemiol Infect. 1995;114:413–421. doi: 10.1017/s0950268800052122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koenraad P M F J, Ayling R, Hazeleger W C, Rombouts F M, Newell D G. The speciation and subtyping of Campylobacter isolates from sewage plants and waste water from a connected poultry abattoir using molecular techniques. Epidemiol Infect. 1995;115:485–494. doi: 10.1017/s0950268800058647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logan R P H, Berg D E. Genetic diversity of Helicobacter pylori. Lancet. 1996;348:1462–1463. doi: 10.1016/s0140-6736(05)65885-0. [DOI] [PubMed] [Google Scholar]
- 14.Penner J L, Hennessy J N. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol. 1980;12:732–737. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salama S M, Bolton F J, Hutchinson D M. Application of a new phagetyping scheme to campylobacters isolated during outbreaks. Epidemiol Infect. 1990;104:405–411. doi: 10.1017/s0950268800047427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shane S M. The significance of Campylobacter jejuni infection in poultry: a review. Avian Pathol. 1992;21:189–213. doi: 10.1080/03079459208418836. [DOI] [PubMed] [Google Scholar]
- 17.Stanley J, Linton D, Sutherland K, Jones C, Owen R J. High-resolution genotyping of Campylobacter coli identifies clones of epidemiologic and evolutionary significance. J Infect Dis. 1995;172:1130–1134. doi: 10.1093/infdis/172.4.1130. [DOI] [PubMed] [Google Scholar]
- 18.Stephens, C. P., S. L. W. On, and J. A. Gibson. An outbreak of infectious hepatitis in commercially reared ostriches associated with Campylobacter coli and Campylobacter jejuni. Vet. Microbiol., in press. [DOI] [PubMed]
- 19.Suzuki Y, Ishihara M, Funabashi M, Suzuki R, Isomora S, Yokochi T. Pulsed field gel electrophoretic analysis of Campylobacter jejuni DNA for use in epidemiological studies. J Infect. 1993;27:39–42. doi: 10.1016/0163-4453(93)93628-h. [DOI] [PubMed] [Google Scholar]
- 20.Taylor D E. Genetic analysis of Campylobacter spp. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C: American Society for Microbiology; 1992. pp. 255–266. [Google Scholar]
- 21.Taylor D E, Eaton M, Chang N, Salama S M. Construction of a Helicobacter pylori genome map and demonstration of diversity at the genome level. J Bacteriol. 1992;174:6800–6806. doi: 10.1128/jb.174.21.6800-6806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Muruay B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Taylor D. Natural transformation in Campylobacter species. J Bacteriol. 1990;172:949–955. doi: 10.1128/jb.172.2.949-955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wareing, D. Personal communication.
- 25.Wassenaar T M, van der Zeijst B A M, Ayling R, Newell D G. Colonization of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. J Gen Microbiol. 1993;139:1171–1175. doi: 10.1099/00221287-139-6-1171. [DOI] [PubMed] [Google Scholar]
- 26.Wassenaar T M, Fry B N, van der Zeijst B A M. Genetic manipulation of Campylobacter: evaluation of natural transformation and electro-transformation. Gene. 1993;132:131–135. doi: 10.1016/0378-1119(93)90525-8. [DOI] [PubMed] [Google Scholar]
- 27.Wassenaar T M, Fry B N, van der Zeijst B A M. Variation of the flagellin gene locus of Campylobacter jejuni by recombination and horizontal gene transfer. Microbiology. 1995;141:95–101. doi: 10.1099/00221287-141-1-95. [DOI] [PubMed] [Google Scholar]
- 28.Yan W, Chang N, Taylor D E. Pulsed-field gel electrophoresis of Campylobacter jejuni and Campylobacter coli genomic DNA and its epidemiologic application. J Infect Dis. 1991;163:1068–1072. doi: 10.1093/infdis/163.5.1068. [DOI] [PubMed] [Google Scholar]