Abstract
An systematic phenotypic screen of the mouse gut microbiome for metabolites with an immunomodulatory effect identified Muribaculum intestinale as one of only two members with an oversized effect on T-cell populations. Here we report the identification and characterization of a lipid, MiCL-1, as the responsible metabolite. MiCL-1 is an 18:1-16:0 cardiolipin, whose close relatives are found on concave lipid surfaces of both mammals and bacteria. MiCL-1 was synthesized to confirm the structural analysis and functionally characterized in cell-based assays. It has a highly restrictive structure–activity profile, as its chain-switched analog fails to induce responses in any of our assays. MiCL-1 robustly induces the production of pro-inflammatory cytokines like TNF-α, IL-6, and IL-23, but has no detectable effect on the anti-inflammatory cytokine IL-10. As is the case with other recently discovered immunomodulatory lipids, MiCL-1 requires functional TLR2 and TLR1 but not TLR6 in cell-based assays.
The innate immune system constantly surveils members of the gut microbiome to detect potential pathogens and generate appropriate immune responses.1,2 This surveillance relies on specialized receptors that detect molecules with structural features associated with pathogens: PAMPS (pathogen-associated molecular patterns). The defining characteristics of these features are imperfectly understood, and we have initiated a program to improve our understanding.
A recent report identified two unrelated strains, Akkermansia muciniphila and Muribaculum intestinale (S24–7), with a similar ability to induce adaptive immune responses during homeostasis.3A. muciniphila has a prominent role in several studies relating gut microbes with health and disease.4−7 In a recent study of A. muciniphila, we reported that a single phosphatidylethanolamine (PE), a15:0-i15:0 PE with different branched chain fatty acids at both the sn-1 and sn-2 positions induced in vitro homeostatic cellular responses.8M. intestinale, a Gram-negative obligate anaerobe recently discovered in the mouse gut microbiome, is less studied.9 It has been associated with inflammatory bowel disease in both mouse and human studies by several research groups.10−13 The link of these two disparate microbes with similar immune responses raises questions about the similarity, if any, of the responsible metabolites and their associated mechanisms.14
We assayed M. intestinale (DSM 28989) cultures with an assay-guided fractionation technique used in earlier studies.8,15,16 The crude cell pellet extract of M. intestinale cultures showed significant pro-inflammatory activity: TNF-α induction was observed in murine bone marrow dendritic cells (mBMDCs). Further purification identified a single active fraction, which was further subjected to a series of chromatographic separations and functional analysis to identify a single compound, which we named MiCL-1 (1) (Figures 1a and 2).
Figure 1.
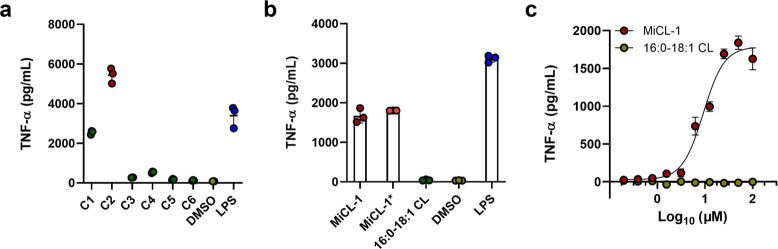
Pro-inflammatory activity of M. intestinale. (a) TNF-α production by mBMDCs stimulated with cell pellet fractions from M. intestinale cultures. (b) TNF-α production of MiCL-1, MiCL-1*, and 16:0-18:1 CL in mBMDCs. (c) Dose–response of TNF-α production in mBMDCs for MiCL-1 and 16:0-18:1 CL. LPS and Pam3CSK4 were used as positive control. Error bars = SD of technical replicates (n = 3 or 4).
Figure 2.
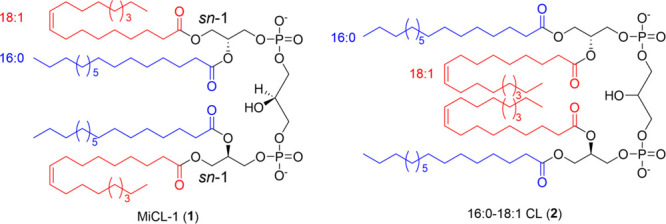
Structures of MiCL-1 (1) and its chain-switched isomer (2).
MiCL-1 was assigned the molecular formula C77H146O17P2 based on high-resolution mass spectrometry (observed [M – H]− at m/z 1403.9913, calc. 1403.9962). A combined analysis of 1H, 13C, and HSQC NMR data identified a pseudosymmetric dimer with two phosphatidylglycerols, two paired carbonyl signals, two paired olefinic methine signals, five paired oxygenated methylene/methine groups, many aliphatic methylene groups, and four virtually identical methyl groups. Careful interpretation of 1H–1H COSY and HMBC data afforded the chemical structure of MiCL-1 as a canonical cardiolipin (CL), with four fatty acid esters attached to the sn-1/sn-1’ and sn-2/sn-2’ positions, three glycerol fragments linked by two phosphate diesters, which were further supported the by 31P NMR data (Figure S8).17 The composition of fatty acids was determined to be oleic acid (18:1) and palmitic acid (16:0) through fatty acid methyl esterification for gas chromatography–mass spectrometry analysis (FAME).18,19 The order of the fatty acids was originally established by selective O-deacylation at the sn-2 and sn-2’ positions for NMR as well as HRMS analysis. Therefore, MiCL-1 is 18:1/16:0/18:1/16:0 cardiolipin, abbreviated 18:1-16:0 CL (Figure 2).
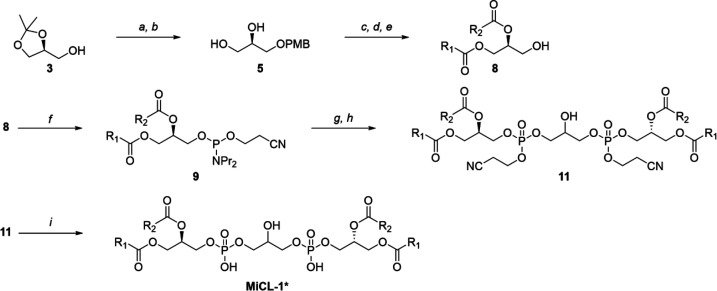
The chain-switched isomer of MiCl-1, 16:0-18:1 CL, is commercially available (Avanti Polar Lipids), and while the purchased material had almost identical spectral and chromatographic behavior to that of MiCL-1 it had no activity in our assays, a result that indicates a remarkably constrained structure–activity relationship. To rule out contamination and/or a flawed structural analysis, we synthesized the proposed structure for MiCL-1, which we designate as MiCL-1*—same structure, different sources. The synthesis is outlined in Scheme 1. A protected glycerol, (+)-1,2-O-isopropylidene-sn-glycerol (3), was converted to PMB ether (4), which was subsequently converted to 1,2-diol (5). Regiospecific esterification of the primary alcohol of 5 with oleoyl chloride led to sn-1-acylated 6. Esterification of the remaining secondary hydroxyl group with palmitic acid provided diacylglycerol 8 after selective deprotection of the PMB group. Reaction of 8 with 2-cyanoethyl-N,N,N′,N′-tetraisopropyl phosphorodiamidite generated the phosphoramidite intermediate 9 as a mixture of diastereomers due to the creation of a stereogenic phosphorus atom. The coupling reaction with intermediate 9 and PMB-protected-1,3-diol followed by subsequent removal of PMB group generated a diastereomeric mixture of protected 18:1-16:0 CL. Finally, deprotection of the cyanoethyl group yielded MiCL-1*.20−25
Scheme 1. Total Synthesis of MiCL-1*.
Reagents: (a) NaH, PMBCl, DMF; (b) PTSA, MeOH; (c) 2,4,6-collidine, oleoyl chloride, DCM; (d) DMAP, EDC HCl, palmitic acid, DCM; (e) DDQ, DCM; (f) 1H-tetrazole, bis(diisopropylamino)(2-cyanoethoxy)phosphine, DCM; (g) PMB-protected glycerol, 1H-tetrazole, DCM/MeCN, then H2O2; (h) DDQ, DCM; (i) DBU, DCM, then AcOH.
Three molecules MiCL-1, MiCL-1*, and 16:0-18:1 CL, were evaluated for TNF-α induction from mBMDCs. MiCL-1 and MiCL-1* have equal and significant activity (EC50 = 9.2 μM), while 16:0-18:1 CL has no detectable activity (Figure 1b and c). Dendritic cells detect bacterial metabolites through toll-like receptor 2 (TLR2) and toll-like receptor 4 (TLR4).26 Receptor specificity was established using mBMDCs from genetically altered tlr2–/– and tlr4–/- mice. MiCL-1 active in wild-type cell assays produced a robust TNF-α induction in mBMDCs lacking TLR4 and but no induction in mBMDCs lacking TLR2 as shown in Figure 3.
Figure 3.
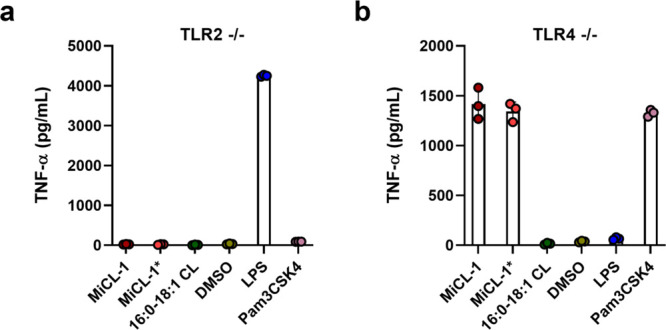
(a) TNF-α production by BMDCs from tlr2–/– mice. (b) TNF-α production by BMDCs from tlr4–/– mice. LPS and Pam3CSK4 were used as positive controls. Error bars are SD of technical replicates (n = 3).
Since M. intestinale was isolated from mice and characterized using in vitro assays with murine cells, it was important to see if assays with human cells behaved similarly. This was accomplished by assaying MiCL-1, MiCL-1*, and 16:0-18:1 CL in human monocyte-derived dendritic cells (MDDCs). Human MDDCs assays for cytokine induction showed robust release of the pro-inflammatory cytokines TNF-α, IL-6, and IL-23, and negligible induction of IL-10, an important anti-inflammatory cytokine (Figure 4).
Figure 4.
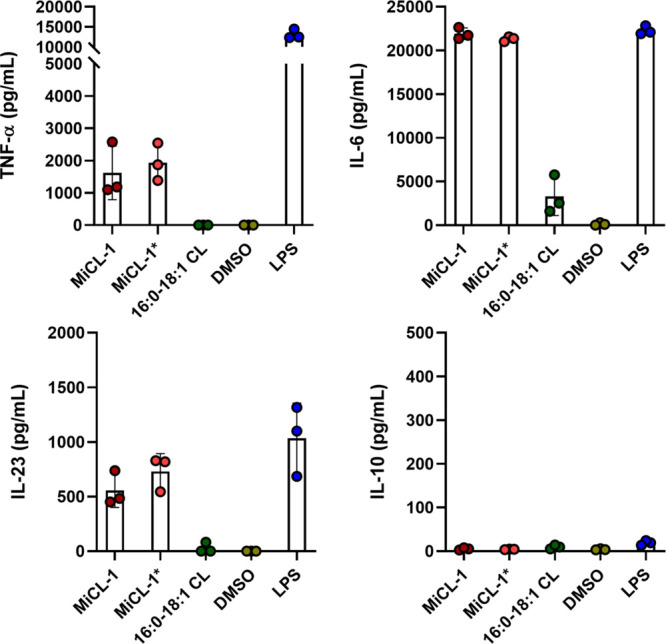
Induction of cytokines (TNF-α, IL-6, IL-23, and IL-10) from human MDDCs activated by MiCL-1, MiCL-1*, and 16:0-18:1 CL. LPS and Pam3CSK4 were used as positive controls. Error bars = SD of technical replicates (n = 3).
TLR2 typically forms a heterodimer with either TLR1 or TLR6. A CRISPR/cas9 knockdown of TLR1 and TLR6 in human MDDCs was used to show that MiCL-1 uses the TLR2-TLR1 signaling pathway for TNF-α induction (Figure 5).
Figure 5.
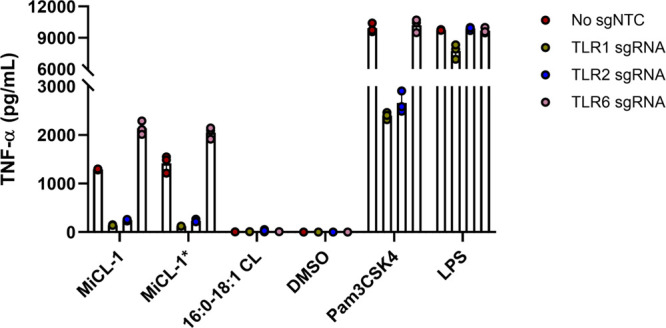
TNF-α induction of CRISPR/Cas9 targeting by human MDDCs treated with MiCL-1, MiCL-1*, and 16:0-18:1 CL. LPS and Pam3CSK4 were used as positive controls. Error bars = SD of technical replicates (n = 3).
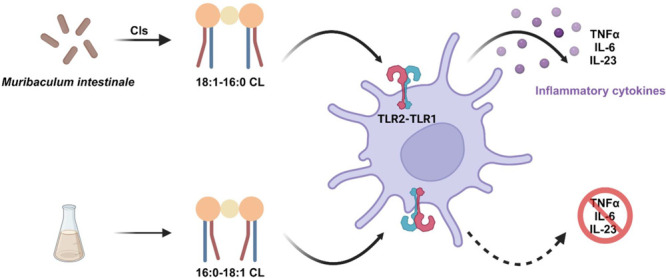
This study was motivated by the similar behavior of two bacterial strains, A. muciniphila and M. intestinale, in an immunomodulatory screen.3 The active metabolite from A. muciniphila had been identified as a15:0-i15:0 PE, a membrane lipid, and this study identified 18:1-16:0 CL, also a membrane lipid, as its counterpart from M. intestinale. What can be learned from a comparison? Both are membrane lipids with no distinctive headgroup; information for immunomodulatory responses is encoded in the acyl chains. In both, the acyl chains show a highly restricted structure–activity relationship (unsaturated fatty acids at sn-1/sn-1’ and a saturated fatty acid at sn-2/sn-2’), and no naturally occurring active metabolites with similar structures were identified in either study. While the lipids belong to different classes, they both use TLR2-TLR1 receptors and have similar EC50 values. They have similar, but not identical, cytokine profiles, and the most significant difference seems to be IL-23 induction.27 An additional and completely unexpected feature of MiCL-1 is its similarity to a recently characterized immunomodulatory metabolite from Streptococcus pyogenes.28 The two, MiCL-1 and SpCL-1, are essentially identical as they are related by switching a C16 saturated fatty acid (16:0) with a C18 saturated fatty acid (18:0), and both use TLR2-TLR1.28 It is likely that the class of cardiolipin-based immunomodulators from human-associated bacteria will continue to expand.
Acknowledgments
This work was funded by NIH R01 AT009708 and NIH R01 AI172147. We thank the Harvard Medical School’s Analytical Chemistry Core (ACC) and East Quad NMR facility for their analytical service. Figures were created with BioRender.com.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c09734.
Supplementary figures, NMR spectra for synthetic compounds and detailed experimental method (PDF)
Author Contributions
# S.B. and Y.-H.S. contributed equally to this work. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare the following competing financial interest(s): Some of the authors have filed a patent application related to the research reported in this article.
Supplementary Material
References
- Thaiss C. A.; Zmora N.; Levy M.; Elinav E. The microbiome and innate immunity. Nature 2016, 535, 65–74. 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- Belkaid Y.; Harrison O. J. Homeostatic Immunity and the Microbiota. Immunity 2017, 46, 562–576. 10.1016/j.immuni.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansaldo E.; Slayden L. C.; Ching K. L.; Koch M. A.; Wolf N. K.; Plichta D. R.; Brown E. M.; Graham D. B.; Xavier R. J.; Moon J. J.; Barton G. M. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 2019, 364, 1179–1184. 10.1126/science.aaw7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plovier H.; Everard A.; Druart C.; Depommier C.; Hul M. V.; Geurts L.; Chilloux J.; Ottman N.; Duparc T.; Lichtenstein L.; Myridakis A.; Delzenne N. M.; Klievink J.; Bhattacharjee A.; van der Ark K. C. H.; Aalvink S.; Martinez L. O.; Dumas M.-E.; Maiter D.; Loumaye A.; Hermans M. P.; Thissen J.-P.; Belzer C.; de Vos W. M.; Cani P. D. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- Everard A.; Belzer C.; Geurts L.; Ouwerkerk J. P.; Druart C.; Bindels L. B.; Guiot Y.; Derrien M.; Muccioli G. G.; Delzenne N. M.; de Vos W. M.; Cani P. D. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. National Acad. Sci. 2013, 110, 9066–9071. 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derosa L.; Routy B.; Thomas A. M.; Iebba V.; Zalcman G.; Fri ard S.; Mazieres J.; Audigier-Valette C.; Moro-Sibilot D.; Goldwasser F.; Silva C. A. C.; Terrisse S.; Bonvalet M.; Scherpereel A.; Pegliasco H.; Richard C.; Ghiringhelli F.; Elkrief A.; Desilets A.; Blanc-Durand F.; Cumbo F.; Blanco A.; Boidot R.; Chevrier S.; Daillère R.; Kroemer G.; Alla L.; Pons N.; Chatelier E. L.; Galleron N.; Roume H.; Dubuisson A.; Bouchard N.; Messaoudene M.; Drubay D.; Deutsch E.; Barlesi F.; Planchard D.; Segata N.; Martinez S.; Zitvogel L.; Soria J.-C.; Besse B. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 2022, 28, 315–324. 10.1038/s41591-021-01655-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depommier C.; Everard A.; Druart C.; Plovier H.; Hul M. V.; Vieira-Silva S.; Falony G.; Raes J.; Maiter D.; Delzenne N. M.; Barsy M. de; Loumaye A.; Hermans M. P.; Thissen J.-P.; Vos W. M. de; Cani P. D. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae M.; Cassilly C. D.; Liu X.; Park S.-M.; Tusi B. K.; Chen X.; Kwon J.; Filipčík P.; Bolze A. S.; Liu Z.; Vlamakis H.; Graham D. B.; Buhrlage S. J.; Xavier R. J.; Clardy J. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature 2022, 608, 168–173. 10.1038/s41586-022-04985-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod K. L.; Wood D. L. A.; Lachner N.; Gellatly S. L.; Daly J. N.; Parsons J. D.; Dal’Molin C. G. O.; Palfreyman R. W.; Nielsen L. K.; Cooper M. A.; Morrison M.; Hansbro P. M.; Hugenholtz P. Genomic characterization of the uncultured Bacteroidales family S24–7 inhabiting the guts of homeothermic animals. Microbiome 2016, 4, 36. 10.1186/s40168-016-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. B.; Luo C.; O’Connell D. J.; Lefkovith A.; Brown E. M.; Yassour M.; Varma M.; Abelin J. G.; Conway K. L.; Jasso G. J.; Matar C. G.; Carr S. A.; Xavier R. J. Antigen discovery and specification of immunodominance hierarchies for MHCII-restricted epitopes. Nat. Med. 2018, 24, 1762–1772. 10.1038/s41591-018-0203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen T. K.; Brown E. M.; Plichta D. R.; Johansen J.; Twar dus S. W.; Delorey T. M.; Lau H.; Vlamakis H.; Moon J. J.; Xavier R. J.; Graham D. B. The CD4+ T cell response to a commensal-derived epitope transitions from a tolerant to an inflammatory state in Crohn’s disease. Immunity 2022, 55, 1909–1923.e6. 10.1016/j.immuni.2022.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobranowski P. A.; Tang C.; Sauvé J. P.; Menzies S. C.; Sly L. M. Compositional changes to the ileal microbiome precede the onset of spontaneous ileitis in SHIP deficient mice. Gut Microbes 2019, 10, 578–598. 10.1080/19490976.2018.1560767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara M. P.; Singleton J. M.; Cadney M. D.; Ruegger P. M.; Borneman J.; Garland T. Early-life effects of juvenile Western diet and exercise on adult gut microbiome composition in mice. J. Exp Biol. 2021, 224, jeb239699. 10.1242/jeb.239699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. B.; Xavier R. J. Conditioning of the immune sys tem by the microbiome. Trends Immunol. 2023, 44, 499–511. 10.1016/j.it.2023.05.002. [DOI] [PubMed] [Google Scholar]
- Henke M. T.; Kenny D. J.; Cassilly C. D.; Vlamakis H.; Xavier R. J.; Clardy J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc. National Acad. Sci. 2019, 116, 12672–12677. 10.1073/pnas.1904099116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon J.; Bae M.; Szamosvári D.; Cassilly C. D.; Bolze A. S.; Jackson D. R.; Xavier R. J.; Clardy J. Collinsella aerofaciens Produces a pH-Responsive Lipid Immunogen. J. Am. Chem. Soc. 2023, 145, 7071–7074. 10.1021/jacs.3c00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinot C.; Gaysinski M.; Thomas O. P.; Ferrier-Pagès C.; Grover R. On the use of 31P NMR for the quantification of hydrosoluble phosphorus-containing compounds in coral host tissues and cultured zooxanthellae. Sci. Rep-uk 2016, 6, 21760. 10.1038/srep21760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren B.; Stenhagen S.; Ryhage R.; Marinder B.-O.; Veige S.; Diczfalusy E. On the Use of Gas Chromatography and Mass Spectroscopy in the Analysis of the Fatty Acids Found in Butter and Margarine. Acta Chem. Scand. 1958, 12, 1351–1351. 10.3891/acta.chem.scand.12-1351a. [DOI] [Google Scholar]
- Hallgren B.; Ryhage R.; Stenhagen E.; Sömme R.; Palm stierna H. The Mass Spectra of Methyl Oleate, Methyl Linoleate, and Methyl Linolenate. Acta Chem. Scand. 1959, 13, 845–847. 10.3891/acta.chem.scand.13-0845. [DOI] [Google Scholar]
- Hebert N.; Beck A.; Lennox R. B.; Just G. A new reagent for the removal of the 4-methoxybenzyl ether: application to the synthesis of unusual macrocyclic and bolaform phosphatidylcholines. J. Org. Chem. 1992, 57, 1777–1783. 10.1021/jo00032a033. [DOI] [Google Scholar]
- Manley P. W.; Tuffin D. P.; Allanson N. M.; Buckle P. E.; Lad N.; Lai S. M. F.; Lunt D. O.; Porter R. A.; Wade P. J. Thromboxane synthase inhibitors. Synthesis and pharmacological activity of (R)-, (S)-, and (.+-.)-2,2-dimethyl-6-[2-(1H-imidazol-1-yl)-1-[[(4-methoxyphenyl)methoxy]methyl]ethoxy]hexanoic acids. J. Med. Chem. 1987, 30, 1812–1818. 10.1021/jm00393a022. [DOI] [PubMed] [Google Scholar]
- Szamosvári D.; Bae M.; Bang S.; Tusi B. K.; Cassilly C. D.; Park S.-M.; Graham D. B.; Xavier R. J.; Clardy J. Lyme Disease, Borrelia burgdorferi, and Lipid Immunogens. J. Am. Chem. Soc. 2022, 144, 2474–2478. 10.1021/jacs.1c12202. [DOI] [PubMed] [Google Scholar]
- Chen J.; Profit A. A.; Prestwich G. D. Synthesis of Photoacti vatable 1,2-O-Diacyl-sn-glycerol Derivatives of 1-l-Phosphatidyl-d-myo-inositol 4,5-Bisphosphate (PtdInsP2) and 3,4,5-Trisphosphate (PtdInsP3). J. Org. Chem. 1996, 61, 6305–6312. 10.1021/jo960895r. [DOI] [PubMed] [Google Scholar]
- Inuki S.; Ohta I.; Ishibashi S.; Takamatsu M.; Fukase K.; Fu jimoto Y. Total Synthesis of Cardiolipins Containing Chiral Cyclopropane Fatty Acids. J. Org. Chem. 2017, 82, 7832–7838. 10.1021/acs.joc.7b00945. [DOI] [PubMed] [Google Scholar]
- Mishra V. K.; Buter J.; Blevins M. S.; Witte M. D.; Rhijn I. V.; Moody D. B.; Brodbelt J. S.; Minnaard A. J. Total Synthesis of an Immunogenic Trehalose Phospholipid from Salmonella Typhi and Elucidation of Its sn-Regiochemistry by Mass Spectrometry. Org. Lett. 2019, 21, 5126–5131. 10.1021/acs.orglett.9b01725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S.; Takeda K. Toll-like receptor signalling. Nat. Rev. Im munol 2004, 4, 499–511. 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Gaffen S. L.; Jain R.; Garg A. V.; Cua D. J. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol 2014, 14, 585–600. 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y. H.; Bang S.; Park S. M.; Ma X.; Cassilly C.; Graham D.; Xavier R.; Clardy J. Revisiting Coley’s Toxins: Immunogenic Cardiolipins from Streptococcus pyogenes. J. Am. Chem. Soc. 2023, 145, 21183–21188. 10.1021/jacs.3c07727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.