Abstract
Legionella-contaminated hot water systems and moist sanitary areas in six hospitals were sampled for amoebae by following a standardized collection protocol. Genus identifications and temperature tolerance determinations were made. Amoebae identified as Hartmannella vermiformis (65%), Echinamoebae spp. (15%), Saccamoebae spp. (12%), and Vahlkampfia spp. (9%) were detected in 29 of 56 (52%) hot water samples. Twenty-three of 49 (47%) swabs obtained from moist areas were amoeba positive. The following genera were identified: Acanthamoeba (22%), Naegleria (22%), Vahlkampfia (20%), Hartmannella (15%), and Vanella (7%). The temperature tolerance of amoebae from hot water systems was strikingly different from that of amoebae from moist areas. At 44°C on agar, 59% of amoebic isolates sampled from hot water systems showed growth. The corresponding value for isolates from moist areas was only 17%. Six Acanthamoeba isolates from the moist areas were considered potential pathogens. Four Hartmannella and two Saccamoeba isolates from hot water could be cultured at 53°C.
Free-living amoebae of the genera Acanthamoeba, Naegleria, and Hartmannella have been isolated from various aquatic habitats in the human environment (2, 5, 14, 20). Additionally, amoebae have been described as carriers of meningoencephalitis and keratitis (13, 24).
It has been shown that, in vitro at least, virulent legionella strains can multiply intracellularly in protozoae after phagocytosis (8, 15, 21). Therefore, it is suggested that the occurrence of amoebae in aquatic habitats may support legionella growth in these ecosystems. In particular, hot water systems can provide an environmental niche for legionellae to multiply to infectious concentrations (7). However, previous reports on isolation of amoebae and legionellae from water samples (1, 10, 16) did not mention sampling procedures in detail. Thus, it remains unclear if a systemic contamination of hot water systems, local terminal contamination, or both cold tap water and hot water were investigated.
In our study, collection of water samples was by a standardized protocol as described by Exner et al. (7) to determine if amoebae are systemically distributed in hot water systems of hospitals, as has been reported for legionellae. To evaluate potential pathogenicity, determinations of the temperature tolerance of amoebae will be presented. Results of comparative investigations will be reported for moist areas of hospitals. Possible sources of organisms found in that biotope are the cold or the hot water system. Organisms in the moist areas colonize at lower temperatures than those in central areas of hot water systems.
MATERIALS AND METHODS
Study sites and sample collection.
Six hospitals took part in this study. Their hot water systems were systemically contaminated with legionellae. Water samples were collected from 56 hot water taps by following a collection technique which allows detection of a systemic contamination as given by Exner et al. (7). Before sampling, the water was allowed to run for 5 to 10 min until the temperature was constant. The temperature was recorded, and a sample volume of 1 liter was collected in sterile glass bottles for microbiological examination. Forty-nine swabs were taken from moist environments within sanitary areas, e.g., wall and floor tiles from bathrooms and showers, the drains of sinks, and water taps. At each area of investigation one swab was taken. About 50% of these areas had not been used recently before they were investigated. The water taps were also part of the hot water sample sites, but the water was not allowed to run. All samples were tested on the same day.
Recovery, determination, and temperature tolerance of amoebae.
Nine hundred milliliters of each water sample was filtered through a cellulose nitrate filter (0.45-μm pore diameter; Sartorius, Göttingen, Germany) with a weak vacuum (flow rate, 1.3 ml/min). The filters were inverted on nonnutrient agar plates (NN-A) according to the method of Page (18), seeded with living Enterobacter amnigenus, and incubated at 30°C. After 3 to 4 days the membranes were removed and the plates were incubated for a further 1 to 2 weeks. Swabs were stroked on NN-A within an area 4 by 4 cm in size and incubated at 30°C for up to 2 weeks.
Amoebic isolates were identified by the morphologic criteria described by Page and Siemensa (19) and characterized with regard to their temperature tolerance (6). All plates were incubated in two independent series for 3 days at different temperatures and then examined for growth. Growth means that a clear migration of trophozoites on the agar surface can be seen and that their isolates can be cultured again. Fifty percent of the amoebic isolates were tested again for temperature tolerance after 3 months of stock culturing on NN-A at 30°C. These isolates showed the same results when the temperature tolerance experiment was repeated.
RESULTS
Amoeba detection frequency.
Amoebae were detected in 29 of 56 (52%) hot water samples and on 23 of 49 (47%) swabs obtained from moist areas.
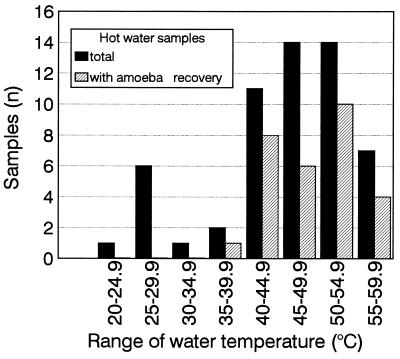
Temperatures of water samples which were cultured for amoeba recovery ranged from 24.7 to 59.5°C (mean, 45.8°C; median, 47.3°C; 95th percentile, 57.3°C). Amoeba isolation frequencies from water samples with different temperature ranges were 73% (40 to 44.9°C), 43% (45 to 49.9°C), 57% (50 to 54.9°C), and 57% (55 to 59.9°C) (Fig. 1). Even at temperatures between 55 and 60°C, four of seven samples were amoeba carriers.
FIG. 1.
Systemic distribution of amoebae in hot water systems from six different hospitals in relation to the water temperature range (900-ml samples were used). The total number of water samples investigated was 56.
Amoeba determination.
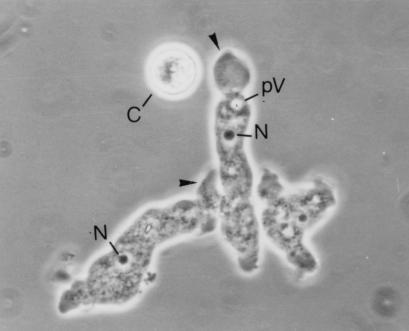
Morphologic characterization of the isolates showed that Hartmanella vermiformis was the dominant species systemically distributed in the hot water systems. From a total of 34 isolates, the following organisms were identified: Hartmannella vermiformis (65%), Echinamoeba spp. (15%), Saccamoeba spp. (12%), and Vahlkampfia spp. (9%). H. vermiformis is shown in Fig. 2.
FIG. 2.
Three trophozoites and one cyst (C) of H. vermiformis (05101). N, nucleus; pV, pulsating vacuole. Arrowheads indicate pseudopodiae formed by the hyaline zone. Magnification, ×1,200.
In swabs taken from moist areas, a greater variety of species was present. From the 41 isolates, organisms of six different genera were identified: Acanthamoeba spp. groups II and III (22%), Naegleria spp. (22%), Vahlkampfia spp. (20%), H. vermiformis (15%), unidentified amoebae (10%), Vannella spp. (7%), and unidentified miniamoebae (5%).
Temperature tolerance of the amoebic isolates.
All amoebic isolates from both biotopes were able to be cultured at room temperature and at 30°C on NN-A. Temperature tolerance of amoebae revealed striking differences between both biotopes studied. At 44°C on agar, 59% of amoebic isolates from hot water systems (total n = 34) showed growth (Hartmannella, 41%; Saccamoeba, 12%; Vahlkampfia, 6%). The corresponding value for the amoebic isolates from the moist areas (total n = 41) was only 17%. The distribution of genera was as follows: Vahlkampfia, 7%; Hartmannella, 5%; Acanthamoeba, 2%; and unidentified amoebae, 2%.
Six strains were able to grow at 53°C on NN-A. These thermotolerant amoebae belonged to the genera Hartmannella (n = 4) and Saccamoeba (n = 2) and have been isolated from a total of six hot water samples with temperatures between 46.5 and 55.7°C.
Six of nine Acanthamoeba isolates from the moist areas were able to grow at 40°C, and one of them even grew at 44°C. None of the Naegleria isolates from the moist areas could be cultured at 42 or 44°C.
DISCUSSION
In our investigations the most common amoeba systemically distributed in hot water systems was H. vermiformis (65%). These results confirm those of other investigations with various water samples from plumbing systems of hospitals (1, 22). Breiman et al. (1) reports that about 71% of the amoeba isolates from drinking water and cooling tower water were of the genus Hartmannella. Besides Hartmannella, both Breiman et al. (1) and Sanden et al. (22) found Acanthamoeba and Vahlkampfia. We also cultured Vahlkampfia from our water samples, but we found no trace of Acanthamoeba. Both Breiman et al. and Sanden et al. (1, 22) probably worked with cold water or mixed water samples, whereas we followed the criteria for the detection of systemic hot water contamination (7). It is strongly suggested that our sample technique detects mainly microorganisms distributed in the central areas of hot water systems. Thus, it is not surprising that our results are only partly in line with other observations (1, 22). The fact that the majority of our samples had temperatures above 45°C supports the conclusion that only organisms with high temperature tolerance may survive. Acanthamoeba and Naegleria show a relatively low temperature tolerance. Strains from these genera which are able to grow at 45°C have rarely been found (3, 6). Our study confirms these reports. We frequently found these genera colonizing moist areas at room temperature but not the hot water system. It is possible that they colonize hot water systems with lower temperatures, cold water systems, or only the pipes near the outlets.
Up to now no pathogenic potential has been described for the genera we isolated from the hot water systems (Hartmannellae, Echinamoebae, Saccamoebae, and Vahlkampfia). The barrier of pathogenicity is apparently not related to temperature, as has been described for the genera Acanthamoeba and Naegleria (9). Therefore, our results indicate that there was no danger of infection via the amoebae present in the investigated hot water. On the other hand, in moist environments within sanitary areas we found six Acanthamoeba strains which must be regarded as potential pathogens because they can grow at 42°C (4). We found these strains colonizing drains, the wall and floor tiles from bathrooms, and shower heads. Although no epidemiological correlation exists, from a hygienic point of view infections may be possible by the inhalation of amoeba-contaminated aerolized water (water spray). None of the Naegleria isolates from the moist areas showed potential pathogenicity (9, 11).
Nevertheless, it should be noted that we have isolated Hartmannella and Saccamoeba strains with extreme temperature tolerance from hot water systems. To our knowledge, it has not been reported in previous studies that amoebae have been cultured at 53°C on NN-A. As a rule, investigations of the temperature tolerance of Hartmannella and other possible apathogenic forms have not been carried out. Only Griffin (9) reported such an investigation. In his study Hartmannella growth was detectable on agar up to 40°C. Kuchta et al. (12) also recognized that H. vermiformis shows a high temperature tolerance in water culture at 55°C in vitro.
The aim of our investigation was to detect amoebae in aquatic habitats where legionellae were known to be systemically distributed (7). Acanthamoeba and Naegleria did not colonize these central areas of the investigated hot water systems at temperatures between 40 and 60°C. Nevertheless, they are often used as host organisms for legionellae in vitro (15, 17). If amoebae really support survival and growth of legionellae in hot water systems, they probably belong to hartmannellae and other thermoresistant forms. Our results are in agreement with those of other investigations (22, 23). A growth-supporting effect of H. vermiformis on legionellae in tap water in vitro has been described by Wadowsky et al. (23). Sanden et al. (22) found that a correlation exists between the occurrence of H. vermiformis and Legionella pneumophila (serogroup 1) in the water systems of hospitals.
REFERENCES
- 1.Breiman R F, Fields B S, Sanden G N, Volmer L J, Meier A, Spika J S. Association of shower use with Legionnaires’ disease. JAMA. 1990;263:2924–2926. [PubMed] [Google Scholar]
- 2.Clark B J, Harkins L S, Munro F A, Devonshire P. Microbial contamination of cases used for storing contact lenses. J Infect. 1994;28:293–304. doi: 10.1016/s0163-4453(94)91893-7. [DOI] [PubMed] [Google Scholar]
- 3.De Jonckheere J F. Pathogenic free-living amoebae in swimming pools: survey in Belgium. Ann Microbiol. 1979;130B:205–212. [PubMed] [Google Scholar]
- 4.De Jonckheere J F. Growth characteristics, cytopathic effect in cell culture, and virulence in mice of 36 type strains belonging to 19 different Acanthamoeba spp. Appl Environ Microbiol. 1980;39:681–685. doi: 10.1128/aem.39.4.681-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Jonckheere J F. Hospital hydrotherapy pools treated with ultra violet light: bad bacteriological quality and presence of thermophilic Naegleria. J Hyg Camb. 1982;88:205–215. doi: 10.1017/s0022172400070078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Jonckheere J F, Van de Voorde H. The distribution of Naegleria fowleri in man-made thermal waters. Am J Trop Med Hyg. 1977;26:10–15. doi: 10.4269/ajtmh.1977.26.10. [DOI] [PubMed] [Google Scholar]
- 7.Exner M, Tuschewitzki G J, Langer B, Wernicke F, Pleischl S. Vorkommen und Bewertung von Legionellen in Krankenhäusern und anderen Großgebäuden. Forum Staedte-Hyg. 1992;43:130–140. [Google Scholar]
- 8.Fields B S, Shotts E B, Feeley J C, Gorman G W, Martin W T. Proliferation of Legionella pneumophila as an intracellular parasite of the ciliated protozoan Tetrahymena pyriformis. Appl Environ Microbiol. 1984;47:467–471. doi: 10.1128/aem.47.3.467-471.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin J L. Temperature tolerance of pathogenic and nonpathogenic free-living amoebas. Science. 1972;178:869–870. doi: 10.1126/science.178.4063.869. [DOI] [PubMed] [Google Scholar]
- 10.Henke M, Seidel K M. Association between Legionella pneumophila and amoebae in water. Isr J Med Sci. 1986;22:690–695. [PubMed] [Google Scholar]
- 11.John D T. Opportunistically pathogenic free-living amoebae in parasitic protozoa. In: Kreier P, Baker J R, editors. Parasitic protozoa. 2nd ed. Vol. 3. San Diego, Calif: Academic Press; 1993. [Google Scholar]
- 12.Kuchta J M, Navratil J S, Shepherd M E, Wadowsky R M, Dowling J N, States S J, Yee R B. Impact of chlorine and heat on the survival of Hartmannella vermiformis and subsequent growth of Legionella pneumophila. Appl Environ Microbiol. 1993;59:4096–4100. doi: 10.1128/aem.59.12.4096-4100.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez A J, Janitschke K. Encephalitis due to Naegleria and Acanthamoeba. Comparison of organisms and diseases. Immun Infekt. 1979;7:57–64. [PubMed] [Google Scholar]
- 14.Michel R, Menn T. Acanthamoebae, naegleriae, and invertebrates isolated from wet areas of physiotherapeutical facilities of hospitals. Zentbl Hyg Umweltmed. 1991;191:423–437. [PubMed] [Google Scholar]
- 15.Moffat J F, Tompkins L S. A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect Immun. 1992;60:296–301. doi: 10.1128/iai.60.1.296-301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nahapetian K, Challemel O, Beurtin D, Dubrou S, Gounon P, Sqinazi F. The intracellular multiplication of Legionella pneumophila in protozoa from hospital plumbing systems. Res Microbiol. 1991;142:677–685. doi: 10.1016/0923-2508(91)90081-k. [DOI] [PubMed] [Google Scholar]
- 17.Newsome A L, Baker R L, Miller R D, Arnold R R. Interactions between Naegleria fowleri and Legionella pneumophila. Infect Immun. 1985;50:449–452. doi: 10.1128/iai.50.2.449-452.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page F C. A new key to freshwater and soil gymnamoebae. Ambleside, Cumbria, United Kingdom: Freshwater Biological Association; 1988. [Google Scholar]
- 19.Page F C, Siemensa F J. Nackte Rhizopoda und Heliozoa. Stuttgart, Germany: Gustav Fischer Verlag; 1991. [Google Scholar]
- 20.Paszko-Kolva C, Yamamoto H, Shahamat M, Sawyer T K, Morris G, Colwell R R. Isolation of amoebae and Pseudomonas and Legionella spp. from eyewash stations. Appl Environ Microbiol. 1991;57:163–167. doi: 10.1128/aem.57.1.163-167.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowbotham T J. Current views on the relationship between amoebae, legionellae and man. Isr J Med Sci. 1986;22:678–689. [PubMed] [Google Scholar]
- 22.Sanden G N, Morrill W E, Fields B S, Breiman R F, Barbaree J M. Incubation of water samples containing amoebae improves detection of legionellae by the culture method. Appl Environ Microbiol. 1992;58:2001–2004. doi: 10.1128/aem.58.6.2001-2004.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadowsky R M, Wilson T M, Kapp N J, West A J, Kuchta J M, States J S, Dowling J N, Yee R B. Multiplication of Legionella spp. in tap water containing Hartmannella vermiformis. Appl Environ Microbiol. 1991;57:1950–1955. doi: 10.1128/aem.57.7.1950-1955.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright P. Abstracts of the International Conference on Biology and Pathogenicity of Free-Living Amoebae. 1989. Acanthamoeba keratitis. [Google Scholar]