Abstract
With the brain, the spinal cord forms the central nervous system. Initially considered a passive relay between the brain and the periphery, the spinal cord is now recognized as being active and plastic. Yet, it remains largely overlooked by the human neuroscience community, in stark contrast with the wealth of research investigating the brain. In this review, we argue that fMRI, traditionally used to image cerebral function, can be extended beyond the brain to help unravel spinal mechanisms involved in human behaviors. To this end, we first outline strategies that have been proposed to tackle the challenges inherent to spinal cord fMRI. Then, we discuss how they have been utilized to provide insights into the functional organization of spinal sensorimotor circuits, highlighting their potential to address fundamental and clinical questions. By summarizing guidelines and applications of spinal cord fMRI, we hope to stimulate and support further research into this promising yet underexplored field.
Keywords: spinal cord, fMRI, neuroimaging, central nervous system, sensorimotor
The spinal cord is a part of the central nervous system. An important one to be precise. Yet, it has long been off the spotlights in neuroscience, merely considered a relay transmitting information between the brain and the body. Nonetheless, over the years, evidence challenging this view has accumulated. The spinal cord is more sophisticated than initially granted, actively participating in sensorimotor processes, affected in various neurological conditions, and capable of neural plasticity. It now appears clear that a thorough characterization of the central nervous system cannot be achieved without insights into the spinal cord functional organization. Accordingly, there is a pressing need for tools enabling noninvasive assessments of spinal activity in humans. In this regard, fMRI, a technique routinely used to measure brain activity, represents a promising approach. However, extending fMRI to the spinal cord is not straightforward and has long remained challenging. Fortunately, technological and methodological advances have emerged to address these hurdles. Today, spinal cord fMRI appears to be an accessible tool allowing neuroscientists and clinicians to delve into the functional organization of the entire central nervous system.
In this review, we first introduce the limitations that have hindered the advancement of the field, along with solutions that have been engineered to tackle them. In doing so, we outline what we consider to be promising acquisition and processing approaches for spinal cord fMRI. We then survey how the use of these strategies has enabled the study of healthy and impaired human spinal cord under a variety of experimental conditions, demonstrating their potential. Finally, we touch upon future avenues for the field.
From Brain to Spinal Cord fMRI: Challenges and Opportunities
Since its advent in the early 1990s (see Bandettini 2012 for review), fMRI has revolutionized noninvasive imaging of human brain function. Interestingly, spinal cord fMRI also emerged around the same period. As early as 1996, researchers sought to image spinal activity during unilateral hand closing (Yoshizawa and others 1996). Despite this early investigation, spinal cord fMRI has not encountered the same success as its brain counterpart. The numbers speak for themselves: to date, the field of spinal cord fMRI has totaled only ~100 publications in humans (Landelle and others 2021). This scarcity stems largely from the additional challenges associated with spinal cord imaging, which have limited interest and progress in the field.
Dimensions: The spinal cord is a long but tiny structure (Box 1). The first implication is that an extended field of view (FOV) would be required to cover its entire rostrocaudal extent, which is technologically burdensome. Second, its small diameter demands high spatial resolution to avoid partial volume effects, with a negative impact on the signal-to-noise ratio (SNR), the latter being proportional to voxel size.
Field inhomogeneities: MRI relies on the application of a homogeneous magnetic field. In the spinal cord, however, the presence of tissue types with disparate magnetic susceptibilities (e.g., bone, fluid) leads to field inhomogeneities resulting in image artifacts, such as distortions or signal dropouts (Finsterbusch 2014).
Physiological noise: The proximity of the lungs, heart, and other visceral organs is a significant source of noise, both from the motion of the cord and from pulsatile flow in the cerebrospinal fluid (CSF) (Piché and others 2009).
Box 1. Spinal Cord Neuroanatomy.
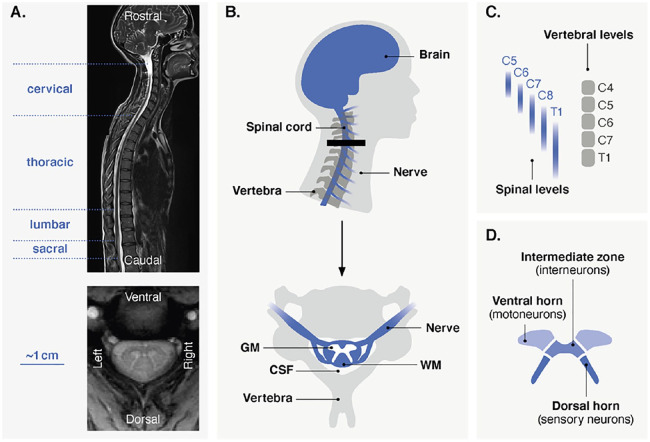
The human spinal cord is a long, curved structure extending from the top of the neck to the lower back (Purves and others 2018). The spinal cord gives rise to 31 pairs of nerves, exiting superior or inferior to the corresponding vertebra and organized in the rostrocaudal direction in four parts: cervical, thoracic, lumbar, and sacral (Fig. 1A, B). The dimensions of the spinal cord vary among these segments, with a transverse diameter (left-right) ranging from 4.7 ± 3.5 mm (population estimate ± 2 SD) at the lowest sacral level (S5) to 13.3 ± 2.2 mm at level C5 in the cervical enlargement (Frostell and others 2016). There is a somatotopic organization along the superior-inferior axis, for motor nerves (myotomes) and sensory nerves (dermatomes), which are linked to different parts of the body (e.g., cervical to upper limbs, lumbar to lower limbs; Box 3). Importantly, the rostrocaudal organization of the spinal cord can be described using two distinct nomenclatures, according to vertebral (bones) and spinal (nerve roots) levels (Fig. 1C). It should be emphasized that spinal levels are not necessarily aligned with the corresponding vertebral bodies, as nerves can exit relatively far from the associated vertebra, with significant variability among individuals (Cadotte and others 2015). Given the purpose of spinal cord fMRI (i.e., to probe neural-related activity), spinal levels should be used to describe fMRI findings.
Figure 1.
(A) Anatomical scans of the human spinal cord. The top image shows a sagittal view (T2 imaging), extending from the brain to the lower part of the back. Spinal levels (nerve roots) are divided into four sections, indicated on the left. The bottom image depicts an axial view (T2* imaging) at the cervical level. (B) Schematic representation of the cervical spinal cord. As seen in the sagittal view (top), the spinal cord extends down from the brain. Nerves branch out from the spinal cord and exit superior or inferior to the vertebra. Importantly, nerve roots and vertebrae are not necessarily aligned and can be described using two nomenclatures (see panel C). The thick black line indicates the approximate position of the axial view (bottom) on which the main structures of the spinal cord and its environment are indicated. GM = gray matter; WM = white matter, CSF = cerebrospinal fluid. (C) Correspondence between spinal (nerve roots) and vertebral (bones) levels in the cervical region. For spinal levels, probabilistic maps are shown to highlight the variability over subjects (Cadotte and others 2015). (D) Subdivisions of the gray matter.
The spinal cord is encircled by vertebrae and intervertebral discs and is surrounded by a cavity called the subarachnoid space, containing the cerebrospinal fluid (CSF) (Fig. 1B). As opposed to the brain, the white matter composed of myelinated axons is found around the gray matter. The gray matter structure, which has a typical butterfly shape, can be divided into different horns (Fig. 1D). The dorsal horns contain neurons processing sensory information, whereas ventral horns contain motor neurons linked to skeletal muscles (Box 3). Interneurons, making connections within or between spinal levels, are also present. Of note, the gray matter can also be divided more finely, for instance according to its laminar organization. Specifically, it can be described using Rexed laminae (10 for each side), which are defined by their cellular structure and function. For the sake of clarity and because the resolution of spinal cord fMRI implies that results are mostly described using gray matter horns, the laminae are not shown in Figure 1.
Over the years, various solutions have been employed to circumvent these limitations. Essentially, the field has been divided into two lines of research leveraging distinct acquisition methods based on different combinations of radiofrequency pulses, namely gradient echo and spin echo sequences. On one hand, spin echo sequences initially attracted a lot of interest, as they are less affected by field inhomogeneities and can thus improve image quality. On the other, most recent studies have opted for gradient echo sequences, primarily using echo planar imaging (i.e., GE-EPI): the standard in brain fMRI. This approach is more sensitive to variations in magnetic susceptibility but offers the benefits of rapid imaging, high signal sensitivity, and reproducibility. While spin echo and gradient echo have both contributed to the development of spinal cord fMRI (for reviews, see Powers and others 2018; Stroman and others 2014), their concurrent deployment led to substantial heterogeneity in the methodological pipelines employed across studies, limiting generalizability.
For consistency, this review focuses on spinal cord fMRI protocols that rely on gradient echo sequences to image the blood oxygenation level–dependent (BOLD) signal (Box 2). Indeed, besides being routinely used in brain fMRI, these protocols have proven to be adaptable to the spinal cord (see Methodological Considerations section). Furthermore, their potential to reliably image spinal activity has been demonstrated by several research groups, using various paradigms (see Investigating the Healthy Spinal Cord and Clinical Potential sections). Although we restrict our discussion to findings derived from gradient echo imaging, we refer to detailed reviews of the existing literature where appropriate.
Box 2. On the Nature of Spinal Signals.
Traditionally, fMRI relies on the blood oxygenation level–dependent (BOLD) signal, a slow proxy for neural activity based on neurovascular coupling. Importantly, studies in animals have investigated the link between spinal neuronal activity and hemodynamic changes. First, spinal neurovascular coupling was confirmed in rats subjected to noxious electrical stimulation (Piché and others 2017). Specifically, spinal hemodynamic changes (i.e., spinal cord blood flow) were found to reflect the underlying neuronal activity, measured by means of local field potentials. More recently, the validity of the BOLD signal in the spinal cord has been underlined in nonhuman primates (Wu and others 2019). BOLD and electrophysiological signals (local field potentials and multiunit spiking) co-localized and exhibited co-varying temporal profiles. Altogether, these findings allude to the genuine nature of the spinal BOLD signal, suggesting that robust spinal cord fMRI is achievable provided that technological hurdles can be overcome.
While all the research presented in this review uses BOLD imaging, it should be noted that an alternative contrast mechanism may be encountered in other studies. Specifically, early spinal cord fMRI works suggested that spin echo sequences could be used to image a non-BOLD contrast mechanism, termed SEEP (signal enhancement by extravascular water protons). Whereas the BOLD signal relies on blood flow, the SEEP contrast has been attributed to changes in tissue water content in regions of neuronal activity based on cellular swelling. Nevertheless, these findings remain controversial, as several groups unsuccessfully attempted to detect reliable activity using this approach (Bouwman and others 2008; Jochimsen and others 2005). Nowadays, even spin echo studies tend to optimize their acquisition parameters for BOLD imaging.
Methodological Considerations
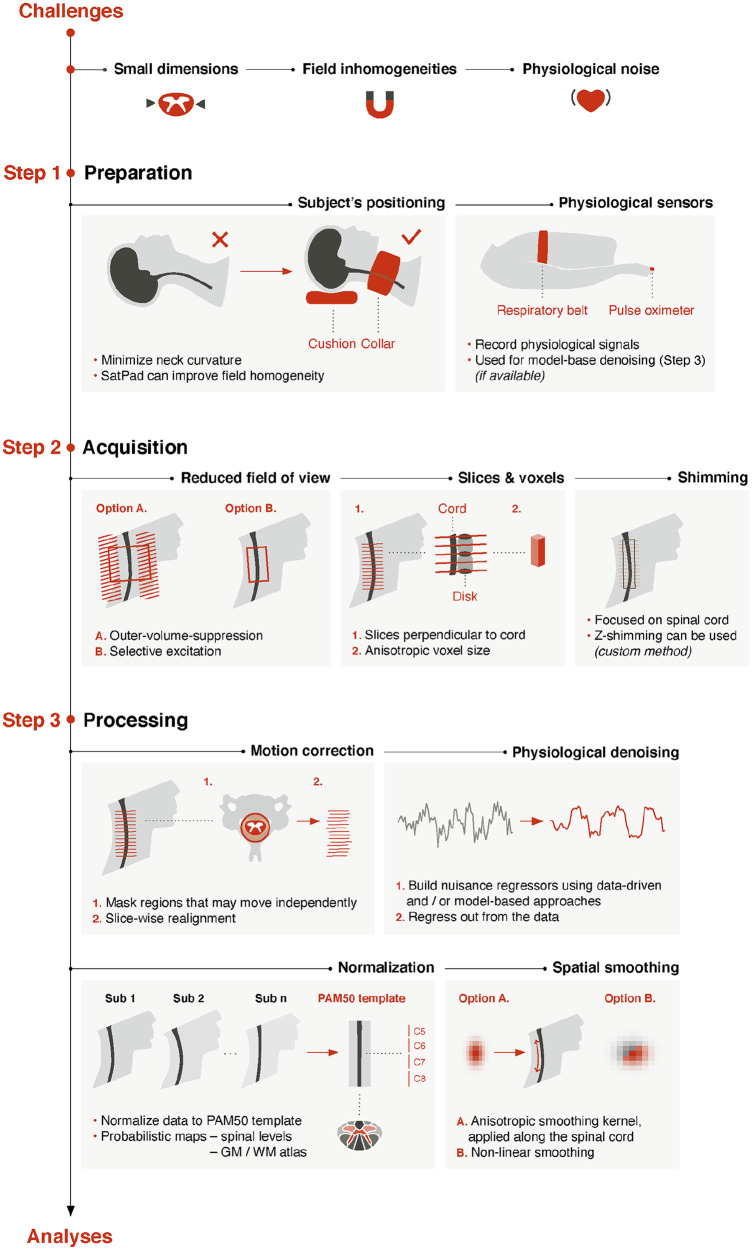
Here we discuss steps that can be taken to address the aforementioned challenges and tailor the fMRI workflow to the spinal cord (Fig. 2). We strongly encourage researchers to follow these best practices in their studies to optimize data quality and obtain reliable signals. While this review focuses on functional imaging, readers can refer to the recently published spine generic protocol for structural scans (e.g., T2-weighted anatomical image) (Cohen-Adad and others 2021).
Figure 2.
Spinal cord fMRI pipeline. Schematic representation of the workflow outlined in this review. Strategies at three stages are considered: preparation, acquisition, and processing. Quality control is essential and should be conducted at each stage. Visual assessments (e.g., signal losses, distortions) can be complemented by signal-to-noise ratio computations. Quality control options are also available in the Spinal Cord Toolbox (e.g., motion correction, registration accuracy) (De Leener and others 2017).
Protocol Specifications
First and foremost: the hardware. Nowadays, most studies are performed using 3-T scanners, for which equipment provided by manufacturers can typically be employed. For instance, fMRI of the upper cord is feasible using a head and neck coil, similar to brain fMRI. In contrast, a spine coil is required for lower regions. Conversely, the lack of specialized hardware has hindered the use of higher field strengths, with only a handful of studies conducted at 7 T (see Barry and others 2018 for review).
The importance of subjects’ positioning should not be overlooked. Minimal curvature of the spine helps align slices perpendicularly to the spinal cord, which minimizes partial volume effects. The use of a collar filled with nonprotonated liquid (e.g., SatPad) can further stabilize the neck, reduce field inhomogeneity, and improve image quality (De Leener and others 2020).
The choice of coverage and resolution is contingent on the research question and technical constraints. Coverage of the entire spinal cord is not easily achievable with current fMRI techniques, and most studies have focused on the larger cervical section. It is possible to take advantage of the tubular structure of the spinal cord by using axial slices with anisotropic voxels (typically using an in-plane resolution of 1 × 1 mm and a slice thickness of 3 to 5 mm). Although this limits the level of detail in the rostrocaudal direction, increasing slice thickness improves the SNR. Moreover, high in-plane resolution best captures anatomical structures such as gray matter horns.
Given its dimensions, reduced FOV acquisition is a compelling strategy to image the spinal cord (see Saritas and others 2014 for review). Briefly, the idea is to limit the extent of the FOV in the anterior-posterior direction. This limits the inclusion of nonspine tissues and thus mitigates the impact of external signals. A straightforward approach, yet with limited suppression efficiency, is to perform outer-volume suppression using saturation pulses applied anterior and posterior to the spinal cord. Alternatively, inner FOV sequences (e.g., ZOOMit for Siemens, FOCUS for GE, iZOOM for Philips) can selectively excite the FOV using dynamic pulses. Both approaches have strengths and weaknesses but can provide similar performances in detecting task and rest activity (Kinany and others 2022).
Finally, a key step of any spinal cord fMRI protocol is to shim the magnetic field (i.e., make it more homogeneous) (see Finsterbusch 2014 for review). The recommended practice is to manually set the shim volume to focus on the spinal cord, an option available on most scanners. However, this cannot fully account for all inhomogeneities, notably at the intervertebral disks, and custom slice-specific z-shimming can be leveraged if available (Finsterbusch and others 2012).
Simultaneous brain and spinal cord fMRI is also feasible, albeit increasingly challenging owing to the differences in ideal MRI protocols for these two regions (see Tinnermann and others 2021a for review). Tailored sequences with distinct brain and spine parameters have shown promising results (Finsterbusch and others 2013; Islam and others 2019) but are not readily available. A more accessible alternative is to enlarge the FOV to include brain regions, although this warrants particular care in selecting appropriate parameters. Again, signals can be improved using a collar filled with nonprotonated liquid (De Leener and others 2020) and/or slice-specific z-shimming (Finsterbusch and others 2012).
Processing and Analysis
In addition to tailored acquisition schemes, spinal cord fMRI calls for dedicated analysis pipelines. Indeed, brain-related routines implemented in standard neuroimaging tools—for example, Statistical Parametric Mapping (SPM) (Ashburner 2012) or the FMRIB Software Library (FSL) (Jenkinson and others 2012)—cannot be fully extended to the spinal cord. Here, we focus on key adjustments that can be made to improve data quality in spinal cord fMRI. These developments have been largely facilitated by the emergence of specialized processing tools, such as the Spinal Cord Toolbox (SCT) (https://spinalcordtoolbox.com; De Leener and others 2017).
After visual inspection of the images, processing typically begins with motion correction. Whereas conventional methods assume that movements can be described using rigid body transformations, this does not hold in the spinal cord. Instead, its articulated structure allows for movements that vary across spinal segments, owing to breathing, swallowing, and so forth. To account for this, motion correction can be done slice-wise, as proposed in the Spinal Cord Toolbox, while excluding regions outside the vertebral column to avoid biases (De Leener and others 2017).
In addition to motion correction, physiological denoising is critical to improve the temporal SNR (see Eippert and others 2017a for review). Briefly, physiological noise can be handled in two ways. On one hand, data-driven approaches such as CompCor (Behzadi and others 2007) directly derive noise components (then regressed out from the data) from fMRI recordings. On the other, model-based approaches rely on the acquisition of physiological recordings during fMRI experiments. These techniques are based on the RETROICOR procedure (retrospective image correction; Glover and others 2000) and have been adapted to the spinal cord (Brooks and others 2008; Kong and others 2012). They use physiological signals to model noise with a Fourier expansion of cardiac and respiratory phases. In practice, this can be conducted using FMRIB Software Library (PNM Toolbox) or statistical parametric mapping (PhysIO Toolbox). A nuisance regressor corresponding to the CSF signal is usually included (Kong and others 2012).
To enable group-level inferences and comparison across studies, images must undergo normalization to a standard space. To this end, the PAM50 template (De Leener and others 2018) was introduced in the Spinal Cord Toolbox, alongside a tailored registration framework. Based on 50 healthy subjects, it covers the entire spinal cord and is available in multiple modalities (T1-, T2-, and T2*-weighted MRI contrasts). Moreover, it uses the same coordinate system as the MNI-ICBM152 brain template, facilitating combined brain and spinal cord fMRI analyses. Associated assets include probabilistic maps of spinal levels and gray and white matter atlas regions, valuable for activation mapping.
Spatial smoothing can be used to enhance SNR, although a tradeoff should be found to limit the reduction in effective resolution. To preserve in-plane resolution and anatomical consistency, an anisotropic smoothing kernel is often used (i.e., small in-plane full-width half maximum and larger rostrocaudal full-width half maximum), applied along the cord to avoid partial volume effect (i.e., in the PAM50 space or using the dedicated Spinal Cord Toolbox’s function). Alternatively, nonlinear smoothing that accounts for tissue type (e.g., using FMRIB Software Library’s SUSAN tool) has been employed to preserve the underlying structure (Weber and others 2020).
Investigating the Healthy Spinal Cord
As noted in the introduction, the field of spinal cord fMRI remains relatively small. Still, over the years, the advances outlined in the previous section have been deployed to image spinal cord activity using a variety of paradigms, hence providing new insights into spinal circuits.
Spinal Cord in Action
Sensory paradigms have, so far, received the most interest, with a considerable amount of research investigating the spinal correlates of pain (see Kolesar and others 2015; Paquette and others 2018; Tinnermann and others 2021a for reviews). Given the anatomical location of peripheral afferent fibers carrying sensory information (Box 3), activations associated with noxious stimuli are primarily expected in the dorsal horn ipsilateral to the side of stimulation, with a segmental distribution of evoked activity mirroring the stimulated dermatome (i.e., somatotopic organization).
Box 3. Spinal Cord Sensorimotor Pathways: Active and Plastic.
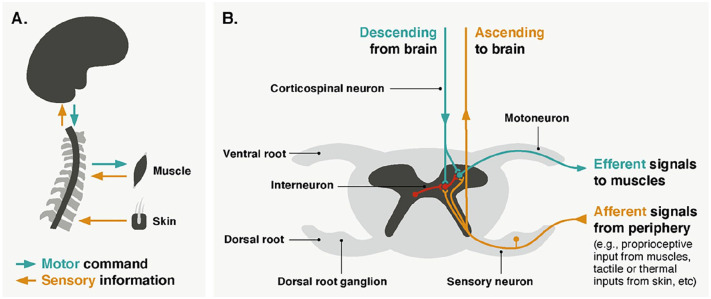
The central nervous system consists of the brain and the spinal cord. At first glance, the latter has the appearance of a long tube (Box 1). In spite of this ostensible simplicity—which has certainly cultivated the view of a passive spinal cord—it actually contains neural structures forming a sophisticated circuitry. In our daily lives, these circuits interact dynamically with supraspinal regions to ensure the vast repertoire of human behaviors.
Let’s imagine a simple motor action: grabbing a glass. What happens at the different levels of the central nervous system to achieve this? Motor commands, originating in the brain, travel down through corticospinal neurons that project to different levels of the spinal cord, depending on which muscles need to be activated (i.e., somatotopic organization) (Box 1, Fig. 3A). Corticospinal neurons can connect to motoneurons in the ventral horns of the spinal cord through direct monosynaptic connections or through indirect connections via interneurons (Nielsen 2016) (Fig. 3B). Spinal motoneurons exit the cord via ventral roots to innervate muscle fibers, sending efferent signals that contract the required skeletal muscles. Yet, this is only one part of the story. At the same time, the spinal cord is continuously processing inputs from the periphery. It integrates the stream of motor commands with afferent sensory feedback, notably through interneuronal circuits, to produce goal-directed behaviors adapted to the specificities of the environment. Interestingly, these interneuronal circuits may also rely on commissural connections, as certain neurons send their axons across the midline to terminate in the contralateral spinal cord, either within the same segment or between spinal levels (Maxwell and Soteropoulos 2020).
Figure 3.
(A) Schematic representation of spinal cord sensorimotor pathways. Descending circuits connect the brain to the periphery and are indicated in green. They are involved in voluntary muscle contraction. Ascending circuits connect the periphery to the brain and are indicated in yellow. They are responsible for transmitting sensory information (e.g., touch, pressure, temperature, nociception, proprioception). Sensory fibers can also evoke reflexes by activating motoneurons directly or through interneurons (see panel B). (B) Schematic representation of spinal sensorimotor circuits (cervical level) displayed on an axial view of the cord. Colors correspond to the different types of neurons (green for motor, yellow for sensory, and red for interneurons). Interneurons crossing the midline are called commissural. Of note, this figure focuses on the sensorimotor circuits of the cervical spinal cord, in which the majority of spinal cord fMRI studies have been performed. As a result, spinal circuits linked to the autonomic system are not shown.
Of course, the spinal cord is not solely engaged in motor control. It also plays a pivotal role in sensory functions (Fig. 3A), including the processing of tactile, pressure, or temperature stimuli (Koch and others 2018). Notably, it acts as the earliest level in the nociceptive transmission pathway, as noxious information from the periphery (e.g., skin) is transmitted to the dorsal horns of the spinal cord (Fig. 3B) before ascending to subcortical and cortical regions, where it may be interpreted as pain. In this context, the spinal cord is also involved in the modulation of nociceptive—and, to a larger extent, sensory—signals, as excitatory and inhibitory interneurons can influence the output of sensory neurons.
Importantly, spinal neuronal circuits support reflexes, in which predetermined patterns of muscle contractions are generated in response to a particular sensory stimulus (Guertin 2013). The simplest form of reflex, mediated by only two neurons, is termed stretch reflex and refers to the involuntary contraction of a muscle in response to its stretching. Specifically, stretch receptors located in the muscle transmit signals to the spinal cord via afferent neurons, which form monosynaptic connections with spinal motoneurons innervating the same muscle. Examples of more complex polysynaptic reflexes include the flexor reflex, occurring to withdraw a limb from a painful stimulus. This reflex arc relies on afferent fibers from nociceptors that synapse with interneurons, which in turn excite motoneurons innervating flexor muscles. It may be accompanied by a crossed-extensor reflex, where the same interneurons excite motoneurons innervating the contralateral extensor muscles, to compensate for the withdrawal of the limb and preserve balance.
In addition to these reflex arcs, observations hint at the ability of the spinal cord to generate more sophisticated semiautonomous behavior. Decades ago, studies demonstrated that spinalized animals (i.e., with a transection of the cord interrupting supraspinal inputs) can produce behaviorally relevant movements such as walking or swimming (Guertin 2013). These behaviors have been shown to rely on networks of spinal neurons called central pattern generators, which are capable of self-generating stereotyped and rhythmic movements. Even more surprising, spinalized cats trained on a treadmill were able to learn to improve their walking patterns (Shurrager and Dykman 1951). In humans as well, the spinal cord is a major site of activity-dependent plasticity in health and disease, challenging the long-standing view of a passive hard-wired organ (Wolpaw and Tennissen 2001).
Note that, besides these sensorimotor pathways, the spinal cord contains visceral motoneurons responsible for the involuntary control of smooth muscles (e.g., related to respiration or heart rate). These neurons are located in the intermediate horn (between ventral and dorsal horns) and connect to ganglionic neurons in the peripheral nervous system, which in turn project to the target tissue. Importantly, intermediate horns are not present in the entire extent of the spinal cord but are primarily found in the thoracic region.
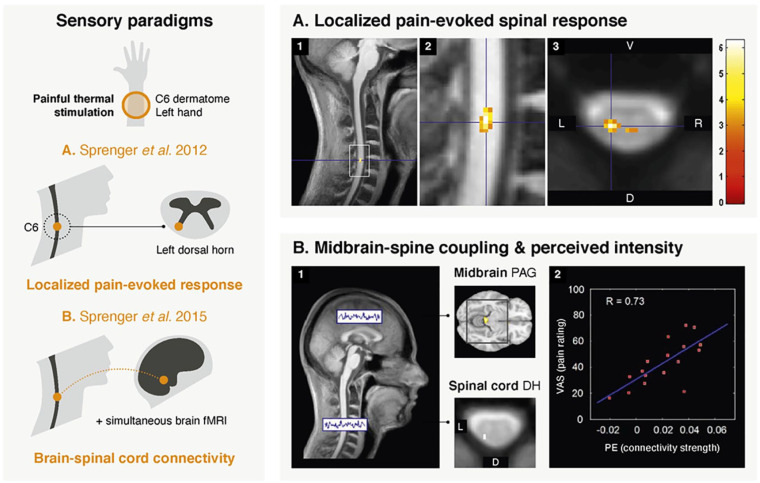
Using thermal stimulation and cervical fMRI, most studies have indeed observed spinal responses localized in the ipsilateral dorsal horn (Eippert and others 2009; Geuter and Büchel 2013; Oliva and others 2022; Sprenger and others 2012; Sprenger and others 2015; Sprenger and others 2018a; Sprenger and others 2018b; Tinnermann and others 2017; Tinnermann and others 2021b; Weber and others 2016a) (Fig. 4A). In addition, all but one study (Weber and others 2016a) captured the somatotopic organization of afferent fibers. Surprisingly, a few studies have reported activations in contralateral and ventral horns (Geuter and Büchel 2013; Sprenger and others 2018b; Weber and others 2016a). These unexpected findings may be due to technical and methodological limitations (e.g., sequence sensitivity, processing and analysis pipeline, intersubject variability). Alternatively, they may reflect genuine activations, possibly related to the engagement of a complex spinal circuitry during nociceptive processing, involving withdrawal reflex arcs, commissural connections, and descending modulation (Box 3). This would echo high-resolution fMRI findings in nonhuman primates, showing that pain-evoked activations extended contralaterally and ventrally (Chen and others 2015; Yang and others 2015).
Figure 4.
Examples of spinal cord fMRI studies using sensory paradigms. (A) The first study (adapted with permission from Sprenger and others 2012) used fMRI to look into the spinal cord response to painful thermal stimulation (applied on the left forearm in dermatome C6). Activation maps correspond to group-level (n = 17) pain-related responses. Panels 1 and 2 show these spinal activations overlaid on the mean structural image of all participants (sagittal views). Panel 3 presents an axial view, displayed on top of the mean functional image. The peak of the BOLD response is seen in the ipsilateral dorsal horn of the C6 spinal level, in line with anatomical expectations. The color bar corresponds to t values, with a visualization threshold set at P < .005 (uncorrected). (B) In the second study (adapted with permission from Sprenger and others 2015), a simultaneous spinal-brain fMRI sequence was deployed during low- and high-intensity painful stimulation of the C6 dermatome of the left forearm (n = 17). A spinal-brain functional connectivity analysis was performed (panel 1) using a seed corresponding to the contrast in the spinal cord (i.e., high intensity > low intensity). The seed was located in the left dorsal horn of the C6 spinal level. Couplings were observed with several brain regions, such as the periaqueductal gray matter (PAG). Moreover, the strength of this spinal-PAG coupling was positively correlated (R = 0.73, panel 2) with the individual mean pain ratings, as illustrated in the scatter plot. D = dorsal; PE = parameter estimates; V = ventral; VAS = visual analog scale (for level of pain).
Capitalizing on the ability of spinal cord fMRI to image pain-evoked responses, researchers set out to explore nociceptive processing using pain modulation studies. In this context, hyperalgesia (i.e., increased sensitivity to pain) has been found to enhance nociceptive spinal activity, whether provoked by a nocebo effect (Geuter and Büchel 2013; Tinnermann and others 2017) or by opioid withdrawal (Sprenger and others 2018a). Conversely, spinal activity turned out to be reduced during analgesia (i.e., decreased sensitivity to pain), which was either induced by placebo (Eippert and others 2009), offset (Sprenger and others 2018b), or cognitive load (Oliva and others 2022; Sprenger and others 2012). In addition to firsthand pain experiences, one study examined spinal responses linked to the observation of others’ pain (Tinnermann and others 2021b). The authors found that observed pain elicited spinal activity at the same spinal level as experienced pain but in a more medial region, suggesting distinct neural processes.
Nociception is also the first application for which simultaneous brain–spinal cord fMRI was tested (see Tinnermann and others 2021a for review). Using a tailored cervical and brain imaging protocol during painful stimulation, Sprenger and colleagues (2015) revealed neural couplings between the spinal cord and brain structures involved in the descending pain modulatory system, such as the periaqueductal gray (PAG) (Fig. 4B). The functional relevance of these interactions was also underscored, as the strength of the fMRI-derived spinal–periaqueductal gray coupling correlated with behavioral pain ratings (Sprenger and others 2015) and was later shown to be modulated by nocebo effects (Tinnermann and others 2017). Simultaneous brain and spinal cord fMRI has been recently complemented by drug-induced neuromodulation (Oliva and others 2022), providing unprecedented insights into an opioidergic descending pathway supporting pain modulation, something that previously had to rely on animal studies.
Aside from nociception, a body of research has been concerned with other facets of somatosensory stimulation (see Landelle and others 2021 for review). Weber and colleagues (2020) attempted to map the spinal correlates of touch using unilateral tactile stimulation of two distinct dermatomes, showing an elicited activation primarily localized in the ipsilateral hemicord. Unexpectedly, however, activity was found in the dorsal and ventral regions and spanned multiple spinal levels, regardless of the stimulated dermatome. While this warrants further investigation, as these results may indicate unwanted reflexive or voluntary muscle contraction during stimulation, the authors suggest that these patterns could presumably arise because of interneuronal processing and the distributed projections of afferent fibers. Of note, tactile stimulation in nonhuman primates elicited activity in the ipsilateral dorsal horn but also in the ipsilateral and contralateral ventral horns, though to a limited extent across slices (Yang and others 2015).
Motor paradigms have also been employed (see Landelle and others 2021 for review), with a variety of upper limb movements, such as fist clenching (Islam and others 2019), finger tapping (Vahdat and others 2015), finger flexion and extension (Barry and others 2021), finger abduction (Kinany and others 2019), wrist flexion (Weber and others 2016b), wrist extension (Kinany and others 2019), and wrist adduction (Kinany and others 2019; Kinany and others 2022). As in sensory studies, imaging was conducted using cervical fMRI, given the innervation of the muscles involved.
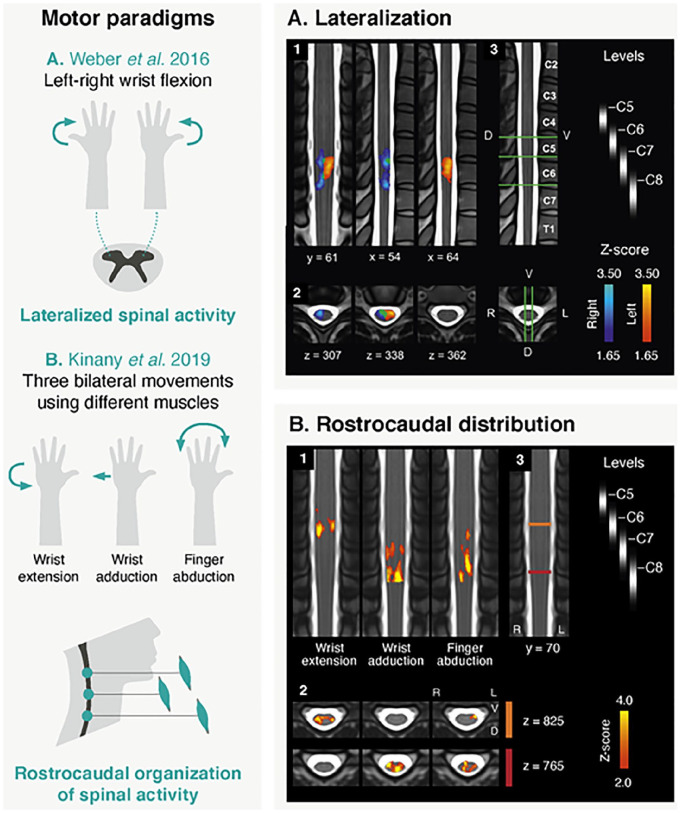
Apart from a few studies evaluating acquisition pipelines (Barry and others 2021; Islam and others 2019; Kinany and others 2022), the primary goal of these works was to shed light on properties of motor-evoked spinal responses. In contrast to sensory paradigms, spinal motor activity is principally expected in the ventral horn ipsilateral to the task (Box 3), at a level dependent on the activated muscles (i.e., myotomes). It should however be noted that movements, especially dynamic ones, may elicit a mixture of motor and sensory (tactile and proprioceptive) processes, possibly resulting in a spread of the activity to dorsal regions. Several studies have reported activity in the ipsilateral hemicord during a unilateral task (Barry and others 2021; Vahdat and others 2015; Weber and others 2016b). In particular, Weber and colleagues (2016b) (Fig. 5A) used a task minimizing complexity (i.e., isometric wrist flexion) and confirmed lateralization at the group and subject levels. Regarding the dorsoventral organization, findings have instead been more equivocal, though a reported tendency for stronger ventral activity suggests greater motoneuron activity relative to sensory input (Barry and others 2021; Kinany and others 2019; Kinany and others 2022; Weber and others 2016b). Finally, along the rostrocaudal direction, activations were in agreement with muscle innervation patterns (Barry and others 2021; Kinany and others 2019; Kinany and others 2022; Vahdat and others 2015; Weber and others 2016b) (Fig. 5B), as confirmed using electromyography-based estimations of spinal activity (Kinany and others 2019).
Figure 5.
Examples of spinal cord fMRI studies using motor paradigms. (A) The first study (adapted with permission from Weber and others 2016b) looked into spinal activations elicited by left and right isometric wrist flexion. Panels 1 (coronal and sagittal views) and 2 (axial views) show group-level activation maps (n = 11) for the left (in red) and right (in blue) contrasts (overlap in green). Maps are thresholded at a Z score >1.65 (cluster-defining threshold of P < .05 to correct for multiple comparisons) and overlaid to the MNI-Poly-AMU template. Overall, a clear lateralization of the activity can be observed, in agreement with anatomical knowledge. Panel 3 shows a sagittal and axial view of the template, with labels of vertebral bodies. Relevant spinal levels are indicated (probabilistic maps). The positions of the slices presented in panels 1 and 2 are also shown (green lines). (B) In the second study (adapted with permission from Kinany and others 2019), the rostrocaudal distribution of spinal activity linked to three upper limb movements is assessed. Group-level activation maps (n = 17) are displayed (coronal and axial views in panel 1 and 2, respectively). Maps are thresholded at a Z score >2 (cluster-defining threshold of P < .01 to correct for multiple comparisons), overlaid to the PAM50 template. Distinct patterns of activations are observed for the three conditions, in line with the myotomes in use (i.e., different muscles are innervated by different spinal levels). Panel 3 shows a coronal view of the template with relevant spinal levels (probabilistic maps). The positions of the two axial slices are indicated (red and orange lines, respectively for the lower and upper slices). D = dorsal; V = ventral.
Importantly, spinal activity is more than a mere reflection of motor output. Notably, Vahdat and colleagues (2015) identified stronger activations during the performance of a complex finger-tapping task as compared with a simple one, even for comparable muscle demands. Furthermore, they leveraged a FOV including the brain and demonstrated that learning-related modulation of spinal activity occurred independently of supraspinal structures. This points to an active contribution of the spinal cord during motor skill acquisition and suggests that it is capable of local plasticity.
Taken together, these studies underscore the potential of fMRI to image sensorimotor pathways in the spinal cord. First, they confirmed basic features of spinal activity. Then, they provided opportunities to delve into the complexity of spinal functional circuits to address fundamental questions about sensorimotor processing.
Restless Spinal Cord
Although task-based paradigms carry a lot of potential to better understand the functioning of the central nervous system, they are only one side of the coin. More than two decades ago, the brain was shown to exhibit meaningful and organized activity, even in the absence of overt task or stimulation (see van den Heuvel and Hulshoff Pol 2010 for review). This laid the foundation for the field of resting state studies. Over the years, the neuroimaging community has shown tremendous interest in investigating the intrinsic brain activity, using functional connectivity analyses to delineate patterns of synchronous activity. This has revealed functionally relevant resting state networks (e.g., auditory, sensorimotor) and provided unique insights into brain organization. These successes led researchers to wonder: are these organized fluctuations a general trait of the central nervous system? Studies extending beyond the cortex are still relatively scarce, but the answer seems to be yes. Indeed, patterns of functional connectivity have now been revealed in various structures of the neural axis, from the brainstem to the cervical spinal cord (see Harrison and others 2021 for review).
From a physiological viewpoint, several mechanisms, likely occurring simultaneously, may indeed generate organized resting state fluctuations in the spinal cord. Activity in the sensory and motor horns may stem from the ongoing descending and ascending communication with the brain or reflect continuous processing of sensory inputs from the periphery (Box 3). Furthermore, local interneuronal circuits may give rise to coherent patterns of activations between horns, within or between hemicords.
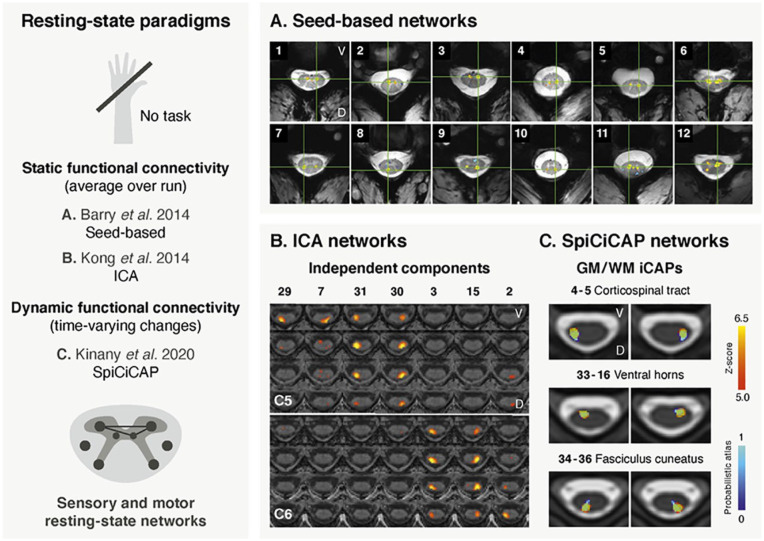
The first conclusive characterization of spinal resting state networks dates from 2014 (Fig. 6A). In this pioneering study, Barry and colleagues (2014) used ultrahigh-field fMRI (7 T) and revealed strong correlations between sensory horns (dorsal-dorsal) and between motor horns (ventral-ventral) using a seed-based approach. These results were corroborated by Kong and colleagues (2014), who extracted sensory and motor networks using an independent component analysis (ICA) at 3 T (Fig. 6B). In this study, however, sensory networks were found to segregate into lateralized components, a discrepancy that may parallel the weaker connectivity strength measured between sensory horns at 7 T (Barry and others 2014). Interestingly, ICA–derived networks exhibited a limited rostrocaudal extent (Kong and others 2014), mirroring the segmental organization of the spinal cord. Following the discovery of spinal resting state networks, active efforts have been made to ascertain their reliability, highlighting their within-subject reproducibility (Barry and others 2016) and their robustness to variations in the processing pipeline (Eippert and others 2017b). Altogether, these findings provided compelling evidence that spontaneous fluctuations are organized in agreement with the structural and functional organization of the spinal cord.
Figure 6.
Examples of spinal cord fMRI studies using resting state paradigms. (A) The first study (adapted with permission from Barry and others 2014) is the first conclusive report of spinal cord resting state networks, which was achieved using a seed-based approach in a 7-T fMRI data set. The figure shows examples of subject-level within-slice resting state connectivity. Correlations (thresholded at P < .001) are overlaid on the corresponding anatomical image. The green crosshair corresponds to the seed’s location. Correlations between ventral (motor) horns can be seen in panels 1 to 6. Correlations between dorsal (sensory) horns are reported in panels 7 to 10. Panels 11 and 12 present other types of less common connectivity patterns. (B) The second study (adapted with permission from Kong and others 2014) retrieved resting state networks using a different approach, namely a group independent component analysis (ICA) in 20 subjects. Dorsal (sensory) networks are presented here, overlaid on an anatomical image. While networks were identified on a region spanning vertebral levels extending from C4 to T1, only levels C5 and C6 are displayed in this figure, for illustration purposes. Each component covers a limited rostrocaudal extent and exhibits lateralized patterns. Maps are thresholding at a false discovery rate of P < 0.05 and arranged by their rostrocaudal position, with each column corresponding to one independent component (number indicated at the top). (C) This third work (adapted with permission from Kinany and others 2020) shows resting state networks that were obtained using a dynamic functional connectivity approach, the SpiCiCAP framework, in a group of 19 participants. In total, 40 fine-grained resting state components, termed iCAPs, were extracted. Six representative iCAPs are presented here (in red), overlaid on the PAM50 template. iCAPs are in agreement with the underlying anatomy, as illustrated by the matching atlas regions (in blue). They correspond to various white matter (WM) regions (e.g., lateral corticospinal tract and fasciculus cuneatus) and gray matter (GM) regions (e.g., ventral horns). D = dorsal; V = ventral.
Several studies have built on these encouraging results, some capturing ipsilateral dorsoventral functional connectivity in addition to the bilateral sensory and motor networks previously reported (Conrad and others 2018; Liu and others 2016b; San Emeterio Nateras and others 2016; Weber and others 2018). While these couplings may be related to anatomical connections between these regions (Box 3), they have been suspected to reflect the proximity of dorsal and ventral horns, particularly in light of their lower robustness (Eippert and others 2017b). Nonetheless, within-hemicord connectivity has been reported in nonhuman primates at 9.4 T (Chen and others 2015).
Besides confirming the presence of organized intrinsic networks in the cervical cord, later research deepened their characterization. Borrowing from graph theory, spinal networks were shown to present small-world properties (Liu and others 2016b), similar to the organization of cortical networks (Bassett and Bullmore 2017). Subsequent work emphasized the functional relevance of these topological features, found to be altered by thermal stimulation (Weber and others 2018).
Importantly, the aforementioned studies relied on static functional connectivity—that is, an average over the scanning session. In contrast, dynamic approaches probe time-varying connectivity patterns. To date, only one study has utilized a dynamic framework (SpiCiCAP) in the spinal cord (Kinany and others 2020), which disentangled spontaneous signals into fine-grained components in gray and white matter, organized along the major ascending and descending spinal pathways (Fig. 6C). Although the white matter has long been ignored in functional neuroimaging, increasing evidence in brain studies supports the existence of reliable BOLD signals in this region (Grajauskas and others 2019). Furthermore, in the spinal cord, the large volume of white matter (Box 1) may have facilitated their detection.
Finally, functional connectivity analyses have recently begun to elucidate networks simultaneously spanning the spinal cord and the brain. In particular, Vahdat and colleagues (2020) have exposed meaningful couplings between the spinal cord and cortical and subcortical regions, in line with anatomical and functional expectations (lateralization and sensory vs. motor segregation). This demonstrates a unified organization of sensorimotor networks across the central nervous system.
In summary, these resting state studies confirmed that the rich content of intrinsic spinal activity can be captured by means of fMRI. They highlighted, with remarkable agreement, that the spinal cord exhibits an organized functional architecture mirroring neuroanatomical and physiological principles, even in the absence of a task.
Clinical Potential
As evidence advocating for the functional relevance of spinal cord fMRI signals accumulated, so did the interest in their clinical usage, for instance to assess spinal residual neuronal function (see Wheeler-Kingshott and others 2014 for review).
Traumatic spinal cord injury (SCI) was, unsurprisingly, the first condition to attract attention, already 20 years ago. Spin echo sequences were deployed in patients with SCI during movements and sensory stimulation, showing that activity could be detected below the site of injury (see Cadotte and others 2018 for review). To the best of our knowledge, only one study to date has deployed a gradient echo sequence to image spinal activity in patients with SCI (Rowald and others 2022). Specifically, fMRI was used in three patients during passive limb mobilization or tendon vibration to map proprioceptive neurons innervating muscles supporting walking and thereby inform preoperative planning for a spinal implant. While resting state studies have not yet been harnessed to study SCI in humans, results in nonhuman primates alluded to their clinical potential (Chen and others 2015), as functional connectivity reflected the functional integrity of the spinal cord over the course of the recovery.
In humans, the clinical potential of functional connectivity has actually been demonstrated in patients with multiple sclerosis, another population with spinal cord abnormalities. Specifically, Conrad and colleagues (2018) examined changes in resting state fluctuations at 7 T. Although they did not detect a difference in average connectivity in patients versus controls, they could show that lesions had a local effect on intrinsic connectivity. This may reflect compensatory changes attributed to white matter damage and/or disrupted interneuronal circuits. See Wheeler-Kingshott and colleagues (2014) for a review of spin echo studies using task-based paradigms in patients with multiple sclerosis.
Besides functional connectivity, regional neural activity can also be assessed, for instance using the resting state amplitude of low-frequency fluctuation (ALFF; from 0.01 Hz to 0.1–0.2 Hz). First, this was done in the context of cervical spondylotic myelopathy (Liu and others 2016a), showing that ALFF values were higher in patients than controls and even more so in severely affected patients. Then, low-frequency fluctuations were investigated in patients with fibromyalgia, one of the most common causes of chronic widespread pain. Martucci and colleagues (2019) initially identified an alteration of the ALFF but not of the average connectivity or the related graph metrics. In a later study, the authors used ALFF to capture the spinal correlates of pain medication (Martucci and others 2021), highlighting that patients taking opioids exhibited ALFF patterns that were more similar to the control group.
Discussion
A “New” Tool
Considerable progress has been made since the emergence of spinal cord fMRI in 1996 (Yoshizawa and others 1996), and converging evidence suggests its potential to image spinal signals. While the field has long lagged behind brain neuroimaging standards, recent years have witnessed a transition toward more systematic and quantitative analyses. In this context, advances on the acquisition side have been paralleled by the development of dedicated processing tools, all of which have been instrumental to establish accessible spinal cord fMRI routines that can be readily deployed in different environments.
From a more general perspective, the broader field of spinal cord imaging has encountered rising interest, with a growing and supportive community. This is notably exemplified by a recent initiative proposing a generic protocol for quantitative spinal cord MRI (i.e., structural imaging), available across vendors and centers (Cohen-Adad and others 2021). Driven by the same intent, it is hoped that the current state of spinal cord fMRI will encourage researchers, even nonexperts, to join forces. Collective efforts will foster the establishment of robust and generalized protocols and further support the development of this burgeoning research stream.
Bringing the Field Forward
It stands to reason that progress and improvements are still to come. As a matter of fact, spinal cord fMRI remains more prone to artifacts than brain fMRI. Efforts could entail new pulse sequences or shimming protocols focused on improving signal strength and homogeneity in the spinal cord. To this end, collaborations with scanner manufacturers could be highly beneficial in promoting the availability and deployment of these novel methods. In addition to efforts aiming to improve gradient echo sequences, a systematic comparison with state-of-the-art spin echo protocols could be fruitful. Indeed, this would help assess their relative strengths and weaknesses, as well as estimate the generalizability of spinal cord fMRI results across acquisition schemes.
It cannot go unnoticed that most spinal cord fMRI research has, so far, been cervical cord fMRI research. In light of this, acquisition schemes extending to thoracic and lumbosacral regions need to be established to achieve an extensive characterization of spinal mechanisms. However, this endeavor entails additional challenges. First, these regions have smaller cross-sectional dimensions (Frostell and others 2016), and acquisition protocols must be validated in these conditions. Then, the environment surrounding the spinal cord varies along the rostrocaudal axis, implying that the impact of different physiological noise sources (e.g., lungs, bowels) should also be assessed. Finally, a concern arises from the relative distances between spinal levels and vertebral bodies, which become more pronounced in caudal regions (Frostell and others 2016). Given this, normalization procedures may need to be adjusted to ensure the accuracy of group-level estimates.
Disentangling Spinal Circuits
While the works presented in this review uncovered the richness of spinal signals during tasks and at rest, they also hinted at their complexity. Indeed, signals were found to reflect sensory, motor, and interneuronal processes. This intricacy may be expected, as most experimental conditions fall short of isolating specific spinal processes. To address this, one potential direction is to better characterize the task itself so that its properties can be directly related to the imaged spinal signals. In other words, we need to obtain objective and quantitative measures of what the subject is actually doing, rather than using predefined and discrete categories. To this end, future work could conduct multimodal experiments in which functional images are acquired with peripheral measurements of task-related parameters (e.g., electromyography or kinematics). Using these concurrent recordings will help build informed task models that better disentangle the different components of the captured activity (e.g. motor vs. proprioceptive signals during dynamic movements; sensory vs. reflexive motor activity during nociception).
An alternative method to delineate spinal circuits is to aim for more controlled experimental paradigms. For instance, the use of brain stimulation can be envisioned to systematically activate descending pathways to the spinal cord. Likewise, functional electrical stimulation and tendon vibration can be deployed to modulate motor and proprioceptive circuits. Furthermore, combining these controlled sensorimotor paradigms with resting state scans could help explain how the functional architecture of the spinal cord is modulated to meet task demands and provide new insights into the origin of spinal intrinsic fluctuations. Simultaneous brain and spinal cord fMRI could also be deployed to achieve a multilevel view of neural processes and discriminate local spinal activity from supraspinal inputs.
Finally, it is worth mentioning that much remains to be learned about the organization and functional role of spinal interneuronal circuits beyond those involved in reflexes and central pattern generators. These circuits likely participate in generating complex spinal cord activity, which cannot be readily deciphered with the level of detail available with current acquisitions and analyses. Indeed, while dividing the cord into ventral, dorsal, and intermediate zones offers a meaningful and straightforward way to describe and interpret spinal cord fMRI results, this does not fully capture the complexity of spinal cord processing. This line of research could be stimulated by technological advances, notably in ultrahigh-field imaging.
Clinical Applications: Perspectives
Clinical studies stand out as an important avenue for investigation. In this review, we present studies underscoring the clinical relevance of spinal cord fMRI in a number of neurological conditions. To enrich these investigations, longitudinal assessments could be carried out to gain insights into disease progression and recovery, thereby improving our capacity for diagnosis and prognosis. One topic that is particularly relevant in this context is that of spinal neuroplasticity, which can occur following damages to sensorimotor pathways (Wolpaw and Tennissen 2001). Joint analyses of spinal activity and clinical indicators could shed new light on the mechanisms mediating adaptive and maladaptive plasticity, possibly highlighting targets for treatment or rehabilitation. In parallel, quantitative MRI could be deployed to relate functional measures to the underlying structure. Given the importance of personalized assessments in the clinical context, it would also be of interest to go beyond group studies. For that purpose, future studies could build on the pipeline introduced in this review to explore the potential of spinal cord fMRI for individual subjects. In doing so, preliminary research in healthy participants should assess the parameters (paradigms, amount of data, etc.) needed to achieve sufficient sensitivity, as well as quantify intra- and intersubject variability. Ultimately, this could pave the way for noninvasive biomarkers of disease progression or treatment response, complementary to those derived using quantitative MRI.
Conclusion
Overall, the findings reported in this review emphasize how fMRI can serve as a powerful tool to peek into the spinal cord’s functional architecture. Despite the numerous challenges encountered along the way, the use of spinal cord fMRI can now provide accessible opportunities to study the healthy and impaired human central nervous system, beyond and in addition to classical brain neuroimaging.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partly funded by the Wyss Center for Bio and Neuroengineering, the Swiss National Centre of Competence in Research (NCCR) Robotics, the grant 2017-205 of the strategic focal area “Personalized Health and Related Technologies” of the ETH Domain, the Bertarelli Foundation, and École Polytechnique Fédérale de Lausanne’s School of Engineering (eSeed grant).
ORCID iDs: Nawal Kinany  https://orcid.org/0000-0003-3804-0991
https://orcid.org/0000-0003-3804-0991
Dimitri Van De Ville  https://orcid.org/0000-0002-2879-3861
https://orcid.org/0000-0002-2879-3861
References
- Ashburner J. 2012. SPM: a history. Neuroimage 62:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini PA. 2012. Twenty years of functional MRI: the science and the stories. Neuroimage 62:575–88. [DOI] [PubMed] [Google Scholar]
- Barry RL, Conrad BN, Maki S, Watchmaker JM, McKeithan LJ, Box BA, and others. 2021. Multi-shot acquisitions for stimulus-evoked spinal cord BOLD fMRI. Magn Reson Med 85:2016–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RL, Rogers BP, Conrad BN, Smith SA, Gore JC. 2016. Reproducibility of resting state spinal cord networks in healthy volunteers at 7 Tesla. Neuroimage 133:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RL, Smith SA, Dula AN, Gore JC. 2014. Resting state functional connectivity in the human spinal cord. Elife 3:e02812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RL, Vannesjo SJ, By S, Gore JC, Smith SA. 2018. Spinal cord MRI at 7T. Neuroimage 168:437–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore ET. 2017. Small-world brain networks revisited. Neuroscientist 23:499–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwman CJC, Wilmink JT, Mess WH, Backes WH. 2008. Spinal cord functional MRI at 3 T: gradient echo echo-planar imaging versus turbo spin echo. Neuroimage 43:288–96. [DOI] [PubMed] [Google Scholar]
- Brooks JCW, Beckmann CF, Miller KL, Wise RG, Porro CA, Tracey I, and others. 2008. Physiological noise modelling for spinal functional magnetic resonance imaging studies. Neuroimage 39:680–92. [DOI] [PubMed] [Google Scholar]
- Cadotte DW, Akbar MA, Fehlings MG, Stroman PW, Cohen-Adad J. 2018. What has been learned from magnetic resonance imaging examination of the injured human spinal cord: a Canadian perspective. J Neurotrauma 35:1942–57. [DOI] [PubMed] [Google Scholar]
- Cadotte DW, Cadotte A, Cohen-Adad J, Fleet D, Livne M, Wilson JR, and others. 2015. Characterizing the location of spinal and vertebral levels in the human cervical spinal cord. AJNR Am J Neuroradiol 36:803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Mishra A, Yang P-F, Wang F, Gore JC. 2015. Injury alters intrinsic functional connectivity within the primate spinal cord. Proc Natl Acad Sci U S A 112:5991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Adad J, Alonso-Ortiz E, Abramovic M, Arneitz C, Atcheson N, Barlow L, and others. 2021. Generic acquisition protocol for quantitative MRI of the spinal cord. Nat Protoc 16:4611–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad BN, Barry RL, Rogers BP, Maki S, Mishra A, Thukral S, and others. 2018. Multiple sclerosis lesions affect intrinsic functional connectivity of the spinal cord. Brain 141:1650–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leener B, Fonov VS, Collins DL, Callot V, Stikov N, Cohen-Adad J. 2018. PAM50: unbiased multimodal template of the brainstem and spinal cord aligned with the ICBM152 space. Neuroimage 165:170–9. [DOI] [PubMed] [Google Scholar]
- De Leener B, Lévy S, Dupont SM, Fonov VS, Stikov N, Louis Collins D, and others. 2017. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage 145:24–43. [DOI] [PubMed] [Google Scholar]
- De Leener B, Soltrand Dahlberg L, Khatibi A, Cohen-Adad J, Doyon J. 2020. Effect of non-protonated perfluorocarbon liquid-filled SatPads on spinal cord MR imaging. In: ISMRM annual meeting proceedings. Concord (CA): International Society for Magnetic Resonance in Medicine. Abstract 1174. [Google Scholar]
- Eippert F, Finsterbusch J, Bingel U, Büchel C. 2009. Direct evidence for spinal cord involvement in placebo analgesia. Science 326:404. [DOI] [PubMed] [Google Scholar]
- Eippert F, Kong Y, Jenkinson M, Tracey I, Brooks JCW. 2017. a. Denoising spinal cord fMRI data: approaches to acquisition and analysis. Neuroimage 154:255–6. [DOI] [PubMed] [Google Scholar]
- Eippert F, Kong Y, Winkler AM, Andersson JL, Finsterbusch J, Büchel C, and others. 2017. b. Investigating resting-state functional connectivity in the cervical spinal cord at 3T. Neuroimage 147:589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterbusch J. 2014. B0 inhomogeneity and shimming. In: Quantitative MRI of the spinal cord. Elsevier. p. 68–90. doi: 10.1016/b978-0-12-396973-6.00006-x [DOI] [Google Scholar]
- Finsterbusch J, Eippert F, Büchel C. 2012. Single, slice-specific z-shim gradient pulses improve T2*-weighted imaging of the spinal cord. Neuroimage 59:2307–15. [DOI] [PubMed] [Google Scholar]
- Finsterbusch J, Sprenger C, Büchel C. 2013. Combined T2*-weighted measurements of the human brain and cervical spinal cord with a dynamic shim update. Neuroimage 79:153–61. [DOI] [PubMed] [Google Scholar]
- Frostell A, Hakim R, Thelin EP, Mattsson P, Svensson M. 2016. A review of the segmental diameter of the healthy human spinal cord. Front Neurol 7:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuter S, Büchel C. 2013. Facilitation of pain in the human spinal cord by nocebo treatment. J Neurosci 33:13784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. 2000. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 44:162–7. [DOI] [PubMed] [Google Scholar]
- Grajauskas LA, Frizzell T, Song X, D’Arcy RCN. 2019. White matter fMRI activation cannot be treated as a nuisance regressor: overcoming a historical blind spot. Front Neurosci 13:1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin PA. 2013. Central pattern generator for locomotion: anatomical, physiological, and pathophysiological considerations. Front Neurol 3:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison OK, Guell X, Klein-Flügge MC, Barry RL. 2021. Structural and resting state functional connectivity beyond the cortex. Neuroimage 240:118379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam H, Law CSW, Weber KA, Mackey SC, Glover GH. 2019. Dynamic per slice shimming for simultaneous brain and spinal cord fMRI. Magn Reson Med 81:825–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. 2012. FSL. Neuroimage 62:782–90. [DOI] [PubMed] [Google Scholar]
- Jochimsen TH, Norris DG, Möller HE. 2005. Is there a change in water proton density associated with functional magnetic resonance imaging? Magn Reson Med 53:470–3. [DOI] [PubMed] [Google Scholar]
- Kinany N, Pirondini E, Martuzzi R, Mattera L, Micera S, Van de Ville D. 2019. Functional imaging of rostrocaudal spinal activity during upper limb motor tasks. Neuroimage 200:590–600. [DOI] [PubMed] [Google Scholar]
- Kinany N, Pirondini E, Mattera L, Martuzzi R, Micera S, Van De Ville D. 2022. Towards reliable spinal cord fMRI: Assessment of common imaging protocols. Neuroimage 250:118964. [DOI] [PubMed] [Google Scholar]
- Kinany N, Pirondini E, Micera S, Van De Ville D. 2020. Dynamic functional connectivity of resting-state spinal cord fMRI reveals fine-grained intrinsic architecture. Neuron 108:424–35.e4. [DOI] [PubMed] [Google Scholar]
- Koch SC, Acton D, Goulding M. 2018. Spinal circuits for touch, pain, and itch. Annu Rev Physiol 80:189–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesar TA, Fiest KM, Smith SD, Kornelsen J. 2015. Assessing nociception by fMRI of the human spinal cord: a systematic review. Magn Reson Insights 8:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y, Eippert F, Beckmann CF, Andersson J, Finsterbusch J, Büchel C, and others. 2014. Intrinsically organized resting state networks in the human spinal cord. Proc Natl Acad Sci U S A 111:18067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y, Jenkinson M, Andersson J, Tracey I, Brooks JCW. 2012. Assessment of physiological noise modelling methods for functional imaging of the spinal cord. Neuroimage 60:1538–49. [DOI] [PubMed] [Google Scholar]
- Landelle C, Lungu O, Vahdat S, Kavounoudias A, Marchand-Pauvert V, De Leener B, and others. 2021. Investigating the human spinal sensorimotor pathways through functional magnetic resonance imaging. Neuroimage 245:118684. [DOI] [PubMed] [Google Scholar]
- Liu X, Qian W, Jin R, Li X, Luk KD, Wu EX, and others. 2016. a. Amplitude of low frequency fluctuation (ALFF) in the cervical spinal cord with stenosis: a resting state fMRI study. PLoS One 11:e0167279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhou F, Li X, Qian W, Cui J, Zhou IY, and others. 2016. b. Organization of the intrinsic functional network in the cervical spinal cord: a resting state functional MRI study. Neuroscience 336:30–8. [DOI] [PubMed] [Google Scholar]
- Martucci KT, Weber KA, 2nd, Mackey SC. 2019. Altered cervical spinal cord resting-state activity in fibromyalgia. Arthritis Rheumatol 71:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martucci KT, Weber KA, 2nd, Mackey SC. 2021. Spinal cord resting state activity in individuals with fibromyalgia who take opioids. Front Neurol 12:694271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell DJ, Soteropoulos DS. 2020. The mammalian spinal commissural system: properties and functions. J Neurophysiol 123:4–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JB. 2016. Human spinal motor control. Annu Rev Neurosci 39:81–101. [DOI] [PubMed] [Google Scholar]
- Oliva V, Hartley-Davies R, Moran R, Pickering AE, Brooks JC. 2022. Simultaneous brain, brainstem, and spinal cord pharmacological-fMRI reveals involvement of an endogenous opioid network in attentional analgesia. Elife 11:e71877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette T, Jeffrey-Gauthier R, Leblond H, PichÉ M. 2018. Functional neuroimaging of nociceptive and pain-related activity in the spinal cord and brain: insights from neurovascular coupling studies. Anat Rec 301:1585–95. [DOI] [PubMed] [Google Scholar]
- Piché M, Cohen-Adad J, Nejad MK, Perlbarg V, Xie G, Beaudoin G, and others. 2009. Characterization of cardiac-related noise in fMRI of the cervical spinal cord. Magn Reson Imaging 27:300–10. [DOI] [PubMed] [Google Scholar]
- Piché M, Paquette T, Leblond H. 2017. Tight neurovascular coupling in the spinal cord during nociceptive stimulation in intact and spinal rats. Neuroscience 355:1–8. [DOI] [PubMed] [Google Scholar]
- Powers JM, Ioachim G, Stroman PW. 2018. Ten key insights into the use of spinal cord fMRI. Brain Sci 8(9):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D, Augustine G, Fitzpatrick D, Hall WC, LaMantia A, Mooney R, and others. 2018. Neuroscience. Sunderland (MA): Sinauer. [Google Scholar]
- Rowald A, Komi S, Demesmaeker R, Baaklini E, Hernandez-Charpak SD, Paoles E, and others. 2022. Activity-dependent spinal cord neuromodulation rapidly restores trunk and leg motor functions after complete paralysis. Nat Med 28:260–71. [DOI] [PubMed] [Google Scholar]
- San Emeterio Nateras O, Yu F, Muir ER, Bazan C, 3rd, Franklin CG, Li W, and others. 2016. Intrinsic resting-state functional connectivity in the human spinal cord at 3.0 T. Radiology 279:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saritas EU, Holdsworth SJ, Bammer R. 2014. Susceptibility artifacts. In: Quantitative MRI of the spinal cord. Elsevier. p. 91–105. [Google Scholar]
- Shurrager PS, Dykman RA. 1951. Walking spinal carnivores. J Comp Physiol Psychol 44:252–62. [DOI] [PubMed] [Google Scholar]
- Sprenger C, Eichler I-C, Eichler L, Zöllner C, Büchel C. 2018. a. Altered signaling in the descending pain-modulatory system after short-term infusion of the μ-opioid agonist remifentanil. J Neurosci 38:2454–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger C, Eippert F, Finsterbusch J, Bingel U, Rose M, Büchel C. 2012. Attention modulates spinal cord responses to pain. Curr Biol 22:1019–22. [DOI] [PubMed] [Google Scholar]
- Sprenger C, Finsterbusch J, Büchel C. 2015. Spinal cord-midbrain functional connectivity is related to perceived pain intensity: a combined spino-cortical FMRI study. J Neurosci 35:4248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger C, Stenmans P, Tinnermann A, Büchel C. 2018. b. Evidence for a spinal involvement in temporal pain contrast enhancement. Neuroimage 183:788–99. [DOI] [PubMed] [Google Scholar]
- Stroman PW, Wheeler-Kingshott C, Bacon M, Schwab JM, Bosma R, Brooks J, and others. 2014. The current state-of-the-art of spinal cord imaging: methods. Neuroimage 84:1070–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinnermann A, Büchel C, Cohen-Adad J. 2021. a. Cortico-spinal imaging to study pain. Neuroimage 224:117439. [DOI] [PubMed] [Google Scholar]
- Tinnermann A, Büchel C, Haaker J. 2021. b. Observation of others’ painful heat stimulation involves responses in the spinal cord. Sci Adv 7(14):eabe8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinnermann A, Geuter S, Sprenger C, Finsterbusch J, Büchel C. 2017. Interactions between brain and spinal cord mediate value effects in nocebo hyperalgesia. Science 358:105–8. [DOI] [PubMed] [Google Scholar]
- Vahdat S, Khatibi A, Lungu O, Finsterbusch J, Büchel C, Cohen-Adad J, and others. 2020. Resting-state brain and spinal cord networks in humans are functionally integrated. PLoS Biol. 18:e3000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahdat S, Lungu O, Cohen-Adad J, Marchand-Pauvert V, Benali H, Doyon J. 2015. Simultaneous brain-cervical cord fMRI reveals intrinsic spinal cord plasticity during motor sequence learning. PLoS Biol. 13:e1002186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. 2010. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 20:519–34. [DOI] [PubMed] [Google Scholar]
- Weber KA, 2nd, Chen Y, Paliwal M, Law CS, Hopkins BS, Mackey S, and others. 2020. Assessing the spatial distribution of cervical spinal cord activity during tactile stimulation of the upper extremity in humans with functional magnetic resonance imaging. Neuroimage 217:116905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KA, 2nd, Chen Y, Wang X, Kahnt T, Parrish TB. 2016. a. Functional magnetic resonance imaging of the cervical spinal cord during thermal stimulation across consecutive runs. Neuroimage 143:267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KA, 2nd, Chen Y, Wang X, Kahnt T, Parrish TB. 2016. b. Lateralization of cervical spinal cord activity during an isometric upper extremity motor task with functional magnetic resonance imaging. Neuroimage 125:233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KA, 2nd, Sentis AI, Bernadel-Huey ON, Chen Y, Wang X, Parrish TB, and others. 2018. Thermal stimulation alters cervical spinal cord functional connectivity in humans. Neuroscience 369:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Kingshott CA, Stroman PW, Schwab JM, Bacon M, Bosma R, Brooks J, and others. 2014. The current state-of-the-art of spinal cord imaging: applications. Neuroimage 84:1082–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, Tennissen AM. 2001. Activity-dependent spinal cord plasticity in health and disease. Annu Rev Neurosci 24:807–43. [DOI] [PubMed] [Google Scholar]
- Wu T-L, Yang P-F, Wang F, Shi Z, Mishra A, Wu R, and others. 2019. Intrinsic functional architecture of the non-human primate spinal cord derived from fMRI and electrophysiology. Nat Commun 10:1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P-F, Wang F, Chen LM. 2015. Differential fMRI activation patterns to noxious heat and tactile stimuli in the primate spinal cord. J Neurosci 35:10493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T, Nose T, Moore GJ, Sillerud LO. 1996. Functional magnetic resonance imaging of motor activation in the human cervical spinal cord. Neuroimage 4:174–82. [DOI] [PubMed] [Google Scholar]