Abstract
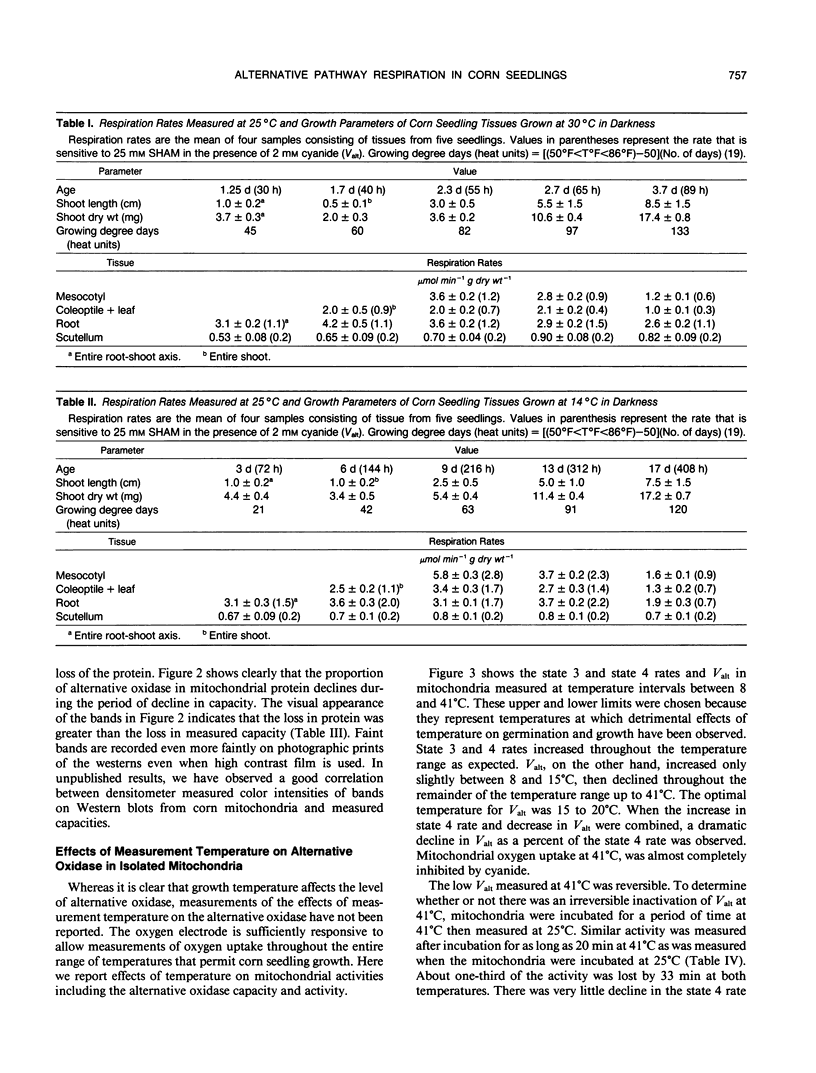
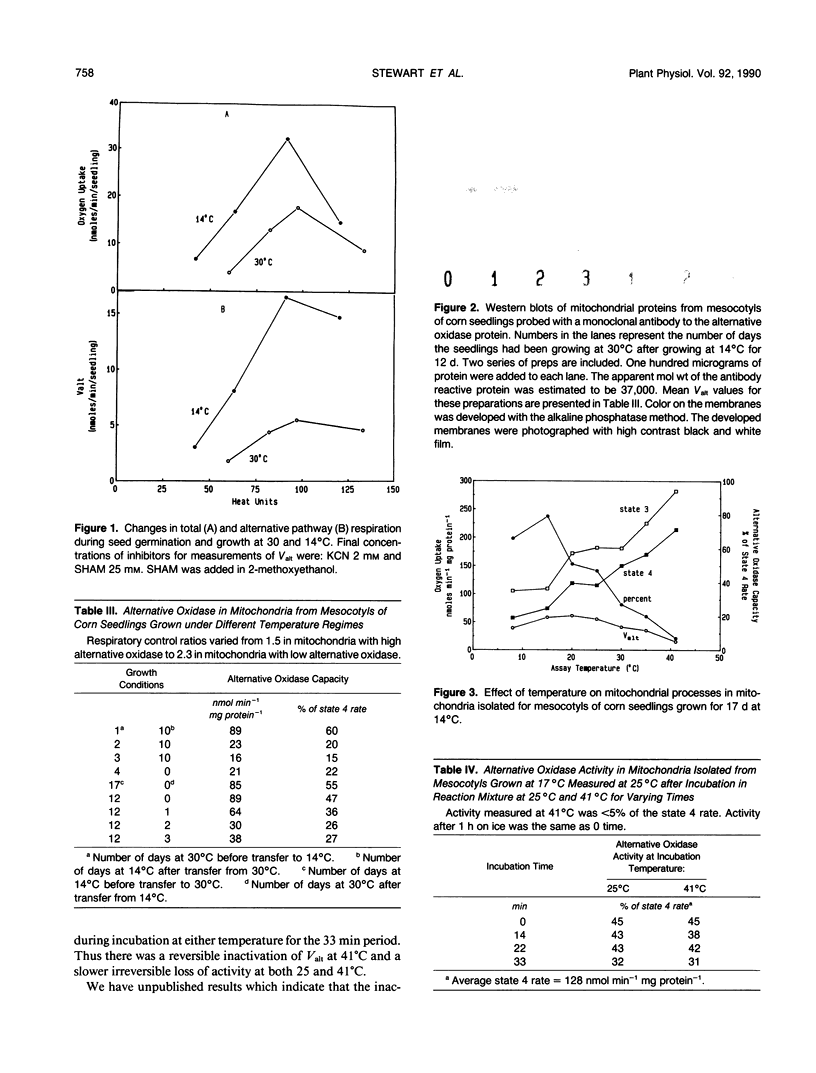
Respiration rates of Zea mays L. seedling tissues grown at 30 and 14°C were measured at 25°C at different stages of seedling growth. Accumulation of heat units was used to define the developmental stages to compare respiration between the two temperatures. At both temperatures, respiration rates of most tissues were highest at the youngest stages, then declined with age. Respiration rates of mesocotyl tissue were the most responsive to temperature, being nearly twofold higher when grown at 14 compared to 30°C. Alternative pathway respiration increased concomitantly with respiration and was higher in mesocotyls grown in the cold. When seedlings were started at 30 then transferred to 14°C, the increase in alternative pathway respiration due to cold was not observed unless the seedlings were transferred before 2 days of growth. Seedlings transferred to 14°C after growth at 30°C for 2 days had the same alternative oxidase capacity as seedlings grown at 30°C. Seedlings grown at 14°C for 10 to 12 days, then transferred to 30°C, lost alternative pathway respiratory capacity over a period of 2 to 3 days. Western blots of mitochondrial proteins indicated that this loss of capacity was due to a loss of the alternative oxidase protein. Some in vitro characteristics of mitochondria were determined. The temperature optimum for measurement of alternative oxidase capacity was 15 to 20°C. At 41°C, very little alternative oxidase was measured, i.e., the mitochondrial oxygen uptake was almost completely sensitive to cyanide. This inactivation at 41°C was reversible. After incubation at 41°C, the alternative oxidase capacity measured at 25°C was the similar to when it was measured at that temperature directly. Isolated mitochondria lost alternative oxidase capacity at the same rate when incubated at 41°C as they did when incubated at 25°C. Increasing the supply of electrons to isolated mitochondria increased the degree of engagement of the alternative pathway, whereas lower temperature decreased the degree of engagement. Lower temperatures did not increase the degree of engagement of the pathway in intact tissues. We interpret these observations to indicate that the greater capacity of alternative oxidase in cold-grown seedlings is a consequence of development at these low temperatures which results in elevated respiration rates. Low temperature itself does not cause greater capacity or engagement of the alternative oxidase in mitochondria that have developed under warm temperatures. Our hypothesis would be that the low growth temperatures require the seedlings to have a higher respiration rate for some reason, e.g., to prevent the accumulation of a toxic metabolite, and that the alternative pathway functions in that respiration.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Day D. A., Moore A. L., Dry I. B., Wiskich J. T., Azcon-Bieto J. Regulation of nonphosphorylating electron transport pathways in soybean cotyledon mitochondria and its implications for fat metabolism. Plant Physiol. 1988 Apr;86(4):1199–1204. doi: 10.1104/pp.86.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., McIntosh L. Characterization and Solubilization of the Alternative Oxidase of Sauromatum guttatum Mitochondria. Plant Physiol. 1986 Sep;82(1):1–6. doi: 10.1104/pp.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., McIntosh L. Identification of the alternative terminal oxidase of higher plant mitochondria. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8399–8403. doi: 10.1073/pnas.84.23.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., Stewart C. R., McCoy C. A., Bonner W. D. Alternative Respiratory Path Capacity in Plant Mitochondria: Effect of Growth Temperature, the Electrochemical Gradient, and Assay pH. Plant Physiol. 1986 Feb;80(2):378–383. doi: 10.1104/pp.80.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiener C. M., Bramlage W. J. Temperature Effects on the Activity of the Alternative Respiratory Pathway in Chill-Sensitive Cucumis sativus. Plant Physiol. 1981 Dec;68(6):1474–1478. doi: 10.1104/pp.68.6.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I., Ehmann A., Melander W. R., Meeuse B. J. Salicylic Acid: a natural inducer of heat production in arum lilies. Science. 1987 Sep 25;237(4822):1601–1602. doi: 10.1126/science.237.4822.1601. [DOI] [PubMed] [Google Scholar]
- Sesay A., Stewart C. R., Shibles R. M. Effects of KCN and Salicylhydroxamic Acid on Respiration of Soybean Leaves at Different Ages. Plant Physiol. 1986 Oct;82(2):443–447. doi: 10.1104/pp.82.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]



