Abstract
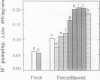
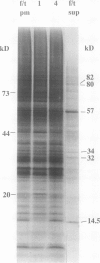
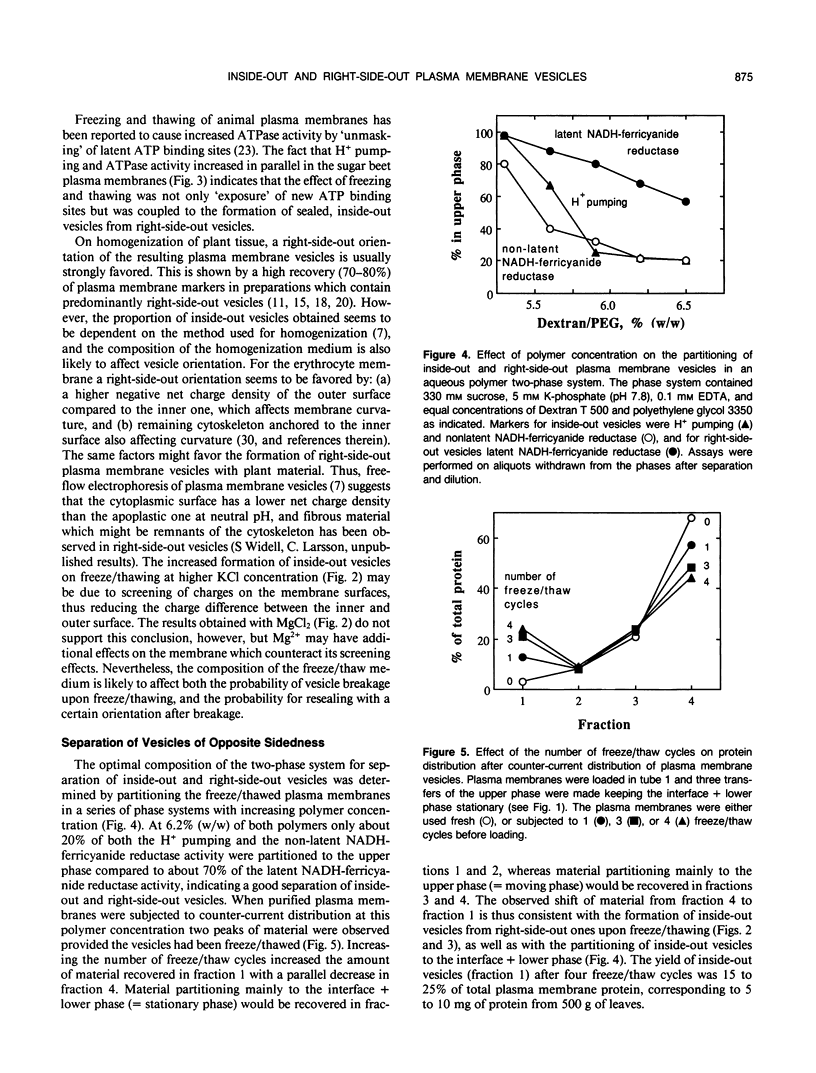
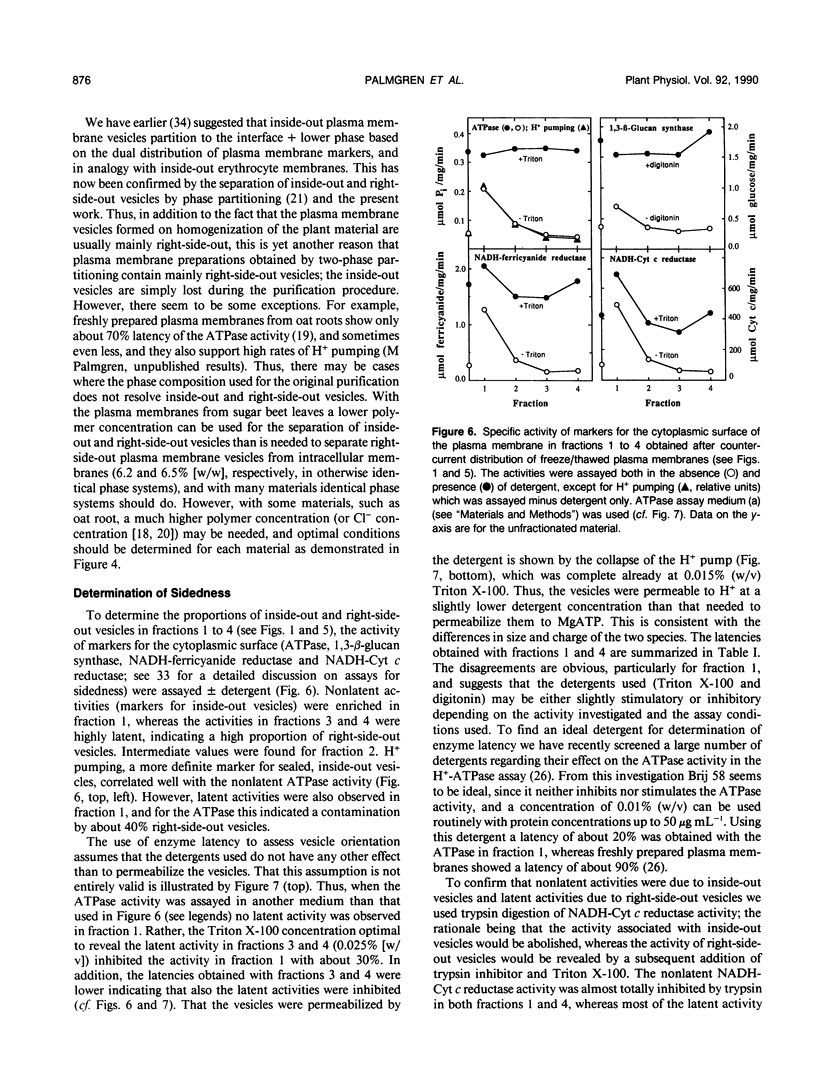
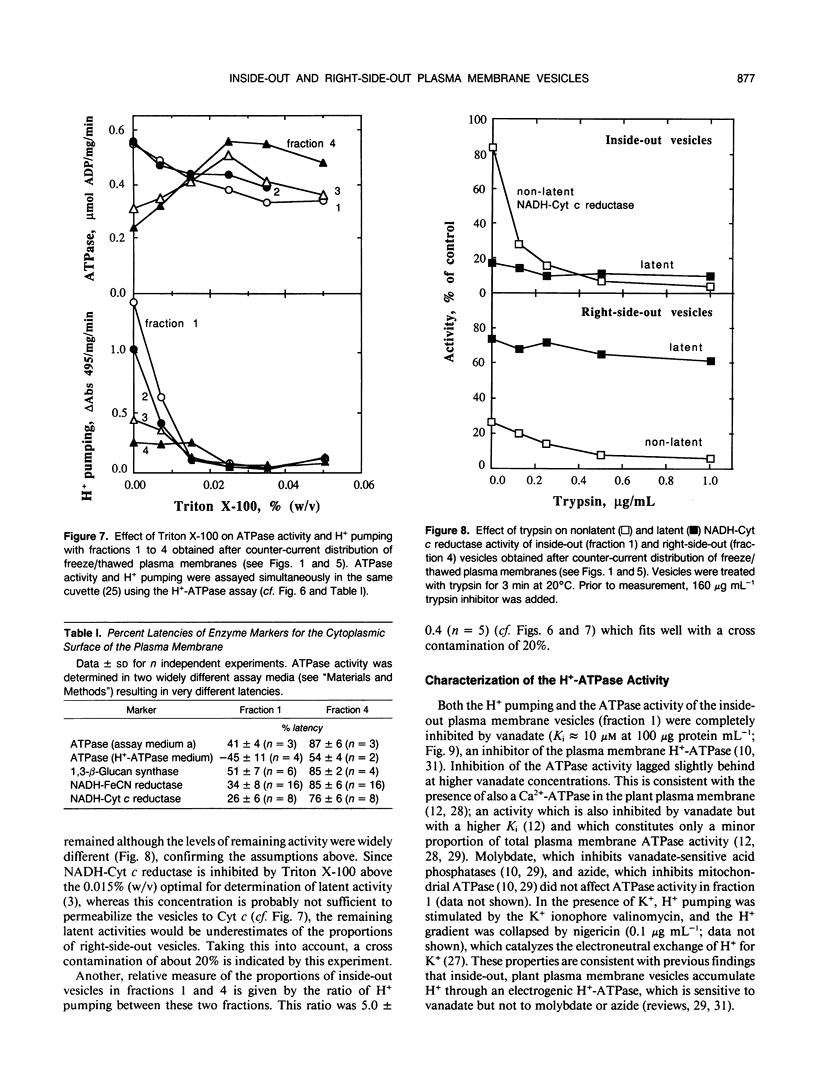
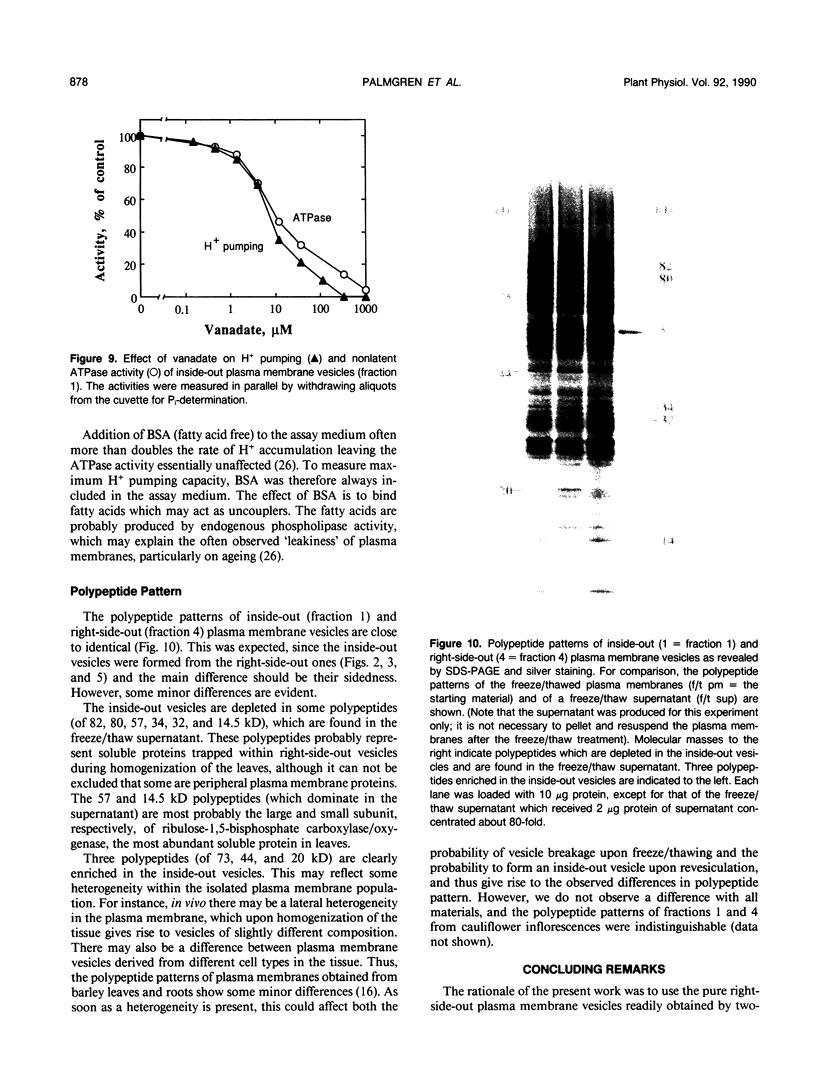
Plasma membrane preparations of high purity (about 95%) are easily obtained by partitioning in aqueous polymer two-phase systems. These preparations, however, mainly contain sealed right-side-out (apoplastic side out) vesicles. Part of these vesicles have been turned inside-out by freezing and thawing, and sealed inside-out and right-side-out vesicles subsequently separated by repeating the phase partition step. Increasing the KCI concentration in the freeze/thaw medium as well as increasing the number of freeze/thaw cycles significantly increased the yield of inside-out vesicles. At optimal conditions, 15 to 25% of total plasma membrane protein was recovered as inside-out vesicles, corresponding to 5 to 10 milligrams of protein from 500 grams of sugar beet (Beta vulgaris L.) leaves. Based on enzyme latency, trypsin inhibition of NADH-cytochrome c reductase, and H+ pumping capacity, a cross-contamination of about 20% between the two fractions of oppositely oriented vesicles was estimated. Thus, preparations containing about 80% inside-out and 80% right-side-out vesicles, respectively, were obtained. ATPase activity and H+ pumping were both completely inhibited by vanadate (Ki ≈ 10 micromolar), indicating that the fractions were completely free from nonplasma membrane ATPases. Furthermore, the polypeptide patterns of the two fractions were close to identical, which shows that the vesicles differed in sidedness only. Thus, preparations of both inside-out and right-side-out plasma membrane vesicles are now available. This permits studies on transport, signal transduction mechanisms, enzyme topology, etc., using plasma membrane vesicles of either orientation.
Full text
PDF
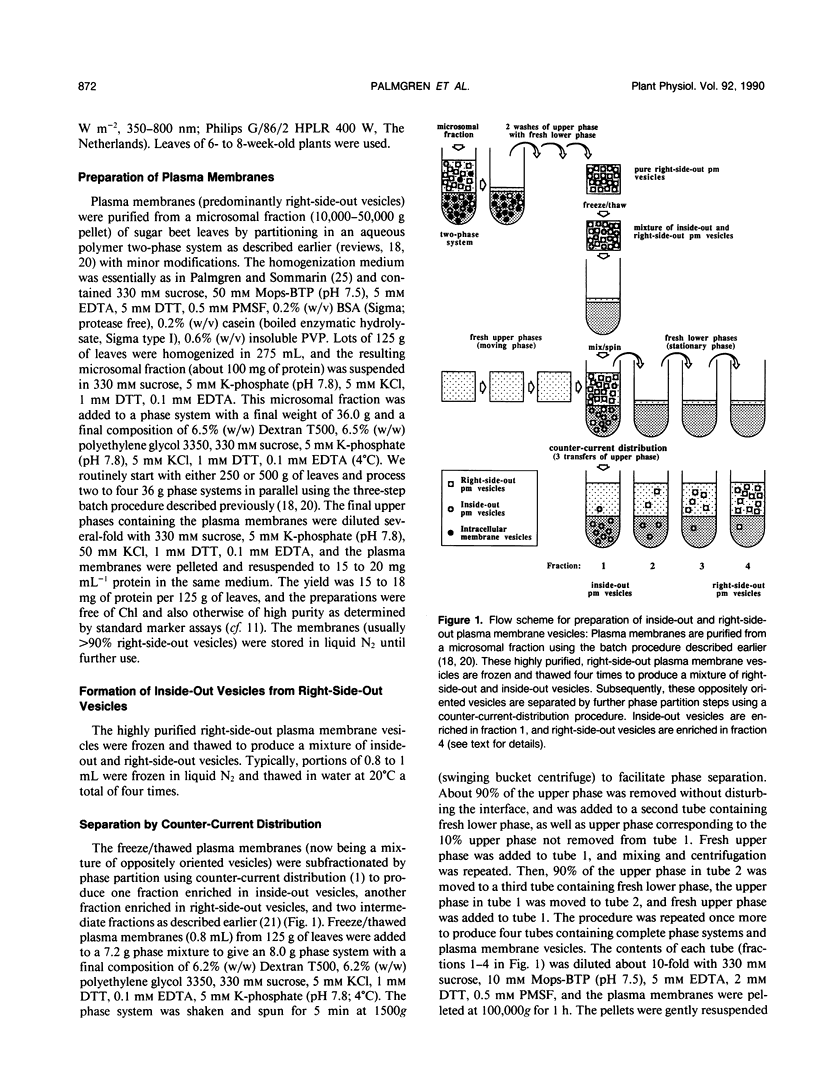



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bearden J. C., Jr Quantitation of submicrogram quantities of protein by an improved protein-dye binding assay. Biochim Biophys Acta. 1978 Apr 26;533(2):525–529. doi: 10.1016/0005-2795(78)90398-7. [DOI] [PubMed] [Google Scholar]
- Brotherus J. R., Jacobsen L., Jørgensen P. L. Soluble and enzymatically stable (Na+ + K+)-ATPase from mammalian kidney consisting predominantly of protomer alpha beta-units. Preparation, assay and reconstitution of active Na+, K+ transport. Biochim Biophys Acta. 1983 Jun 10;731(2):290–303. doi: 10.1016/0005-2736(83)90021-4. [DOI] [PubMed] [Google Scholar]
- Canut H., Brightman A., Boudet A. M., Morré D. J. Plasma membrane vesicles of opposite sidedness from soybean hypocotyls by preparative free-flow electrophoresis. Plant Physiol. 1988 Feb;86(2):631–637. doi: 10.1104/pp.86.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePierre J. W., Ernster L. Enzyme topology of intracellular membranes. Annu Rev Biochem. 1977;46:201–262. doi: 10.1146/annurev.bi.46.070177.001221. [DOI] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner L. E., Kjellbom P., Larsson C., Møller I. M. Surface properties of right side-out plasma membrane vesicles isolated from barley roots and leaves. Plant Physiol. 1985 Sep;79(1):72–79. doi: 10.1104/pp.79.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moller O. J. Activation by freezing of (Na++K+)-ATPase in a microsomal fraction from ox kidney cortex. Exp Cell Res. 1971 Oct;68(2):347–355. doi: 10.1016/0014-4827(71)90160-1. [DOI] [PubMed] [Google Scholar]
- Nørby J. G. Coupled assay of Na+,K+-ATPase activity. Methods Enzymol. 1988;156:116–119. doi: 10.1016/0076-6879(88)56014-7. [DOI] [PubMed] [Google Scholar]
- Palmgren M. G., Sommarin M. Lysophosphatidylcholine stimulates ATP dependent proton accumulation in isolated oat root plasma membrane vesicles. Plant Physiol. 1989 Jul;90(3):1009–1014. doi: 10.1104/pp.90.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren M. G., Sommarin M., Ulvskov P., Larsson C. Effect of detergents on the H(+)-ATPase activity of inside-out and right-side-out plant plasma membrane vesicles. Biochim Biophys Acta. 1990 Jan 29;1021(2):133–140. doi: 10.1016/0005-2736(90)90025-j. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Widell S., Lundborg T., Larsson C. Plasma membranes from oats prepared by partition in an aqueous polymer two-phase system : on the use of light-induced cytochrome B reduction as a marker for the plasma membrane. Plant Physiol. 1982 Nov;70(5):1429–1435. doi: 10.1104/pp.70.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]