Abstract
A valid measure of quality of life is important for clinical goal setting and for evaluating interventions. In the amnestic dementias, proxy-raters (e.g. friends, families, clinicians) typically rate quality of life lower than the self-ratings given by the person with dementia – a proxy bias. This study investigated whether the same proxy bias occurs in Primary Progressive Aphasia (PPA), a language-led dementia.
Quality of life was measured in 18 individuals with PPA using self-ratings, and proxy-ratings by their main communication partner, using the Quality of Life in Alzheimer’s Disease Scale.
There was no strong evidence for proxy bias at a group level, with no consistent pattern across dyads, where proxy- and self-ratings did not show good levels of agreement. We suggest that self-ratings and proxy-ratings of quality of life in PPA are not interchangeable. Higher-powered investigation of the patterns observed here is warranted in future studies.
Keywords: dementia, aphasia, primary progressive aphasia, wellbeing, quality of life, proxy, ratings
Significance Statement
• This study adds to our understanding of quality of life measurement in progressive communication impairments.
• This is the first study to investigate proxy bias in quality of life ratings in Primary Progressive Aphasia (PPA).
• Self-ratings and proxy-ratings of the quality of life of the person with PPA were not interchangeable.
• Predictors of proxy bias (lower rating of person with PPA’s quality of life) were similar to those seen in dementia and aphasia populations, but need replication in a larger study.
• Valid measures of quality of life in PPA will rely on triangulation of information from different sources.
In clinical settings, assessing quality of life can ensure goals are patient-centered, and can measure the impact of interventions. 1 In research settings, it is useful to be able to determine the impact of chronic or degenerative disease over time, as well as the effect of treatments on quality of life. 2 As a construct, quality of life focuses heavily on an individual’s own subjective experiences and perspectives 3 and subjective measures of quality of life may not always relate directly to objective measures such as disease severity. 4 Consequently, self-reporting of quality of life is the most appropriate methodology. However, self-rated quality of life can be challenging to obtain, and/or the results can be unreliable in populations with impaired cognition and/or language.2,5,6
Our team has previously highlighted potential challenges when assessing quality of life in Primary Progressive Aphasia (PPA). 7 PPA is a rare, language-led dementia which is characterized by progressive language impairment in the early to mid-stages. 8
Measuring Quality of Life and Proxy Bias
The challenges associated with self-reporting quality of life in other conditions similar to PPA, such as amnestic dementias, are often dealt with by instead asking a close communication partner, family member, friend, or even health care professional – a proxy – to answer on behalf of the person with the condition. This is often logistically easier and quicker. It also circumvents the fact that over the course of longitudinal studies, individuals with progressive conditions may reach a point where they can no longer provide self-ratings. However, when this approach is used, a ‘proxy bias’ can occur, whereby proxies tend to rate quality of life as being lower than the person living with the condition rates it themself.
This proxy bias is well establish in the amnestic dementia literature across different quality of life instruments, in different countries and across different carer types (see Supplementary Material A, for an overview).9-21 PPA is often associated with Frontotemporal Lobar Degeneration (FTLD) pathology. 8 Two studies investigate proxy bias in FTLD,11,14 however, neither distinguishes which participants or subgroups presented with the language variant of FTLD (i.e., PPA) rather than the behavioral variant. Hence, unfortunately, these studies do not advance our understanding of proxy bias in PPA specifically.
Given that PPA includes language symptoms similar to stroke-aphasia, it is of interest that conclusions have been varied regarding the presence of a proxy bias in stroke-aphasia. Proxy-raters have been noted to have a significant negative bias for their partner with aphasia’s global quality of life, health-related quality of life, and particularly the domains of pain and overall health. Conversely, ratings for other domains including emotions, autonomy and purpose were closer between the proxy-raters and raters with aphasia. 5 Two subsequent studies using a measure of quality of life specifically designed for individuals with aphasia both noted that although proxy-ratings were significantly lower than self-ratings, small effect sizes indicated that differences were not large enough to invalidate the information given by the proxy-raters. Relatively large standard deviations also suggested that any conclusions about the lack of a strong proxy bias at the group level may not hold true at a dyad level.22,23
Predictors of Proxy Bias
Across the literature on predictors of proxy bias in dementia, study designs and results vary considerably. Characteristics of the person with dementia that have been associated with proxy bias include: reduced independence in daily activities, 6 more severe disease, 17 reduced insight, 21 depression, 24 behavioral disturbance6,12 and potentially cognition (which may have an indirect or non-linear relationship with proxy bias and requires further inquiry).6,12,18 Characteristics of the proxy which have been associated with the presence of proxy bias in dementia include greater caregiver burden 6 and relationship to the person with dementia, where proxy bias is larger for (adult) child-raters than spouse raters.13,15
In the stroke-aphasia literature, time living with aphasia has been suggested as a potential mediator of proxy bias: the longer dyads live with aphasia, the more likely they are to agree with each other’s ratings.22,23
Explanations for Proxy Bias
The disability paradox has been widely cited as a theory that can account for proxy bias, proposing that people living with chronic disability can adjust to living with their condition over time and potentially reduce their expectations for quality of life (while presumably, the same does not happen for their partner). 25 Further, variables related to self-rated quality of life are often different from those related to proxy-rated quality of life. This supports the theory that self-rated and proxy-rated quality of life are not the same construct, 12 and may be guided by (different) subjective perceptions, which could account for observed differences in self vs proxy ratings.
Aim and Rationale for the Current Study
Given that PPA is a condition that presents challenges for obtaining insight into quality of life through self-report, especially later in the progression of the disease, it is important to understand the role and validity of proxy-reports. The present study aimed to provide the first insight into proxy bias in PPA, contrasting quality of life ratings by a sample of individuals with PPA with those given by their proxies. As noted above, while studies on proxy bias in amnestic dementia consistently find a proxy bias, studies on stroke-aphasia have mixed findings, therefore, the primary research question of the current study was “Does proxy bias also occur for quality of life ratings in PPA?”
Methods
Ethical approval was obtained in Australia via the human research ethics committees at Macquarie University, Sydney; South Eastern Sydney Local Health District, Sydney and Mater Research, Brisbane.
Recruitment
People with PPA and their proxies (conceptualized in this study as their main communication partners) were recruited using a convenience sample approach via speech pathologists and neurologists working with PPA in Australia and the UK over a period from December 2016 to November 2021. In addition to distribution of flyers via the authors’ professional networks, the first author also advertised the study through an online presentation for the rare dementia support group via University College, London, UK. After the start of the COVID-19 pandemic, assessment had to occur online, enabling the participation of dyads from interstate in Australia and overseas.
People with PPA were eligible if they were aged 45 to 85 years, had a formal diagnosis of PPA 8 from a suitably qualified health professional, had proficient English and were able to give informed consent (with aphasia-appropriate communication support). Eighteen dyads contacted the research team with an interest in the study and all subsequently participated. Nine participants with PPA and seven proxies were seen face-to-face while nine participants with PPA and eleven proxies were seen online via telehealth.
Materials
Background Assessments
For all participants with PPA, demographic details and performance on a number of language and cognitive tests were collected, including the Addenbrooke’s Cognitive Examination-III. 26 For proxy participants, brief demographic details were collected with the Cambridge Behavioural Inventory-Revised Edition, 27 a measure of proxy-rated behavioral symptoms with reference to the person with PPA.
Quality of Life in Alzheimer’s Disease Scale and Administration
The QOL-AD 28 was chosen for this replication study as it was designed for both self-report and proxy use, and has been used in numerous studies that replicate proxy bias across different types of dementia including Alzheimer’s Disease, young-onset dementia and fronto-temporal dementia.6,11,12,14-16 As far as we are aware, the QOL-AD has only previously been used in one study with individuals with PPA. 29 However, it is often used clinically by geriatricians and neurologists, the main medical specialists who diagnose and manage PPA, so understanding more about its application in PPA is of clinical relevance. Measures of QOL designed for stroke-aphasia may also prove useful in PPA, particularly in speech pathology and goal-setting contexts, as we have discussed in a previous review. 7 However, many were not designed to be used by a proxy or have a large focus on physical symptoms, so these were less appropriate to achieve the primary aim of this study.
The QOL-AD is a brief, 13-item questionnaire which assesses 12 domains of quality of life followed by an overall quality of life domain for item 13. Each item has four possible response options: Poor, Fair, Good and Excellent, which are then converted to interval data (Poor = 1, Excellent = 4) with a minimum possible score of 13 and a maximum of 52. Participants were informed “This test is usually used for people who have Alzheimer’s. We’re using it in this study to see if it is useful with people who have PPA”.
The assessment was administered with participants with PPA by the first author, a trained speech pathologist, using the standard conversational script. In aphasia settings, it is standard practice to adapt written materials so they are ‘aphasia friendly’, incorporating modifications such as large text, sans serif fonts, bolding for keywords and supporting images.30-33 Consequently, and given that the QOL-AD’s psychometric properties have not yet been established in the PPA population, we adapted the presentation of the scale: Each question was presented on a single page in large font, the response options were repeated on every page and a series of ticks and crosses were added as visual aids to interpret the written response descriptors. For proxies, the unmodified questionnaire was explained briefly then filled in independently by these participants, who could seek clarification if required.
Analysis
Across the dementia and aphasia literature, proxy bias is typically calculated by paired-samples t-test between the two rater groups, with effect size or a Bland-Altman plot bias line. The degree of rater agreement has been measured via intra-class correlations (ICC) or using the level of agreement lines on a Bland-Altman plot (also see Supplementary Material A). We calculated proxy bias using a one-tailed, paired t-test to determine if self-rated quality of life was higher than proxy-rated quality of life, and a Bayesian one-tailed, paired t-test (using default (flat) priors) to assess evidence for the null hypothesis, as well as visual inspection of the Bland-Altman plot bias. A Bland-Altman plot 34 was chosen rather than a correlation calculation as it provides greater detail on both the inter and intra-rater discrepancies in rating, rather than solely determining if a linear relationship is present.20,35,36 We interpreted agreement via the limits of agreement on the Bland-Altman plot. Given the small group size and heterogeneous nature of PPA, we also analyzed the differences in ratings for each dyad in the study individually, using Kappa coefficients – a measure of inter-rater reliability which corrects for the possibility that raters agree by chance. 37
Finally, a number of exploratory analyses were performed in order to investigate potential relationships between quality of life, proxy bias and demographic, language and cognitive variables. Given the small sample size, and the fact that not all variables met normality assumption checks, all correlations were run as non-parametric Spearman’s correlations. We discuss any correlations where α = <.05, however, we also investigated which of these correlations would survive Holm-Bonferroni correction for multiple comparisons. 38
Results
Demographics and Participant Characteristics
Participant’s demographic details, along with quality of life ratings, are detailed in Table 1. Participants with PPA included six women and twelve men, who ranged from 55 to 81 years of age. Years of education ranged between 10 and 21 years, with 16/18 completing school and 8/18 having university level education. Proxy participants included fifteen women and three men, who ranged in age from 25 to 86 years. Years of education ranged between 11 and 19 years, with 15/18 completing school and 8/18 having university level education. Fourteen of these were spouses or long-term partners, while two daughters, one brother and one friend were also included.
Table 1.
Participant Demographics and Quality of Life Scores.
| pwPPA | Proxy | Quality of Life | Relationship | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dyad # | Age | Gender | Variant | Time since onset | Self-Rated QOL | ACE-III Score | Age | Gender | Proxy-Rated QOL | CBI Proxy Rating | QOL Difference (SR-PR) | QOL Mean | Relationship | Length of Relationship |
| 1 | 73 | Male | NonFluent | 48 | 34 | 93 | 72 | Female | 44 | 24 | −10 | 39 | Spouse | 58 |
| 2 | 73 | Male | NonFluent | 21 | 37 | 95 | 73 | Female | 44 | 20 | −7 | 40.5 | Spouse | 12 |
| 3 | 79 | Male | NonFluent a | 60 | 26 | 74 | 70 | Male | 31.4 | 45 | −5.4 | 26.5 | Brother | 70 |
| 4 | 81 | Male | NonFluent | 24 | 45 | 89 | 78 | Female | 47 | 8 | −2 | 46 | Spouse | 57 |
| 5 | 73 | Male | NonFluent | 42 | 37 | 77 | 70 | Female | 39 | 57 | −2 | 38 | Spouse | 49 |
| 6 | 77 | Male | Logopenic | 36 | 34 | 36 | 64 | Female | 36 | 68 | −2 | 35 | Spouse | 28 |
| 7 | 79 | Male | NonFluent | 60 | 37 | 90 | 75 | Female | 38 | 0 | −1 | 37.5 | Spouse | 38 |
| 8 | 77 | Male | Logopenic | 30 | 25 | 90 | 71 | Female | 25 | 61 | 0 | 25 | Spouse | 46 |
| 9 | 71 | Male | Semantic | 66 | 37 | 43 | 62 | Female | 37 | 79 | 0 | 37 | Spouse | 9 |
| 10 | 55 | Female | Semantic | 60 | 37 | 90 | 68 | Female | 37 | 18 | 0 | 37 | Friend | 36 |
| 11 | 66 | Male | Logopenic | 44 | 43 | 69 | 62 | Female | 42 | 20 | 1 | 42.5 | Spouse | 40 |
| 12 | 68 | Male | NonFluent | 48 | 33 | 19 | 71 | Female | 31 | 54 | 2 | 32 | Spouse | 36 |
| 13 | 69 | Female | Logopenic | 17 | 40 | 31 | 70 | Female | 32 | 64 | 8 | 36 | Spouse | “long time” |
| 14 | 77 | Female | NonFluent | 24 | 46.6 | 94 | 49 | Female | 37.9 | 24 | 8.7 | 42.25 | Daughter | 49 |
| 15 | 79 | Female | NonFluent | 24 | 45 | 61 | 86 | Male | 36 | 50 | 9 | 40.5 | Spouse | 57 |
| 16 | 60 | Female | Mixed | 18 | 45 | 60 | 61 | Male | 36 | 46 | 9 | 40.5 | Spouse | 41 |
| 17 | 60 | Male | Semantic | 30 | 49 | 60 | 55 | Female | 35 | 49 | 14 | 42 | Spouse | 26 |
| 18 | 64 | Female | Logopenic | 14 | 47.7 | 81 | 25 | Female | 32.5 | 33 | 15.2 | 40.1 | Daughter | 25 |
aThe person with PPA, their family and regular speech pathologist reported the patient was diagnosed only with “PPA”. Based on the language assessment, our team determined that the profile was most consistent with a working diagnosis of non-fluent variant PPA.
bSpouse – refers to either a married or long term unmarried, live-in partner.
CBI-R - Cambridge Behavioural Inventory (Revised edition). 27 Scores are out of 180 and higher scores indicate a greater number of abnormal neuro-psychiatric or behavioral symptoms.
ACE-III - Addenbrooke’s Cognitive Examination third edition. 26 A cognitive screening test suitable for people with PPA. Scores are out of 100 and higher scores indicate better cognitive function.
Participants with PPA had a heterogeneous range of presentations, with ACE-III (cognition) scores ranging from 19 to 98 out of 100, and represented all variants according to the international consensus criteria classification system. 8 The participants’ linguistic and cognitive profiles are further detailed in Supplementary Material B. They were 14 to 66 months since the onset of noticeable PPA symptoms. As such, their language and cognitive presentations and severity of PPA differed significantly across the sample. However, all participants were able to understand and consent to the study and express themselves in some way, even if this was through the use of writing, answering closed questions or non-verbal communication.
In all but once case, participants were referred into the study with a formally diagnosed variant of PPA as made by a neurologist or geriatrician. One participant had a confirmed diagnosis of PPA known to his family and speech pathologist, however, the variant was unknown. Upon testing, he presented with an apraxia and agrammatism in line with the international consensus criteria for non-fluent variant, 8 as noted in Table 1.
All participants were of white, Caucasian backgrounds, and all but two participants with PPA had monolingual English-speaking backgrounds. One participant had Afrikaans and German as their native languages while another had Finnish. Anecdotally, this homogeneity in a small convenience sample is a reflection of those individuals who tend to be diagnosed with PPA in Australia and the UK at this time. The fact that people from minority and multilingual backgrounds may be less likely to be correctly diagnosed, or identified early in the disease has been highlighted in one study from India on PPA 42 as well as international studies on Alzheimer’s, frontotemporal and other dementias.43-45
There were missing data for three dyads on the item “How do you feel about your marriage?” The administration guidelines allow participants to rate their closest relationship instead if they are not married, or the item is scored as missing if there is any uncertainty. 28 Two participants with PPA and three proxies (from three dyads) declined to answer this item. Where group averages for a specific item are reported (e.g. Figure 3), these three dyads were omitted for that item. Where overall QOL-AD scores are reported, scores for these dyads were calculated pro-rata such that their total rating scores remain out of 52. We did not administer the GDS-15 to Participant 5, as he had previously been formally diagnosed with depression and was prone to distress when discussing his mood.
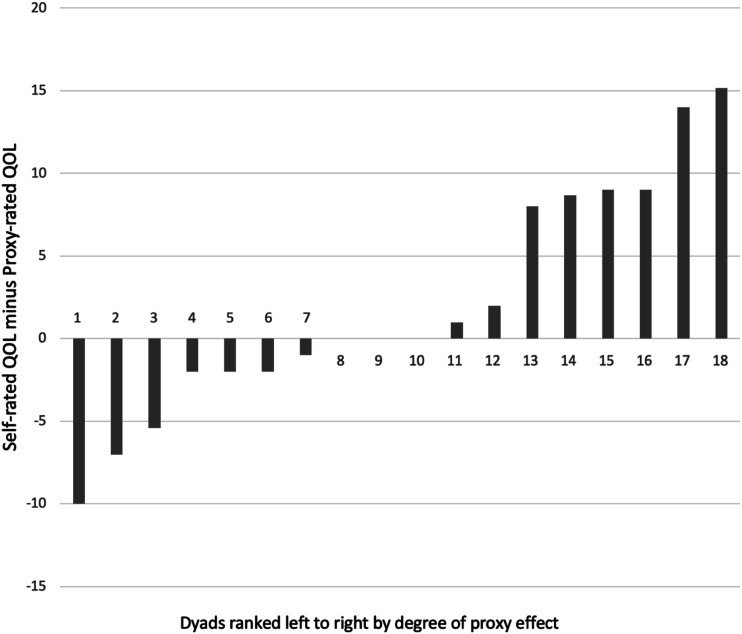
Figure 3.
Differences in quality of life ratings across dyads. Note: Dyads have been ordered by degree of proxy bias, i.e. the left side of the figure shows dyads where the person with PPA rated their quality of life lower than their proxy did (a reverse proxy bias) and the right side shows dyads where the person with PPA rated their quality of life higher than their proxy (greatest proxy bias).
Descriptive Statistics
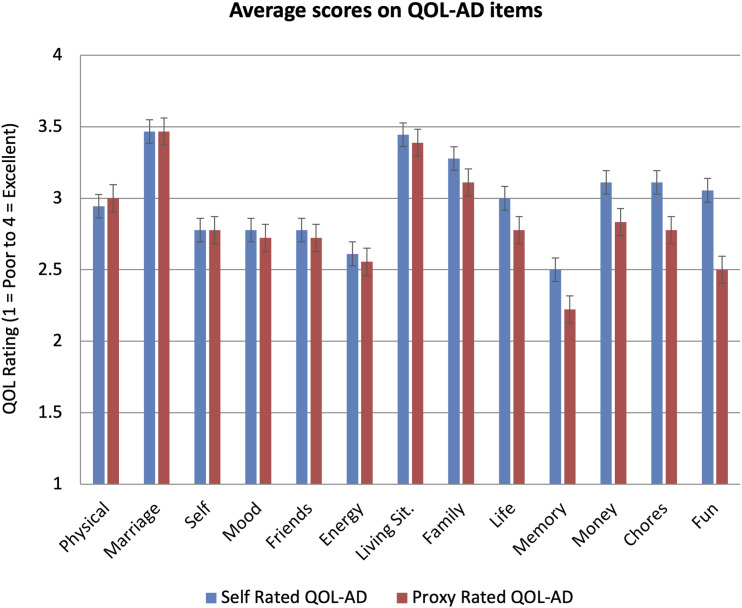
Self-rated quality of life ranged from 25-49 with an average of 38.8 (SD = 7.0, Median = 37, n = 18). Proxy-rated quality of life ranged from 25-47 with an average of 36.7 (SD = 5.4, Median = 36.5, n = 18). As can be seen in Figure 1, the lowest rated items for the self-rated group (i.e. ratings by the individuals with PPA) were ‘memory’ and ‘energy levels’, while for the proxy-rated group (i.e. ratings by the proxies) they were ‘memory’ and ‘fun’, closely followed by ‘energy levels’. The largest difference between self-ratings and proxy-ratings across the groups was on the item ‘fun’, where participants were asked “How do you feel about your ability to do things for fun, that you enjoy?“, with self-ratings being significantly higher on average than proxy-ratings (Wilcoxon one-tailed t-test = 73.00, P = .026, df = 12). Differences on remaining items were not significant.
Figure 1.
Average Ratings for Each QOL-AD Item for Ratings by People with PPA (self-rated QOL-AD) and their Proxies. Note: Figure is ordered from the item with least advantage for self-rated scores over proxy-rated scores (i.e., least proxy bias) to most advantage (i.e. greatest proxy bias). Bars indicate standard error. Y-axis shows rating scale for each item where 1 = Poor, 2 = Fair, 3 = Good and 4 = Excellent. For the item ‘Marriage’, n = 15 whereas for all other items n = 18, due to three dyads not being married and declining to rate the item on another proxy.
Proxy Bias Analyses
Group-Level Analysis
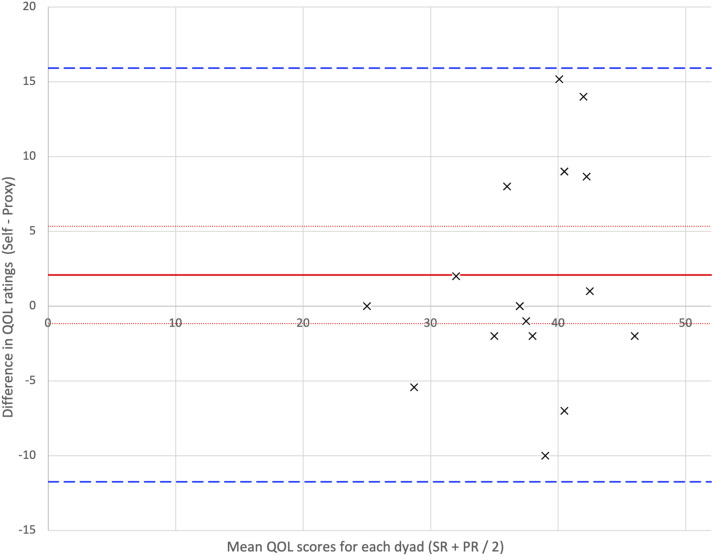
Self-rated quality of life was not strongly, nor significantly, correlated with proxy-rated quality of life (Rho = .209, P = .405). Following a Shapiro-Wilk test, which did not show evidence of non-normality (W = .942, P = .317), the paired samples, one-tailed, t-test was not significant (t = 1.250, P = .114). A paired-samples, one-tailed, Bayesian t-test showed no evidence that self-ratings were higher than proxy-ratings, and anecdotal evidence supporting the ratings of the two groups being the same (BF0+ = 1.202). These results were also supported by the Bland-Altman plot, as displayed in Figure 2.
Figure 2.
Bland-Altman plot for QOL-AD total scores rated by people with PPA and proxies. Note: Bias: The average difference between patient and proxy (or ‘bias’) is indicated by the red line with accompanying confidence intervals (dotted red lines). A difference of 0 (the x-axis) would indicate perfect agreement. If the x-axis falls outside of the confidence interval of the bias, this would indicate significant rater bias is occurring; significant rater bias is not seen in this plot. Agreement: Limits of agreement (green dashed lines) indicate the area from +1.96 SD to -1.96 SD, within which 95% of the data lie, and require clinical interpretation.
Regarding the difference between the two groups, the bias (red line; where bias = self-rated quality of life minus proxy-rated quality of life = 2.08, 95% CI = 3.26) was higher than zero, indicating that self-rated quality of life across the group was higher than proxy-rated QOL. However, the zero difference x-axis fell within the 95% confidence interval for the bias. This indicates there was no significant bias occurring between the self-ratings and proxy-ratings of quality of life. Regarding rater agreement, the standard deviation of the bias (7.05) was used to calculate the upper (15.90) and lower (−11.75) ‘limits of agreement’ (green dashed lines), which denote the area within which 95% of the results lie. This means that, within this sample, a given person with PPA could potentially rate themselves 16 points higher or 12 points lower on the QOL-AD scale than their proxy would; in other words, the range of ratings their proxy might give was large.
Dyad-Level Analysis
Given that there was no strong evidence (on t-test or examination of Bland-Altman plot bias) of a proxy bias occurring at a group level, visual inspection of the difference scores per dyad was undertaken. As seen in Figure 3, the discrepancy between self-ratings and proxy-ratings differed in direction and magnitude across the dyads. Table 2 shows the Kappa Coefficients and their interpretations per dyad. Degree of agreement between dyads ranged from ‘no agreement’ to ‘moderate’.
Table 2.
Degree of Agreement in QOL-AD Ratings for Each Dyad.
| Dyad | Kappa Coefficient (k) | Degree of Agreement a |
|---|---|---|
| 1 | −.13 | No agreement |
| 2 | .12 | None – Slight |
| 3 | −.03 | No agreement |
| 4 | −.21 | No agreement |
| 5 | .16 | None – Slight |
| 6 | −.03 | No agreement |
| 7 | .33 | Fair |
| 8 | .36 | Fair |
| 9 | 0.4 | Fair |
| 10 | .43 | Moderate |
| 11 | .36 | Fair |
| 12 | −0.1 | No agreement |
| 13 | .39 | Fair |
| 14 | .25 | Fair |
| 15 | .05 | None – Slight |
| 16 | .06 | None – Slight |
| 17 | −0.1 | No agreement |
| 18 | −.03 | No agreement |
aCohen’s original suggestions for interpretation of coefficient strengths. 37
Exploratory Analyses: Variables Potentially Associated With the Proxy Bias and QOL Ratings
Table 3 displays correlations between variables of interest identified from previous literature (as noted in the Introduction) and degree of proxy bias per dyad and quality of life. An inter-correlation matrix with all variables included in the study is included in Supplementary Materials C.
Table 3.
Spearman’s Correlations for Variables of Interest with Proxy Bias and Quality of Life.
| Rho | p | ||
|---|---|---|---|
| aDegree of Proxy bias | Relationship type+ | −.453 | .078 |
| Time since PPA symptoms | −.458 | .056 | |
| Depression score b | −.428 | .087 | |
| Proxy-rated behavior | .188 | .455 | |
| Cognition | −.385 | .115 | |
| Self-rated QOL | Relationship type+ | −.497 | .050 * |
| Depression score b | −.397 | .114 | |
| Proxy-rated behavior | −.249 | .320 | |
| Cognition | −.01 | .967 | |
| Proxy-rated QOL | Partner years of education | .142 | .599 |
| Depression score b | −.086 | .743 | |
| Proxy-rated behavior | −.584 | .011 * | |
| Cognition | .52 | .027 * |
N = 18 unless otherwise indicated (and includes adult-child-raters).
aSelf-rated minus proxy-rated quality of life.
bN = 17 for the depression correlations as the depression scale was not administered with one participant.
Significant P-values where α = ≤.05 are as indicated by *.
*Significant P-values following Holm-Bonferroni correction are as indicated by shaded cell.
Note. Relationship Type: Where 0 = adult-child-rater and 1 = partner-rater (N = 16 as two dyads were not classified as either and were not included in this variable). Depression Score: Geriatric Depression Scale-15. 46 Higher scores indicate more symptoms of depression. Proxy-Rated Behavior: Cambridge Behavioural Inventory (Revised edition). 27 Higher scores indicate a greater number of abnormal neuro-psychiatric or behavioral symptoms. Cognition (/Disease severity): Addenbrooke’s Cognitive Examination third edition. 26 A cognitive screening test suitable for people with PPA. Higher scores indicate better cognitive function.
Variables Associated With Proxy Bias
None of the variables identified in the previous literature on proxy bias in other populations (disease severity/cognition, depression, behavioral changes, and relationship type (partner vs adult-child)) were significantly nor substantially correlated with degree of proxy bias (Table 3). When presence vs absence of depression was instead analyzed as a categorical variable (above vs below cut off on the GDS), there remained no relationship with degree of proxy bias (H(1) .709, P = .4, df = 1). This is despite the fact that Participant 5 – who had a diagnosis of depression but did not complete the GDS – was included in this analysis.
When we examined the full correlation matrix (Supplementary Materials C), we noted that other variables – which had not been planned a priori for examination based on previous literature – were significantly correlated with a greater degree of proxy bias: fewer years of education for the person with PPA (Rho = −.557, P = .016), the person with PPA being female (Rho = −.639, P = .04), younger age of both the proxy (Rho = −.533, P = .023), and the person with PPA (Rho = −.481, P = .043).
Since younger age of the proxy was related to greater degree of proxy bias, and a previous dementia study had demonstrated that spouse raters tend to rate quality of life differently to adult child-raters, 13 we further investigated this finding by removing the two adult-child-raters from the data set and re-running the correlations (participants 14 and 18, who were younger than all other proxy-raters at 49 and 25 years, respectively). In this subgroup, younger age of the proxy was no longer correlated with proxy bias, suggesting that the adult-child-raters were driving the correlation between younger age of proxy and greater proxy bias in the full set of dyads. Additionally, lower language ability (Rho = −.5, P = .048) and lower cognition of the people with PPA (Rho = −.611, P = .012) was significantly correlated with a greater degree of proxy bias in this sub-group analysis.
Variables Associated With Self-Rated Quality of Life
For self-rated quality of life, we investigated the following variables of interest based on the previous literature: cognition, depression, behavioral changes and relationship type (partner vs adult-child), Table 3. Higher self-rated quality of life was significantly correlated with the proxy being a child not a partner, although this would not survive Holm-Bonferroni correction. Depression score on the GDS-15 was weakly (negatively) correlated with self-rated quality of life. However, when treated as a categorical variable, the group with depression had significantly lower self-rated quality of life than the group without depression (H(1), 4.399, P = .036, N = 18).
In the full correlation matrix, higher quality of life ratings by the person with PPA were also significantly correlated with: less time since onset of PPA symptoms (Rho = −.58, P = .012) and being female (Rho = −.464, P = .024).
Variables Associated With Proxy-Rated Quality of Life
For proxy-rated quality of life, we investigated the following variables of interest: cognition, depression, behavioral changes in the person with PPA, and proxy education levels. Higher proxy-rated quality of life was significantly related to fewer behavioral changes. It was also moderately correlated with better cognition in the person with PPA but this did not survive correction for multiple comparisons. Presence of depression as a categorical variable also was not significantly related to differences in proxy-rated quality of life (H(1) 1.652, P = .199, N = 18). The full correlation matrix also showed that higher proxy-rated quality of life was significantly correlated with better language performance (Rho = .567, P = .014).
Discussion
This is the first (cross-sectional) study we are aware of that compares how people with PPA and a proxy (their main communication partner) rate the person with PPA’s quality of life. Self-rated and proxy-rated quality of life scores each had a wide range across the group. There was no statistical evidence for a consistent proxy bias at a group level, although there was also no strong evidence for the null hypothesis (of no proxy bias) in Bayesian analyses, and, across the dyads there was considerable variation in the degree (and direction) of differences between the ratings of people with PPA and their proxies.
Reports of Quality of Life
We found that reports of quality of life were heterogeneous even in this relatively small sample of individuals with PPA. Notably, no participants self-rated their overall quality of life as ‘poor’, with ratings falling between ‘fair’ and ‘excellent’ using the QOL-AD descriptors. For both people with PPA and their proxies, total QOL-AD scores (maximum 52) ranged from the high forties (most items rated as ‘excellent’) down to the mid-twenties (reflecting most items rated as fair, or some items rated as ‘poor’ balanced by other ‘good’ or ‘excellent’ ratings).
People with PPA and their proxies tended to rate the quality of life domains of ‘memory’ and ‘energy’ similarly within dyads. This makes sense given that word finding difficulties, effort and fatigue are associated with communication activities in PPA, as well as emerging memory difficulties. These symptoms are all likely to be observable by both parties.8,47
The ability to have fun, meanwhile, was rated significantly lower by proxies than people with PPA, as also has been observed to occur with spouse raters of people with dementia. 13 The experience of fun, of course, is less transparent to observers than speech errors. One possible explanation is that what constitutes fun or is enjoyable might change over time for the person with PPA, as their condition progresses, in a process potentially akin to the disability paradox. 25 In contrast, proxies may continue to evaluate the person with PPA against previously enjoyable activities or acts of independence which are no longer options due to disease progression.
Why Did We Not Find a Proxy Bias?
The lack of a statistically reliable proxy bias, in this small sample, is contrary to the proxy bias occurring consistently in quality of life ratings for people with amnestic presentations of dementia. Many of the factors thought to affect the proxy bias in amnestic dementias, such as reduced insight, 21 behavioral disturbances6,12 and reduced independence in daily activities 6 are less likely to apply to individuals with early and mid-stages of PPA, when communication is the primary presenting concern. This may go some way to explaining the lack of consistent proxy bias in PPA in this study.
Instead, our results were more closely aligned with the minimal proxy bias reported in some studies of stroke-aphasia.22,23 On the one hand, this is unsurprising given that stroke-aphasia and PPA are alike in that they involve communication impairment and are both extremely variable in presentation. On the other hand, individuals with PPA tend to have a high degree of insight into their current communication decline and particularly that there is a prognosis of decline. 48 This prognosis differs markedly from individuals with stroke-aphasia who can potentially look toward to the future with a positive outlook. 49 However, the QOL-AD conversational script states “We want to find out how you feel about your current situation in each area” and the scale has no items which pertain to the future. Consequently, this may bias raters towards evaluating quality of life based on the present moment and without much consideration to the future prognosis of decline. Hence, despite the differences in prognosis between stroke-aphasia and PPA, because of the wording of the QOL-AD, the responses are not greatly affected by these differences.
Different dyads demonstrated different degrees of agreement with each other’s overall scores, and in both directions, such that either party might rate overall quality of life as higher. This suggests that group level results may not reflect individual dyad’s ratings well, and future studies investigating proxy bias in PPA should not presume anything about individual dyads based only on group trends. However, this exploratory study lacked power, and investigation of proxy bias in a larger sample of people with PPA would be warranted to confirm these results.
Rater Agreement
The limits of agreement on the Bland-Altman plot indicated that, for a given participant with PPA, the range of scores within which their proxy might rate was quite large relative to the range of 40 (ratings from 13 to 52) available on the QOL-AD. This suggests that proxy-ratings on the QOL-AD in this study were not interchangeable with self-ratings, even if the statistical tests for proxy bias were non-significant at a group level. It is not possible, in this sample, to predict whether someone’s proxy will over or under-estimate their self-rating. On the basis of this, our clinical recommendation would be to always attempt to collect a self-rating of a person with PPA’s quality of life. This can be achieved when the person is able to use the instrument, with appropriate scaffolding as required, or, alternatively, responses can be gathered using more open ended case history or interview-style questions on wellbeing.7,50
Unlike the Bland-Altman plot analysis, which subtracts one rater’s total score from the other, the Kappa Coefficient calculation takes into account variability across each item on the QOL-AD, so it is a more sensitive indicator of agreement when a particular dyad is considered individually. The fact that only one of 18 dyads reached moderate agreement, again supports our recommendation that self- and proxy-ratings on the QOL-AD should not be considered interchangeable in PPA.
Potential Predictors of Proxy Bias
This study’s results highlighted that although some dyads demonstrated a pattern of ratings of quality of life in the direction that would be expected if there was a proxy bias (i.e., proxy-ratings lower than self-ratings), others did not, or even showed the reverse pattern (see Figure 3). It was therefore important to explore what might have been underpinning these differences across dyads.
Unlike in studies of proxy bias in amnestic dementias, depression, behavioral changes and relationship type were not correlated with proxy bias in this PPA sample. The lack of association with behavioral changes could be explained by the fact that behavioral changes are less common in the early and mid stages of PPA compared with Alzheimer’s. This was reflected in the relatively low scores on the Cambridge Behavioural Inventory (Mean = 40, Median = 45.5, Range 0-79, higher scores out of 180 indicate more behavioral and neuropsychiatric changes).
Greater proxy bias was significantly and moderately negatively correlated with age of the proxy in our sample, in the post-hoc review of the full correlation matrix. We hypothesized that this occurred in large part because the two adult-child raters (both daughters) were the youngest members of the proxy group and each demonstrated a large proxy bias. This hypothesis was supported by the fact that the correlation was no longer statistically reliable when these dyads were removed. Relationship type (adult-child rater or partner rater) also approached significance in the planned investigation of variables potentially associated with proxy bias. In one study on amnestic dementia, daughter caregivers have been reported to have the most negative perception of the quality of life of the person with dementia (compared to spouses and sons). 13 The authors suggested that perception of quality of life may become more negative as the relationship to the patient becomes further removed (i.e. spouse, then child, followed by other family member). This is perhaps because caring for one’s spouse in older age is more expected in terms of societal roles than caring for parents as an adult-child on top of other life and carer responsibilities, increasing caring burden. 13 The burden falling to (adult) child caregivers of individuals with PPA and the associated mental health consequences require further investigation.
After removing the daughter-raters from the analysis, poorer cognition and poorer language ability of the people with PPA were also correlated with a greater degree of proxy bias. This suggests that outwardly observable symptoms of PPA may predict proxy bias (as they do in amnestic dementias), but only for spouses or similar aged raters, with different predictors relevant for adult-child-raters. Further exploration of this question is required given the small sample here. However, should it prove to be a robust finding that there is a consistent proxy bias for observable behaviors, and a less consistent pattern for other aspects of well-being, then this information would greatly assist in interpreting proxy ratings.
Greater degree of proxy bias was correlated with the individual with PPA being female, younger and having fewer years of education. The small sample size and fact that these associations have not been widely reported in the dementia literature, make these factors difficult to interpret. Until these relationships are better understood, clinicians might be conservative in interpreting proxy reports for younger female individuals with PPA and triangulate them with other sources of information.
We noted that where proxy-ratings of quality of life were higher than self-ratings (i.e., a reverse proxy bias), the individuals with PPA were those who had mild presentations of non-fluent PPA characterized by effortful speech and fatigue. 51 It is feasible that in the earlier stages of non-fluent PPA, friends and family are less able to perceive the extent of an individual with PPA’s mental fatigue or the effort associated with communication, resulting in them not rating quality of life as low as the affected individual. There was insufficient data to examine differences across the three PPA variants in this small study. A post-hoc correlation of the difference in dyad scores with variant as a collapsed binary variable (non-fluent PPA vs other PPA) revealed a moderate but only marginally significant relationship (Rho = −.452, P = .06). One of the participants with semantic variant PPA (participant 17) rated his quality of life as high and gave the impression that he was certain in his responses. His wife later commented (to the researchers) that she disagreed with his ratings and that he was trying to impress the researcher with high scores. It is well established that people with semantic variant PPA can have reduced insight and emotion processing.52,53 For any the participants who scored poorly on the semantic subscales administered, regardless of variant, it should be considered how easily they are able to understand the concept of quality of life. This line of investigation is being explored by our team in forthcoming work on comprehension of abstract concepts in PPA. 54
It may be fruitful for future studies to investigate the intersection of variant, clinical presentation, PPA stage and the extent to which influences on quality-of-life ratings are observable, with regard to differences in proxy- and self-ratings of quality of life in PPA.
In populations where proxy bias has been more robustly demonstrated, it is often related to the disability paradox, in which resilience and adaptation may alter expectations for quality of life over time.15,25 However, the fact that we observed dyads in which there was a proxy bias and others in which there was a reverse proxy bias would suggest that, if the disability paradox occurs in PPA, it is not universal. In a forthcoming qualitative study, we found that 10 individuals with PPA demonstrated mixed experiences with resilience and a positive outlook in some individuals contrasting with low mood and a focus on future decline in others. 55 Qualitative work with proxies will also more clearly illuminate the processes behind the individual differences found in the current study.
Are Self and Proxy-Ratings Different Constructs?
In this study, higher self-rated quality of life was significantly correlated with less time since onset of PPA symptoms and being female. It was also correlated with having an adult-child rather than a partner as a proxy rater. Given only two adult-child raters in the sample, and the opposite finding in dementia with regard to the relationship of the proxy to the person with dementia, 13 this latter result from our study requires further confirmation. Those without depression, unsurprisingly, had higher self-rated quality of life, as has been reported in amnestic dementias13,15,16,18,21,24 and stroke-aphasia. 56
In addition, as noted above, we found moderate evidence that higher proxy-rated quality of life was significantly correlated with observable symptoms of PPA. These included better cognition, and fewer behavioral changes, as occurs in the amnestic dementia research,15,16,21 as well as better language performance.
The fact that the variables that correlated with self-rated and proxy-rated quality of life were different, lends weight to the argument that these are different constructs 12 : people with PPA and their proxies are potentially not evaluating the same thing when they rate quality of life. It is possible that proxies are evaluating quality of life by comparing their loved one to their former, pre-PPA, self.
Future Directions
The findings presented here are preliminary due to the small sample size, and will benefit from replication in a better-powered study. We used the QOL-AD in this study because it is widely and freely available, commonly used by geriatricians, general practitioners and neurologists, short and straightforward to administer, and has standardized cues for supporting people to respond. In future work, however, examining proxy bias in PPA using more than one quality of life instrument may be helpful since in previous stroke-aphasia proxy findings have differed with different instruments. Future research may also benefit from investigating a wider range of predictor variables, such as insight and caregiver burden. Exploring the role of perspective-taking in proxies may also be worthwhile, as some research has indicated that asking a family member to imagine how their loved one would rate their own quality of life can potentially reduce proxy bias in amnestic dementias.15,57
Given the nature of linguistic and cognitive decline in PPA, the PPA field, like the dementia field, will benefit from longitudinal studies on measurement issues in quality of life. Patient and proxy perspectives on how they would prefer to be asked about their quality of life, and/or the development of a co-designed, condition-specific quality of life scale, could also prove valuable in establishing best practice in the future.
Concluding Remarks
Measurement of proxy-rated quality of life will always be warranted in some contexts, particularly when an individual with PPA is unable to provide self-ratings or it becomes challenging to do so over time in a longitudinal study. Family and friends are also likely to be well informed and provide clinically relevant information in their ratings. However, this study, albeit preliminary, suggests that self and proxy-ratings are not directly interchangeable in PPA, and quite possibly do not reflect the same construct, at least on the QOL-AD measure. Consequently proxy-rated measures alone need to be interpreted with caution, as the proxy-rating may be lower, higher or similar to a rating provided by the person with PPA themselves. Where possible, it is probably wise to collect both perspectives and triangulate these as clinically appropriate.
Supplemental Material
Supplemental Material for Quality of Life Ratings and Proxy Bias in Primary Progressive Aphasia: Two Sides to the Story? by Leanne Ruggero, Karen Croot, Lyndsey Nickels in American Journal of Alzheimer's Disease & Other Dementias®
Supplemental Material for Quality of Life Ratings and Proxy Bias in Primary Progressive Aphasia: Two Sides to the Story? by Leanne Ruggero, Karen Croot, Lyndsey Nickels in American Journal of Alzheimer's Disease & Other Dementias®
Supplemental Material for Quality of Life Ratings and Proxy Bias in Primary Progressive Aphasia: Two Sides to the Story? by Leanne Ruggero, Karen Croot, Lyndsey Nickels in American Journal of Alzheimer's Disease & Other Dementias®
Acknowledgments
Many thanks to the participants with PPA and their friends and family members involved in this study. We are also grateful to those colleagues who assisted with ethics and recruitment on this study: Dr Cathleen Taylor-Rubin and War Memorial Hospital, Sydney, Australia, Professor Peter Nestor, Caitlin McElligott, Dr Jade Cartwright, Ffion Walker, Dr Trudy Krajenbrink, Rosemary Townsend and the Rare Dementia team at UCL.
Appendix.
Abbreviations
- ACE-III
Addenbrooke’s Cognitive Examination-III (Hsieh et al., 2013)
- CBI-R
Cambridge Behavioural Inventory-Revised Edition (Wear et al., 2008)
- FTLD
Fronto-temporal Lobar Degeneration
- GDS-15
Geriatric Depression Scale 15 item version (Sheikh & Yesavage, 1986)
- ICC
Intraclass Correlations
- PPA
Primary Progressive Aphasia
- QOL
Quality of Life
- QOL-AD
Quality of Life in Alzheimer’s Disease Scale (Logsdon et al., 1999)
- SydBat
The Sydney Language Battery (Savage et al., 2013)
- WAIS-IV
Weschler Adult Intelligence Scale-IV (Wechsler, 2008)
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Leanne Ruggero was supported by an Australian Government Research Training Program Scholarship and a Macquarie University Research Training Pathway Stipend Scholarship during the preparation of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Leanne Ruggero https://orcid.org/0000-0002-8278-8174
Karen Croot https://orcid.org/0000-0001-9459-2913
Lyndsey Nickels https://orcid.org/0000-0002-0311-3524
References
- 1.Hilari K, Cruice M, Sorin-Peters R, Worrall LE. Quality of life in aphasia: State of the art. Folia Phoniatr Logop. 2015;67(3):114-118. doi: 10.1159/000440997 [DOI] [PubMed] [Google Scholar]
- 2.Ready RE, Ott BR. Quality of life measures for dementia. Health Qual Life Outcome. 2003;1:11-19. doi: 10.1186/1477-7525-1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Constitution of the World Health Organization. WHO; 1946. www.who.int/governance/eb/who_constitution_en.pdf [Google Scholar]
- 4.Allison PJ, Locker D, Feine JS. Quality of life: A dynamic construct. Soc Sci Med. 1997;45(2):221-230. doi: 10.1016/S0277-9536(96)00339-5 [DOI] [PubMed] [Google Scholar]
- 5.Cruice M, Worrall L, Hickson L, Murison R. Measuring quality of life: Comparing family members’ and friends’ ratings with those of their aphasic partners. Aphasiology. 2005;19(2):111-129. doi: 10.1080/02687030444000651 [DOI] [Google Scholar]
- 6.Zhao H, Novella JL, Dramé M, et al. Factors associated with caregivers’ underestimation of quality of life in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2012;33(1):11-17. doi: 10.1159/000333070 [DOI] [PubMed] [Google Scholar]
- 7.Ruggero L, Nickels L, Croot K. Quality of life in primary progressive aphasia: What do we know and what can we do next? Aphasiology. 2019;33(5):498-519. doi: 10.1080/02687038.2019.1568135 [DOI] [Google Scholar]
- 8.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006-1014. doi: 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arlt S, Hornung J, Eichenlaub M, Jahn H, Bullinger M, Petersen C. The patient with dementia, the caregiver and the doctor: cognition, depression and quality of life from three perspectives. Int J Geriatr Psychiatry. 2008;23:604-610. doi: 10.1002/gps [DOI] [PubMed] [Google Scholar]
- 10.Arons AM, Krabbe PF, Schölzel-Dorenbos CJ, Van Der Wilt GJ, Rikkert MGMO. Quality of life in dementia: A study on proxy bias. BMC Med Res Methodol. 2013;13(1):110. doi: 10.1186/1471-2288-13-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YT, Nelis SM, Quinn C, et al. Factors associated with self- and informant ratings of quality of life, well-being and life satisfaction in people with mild-to-moderate dementia: Results from the improving the experience of dementia and enhancing active life programme. Age Ageing. 2020;49(3):446-452. doi: 10.1093/ageing/afz177 [DOI] [PubMed] [Google Scholar]
- 12.Huang H-L, Chang MY, Tang JS-H, Chiu Y-C, Weng L-C. Determinants of the discrepancy in patient- and caregiver-rated quality of life for persons with dementia. J Clin Nurs. 2009;18(22):3107-3118. doi: 10.1111/j.1365-2702.2008.02537.x [DOI] [PubMed] [Google Scholar]
- 13.Conde-Sala JL, Garre-Olmo J, Turró-Garriga O, Vilalta-Franch J, López-Pousa S. Quality of life of patients with Alzheimer’s Disease: Differential perceptions between spouse and adult child caregivers. Dement Geriatr Cogn Disord. 2010;29(2):97-108. doi: 10.1159/000272423 [DOI] [PubMed] [Google Scholar]
- 14.Bakker C, De Vugt ME, van Vliet D, et al. Unmet needs and health-related quality of life in young-onset dementia. Am J Geriatr Psychiatry. 2014;22(11):1121-1130. doi: 10.1016/j.jagp.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 15.Bosboom PR, Alfonso H, Eaton J, Almeida OP. Quality of life in Alzheimer’s disease: Different factors associated with complementary ratings by patients and family carers. Int Psychogeriatr. 2012;24(5):708-721. doi: 10.1017/S1041610211002493 [DOI] [PubMed] [Google Scholar]
- 16.Bruvik FK, Ulstein ID, Ranhoff AH, Engedal K. The quality of life of people with dementia and their family carers. Dement Geriatr Cogn Disord. 2012;34(1):7-14. doi: 10.1159/000341584 [DOI] [PubMed] [Google Scholar]
- 17.Jacob L, Han JW, Kim TH, et al. How different are quality of life ratings for people with dementia reported by their family caregivers from those reported by the patients themselves? Tales A. J Alzheimers Dis. 2017;55(1):259-267. doi: 10.3233/JAD-160538 [DOI] [PubMed] [Google Scholar]
- 18.Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med. 2002;64(3):510-519. doi: 10.1097/00006842-200205000-00016 [DOI] [PubMed] [Google Scholar]
- 19.Ready RE, Ott BR, Grace J. Patient versus informant perspectives of Quality of Life in mild cognitive impairment and Alzheimer’s disease. Int J Geriatr Psychiatry. 2004;19(3):256-265. doi: 10.1002/gps.1075 [DOI] [PubMed] [Google Scholar]
- 20.Römhild J, Fleischer S, Meyer G, et al. Inter-rater agreement of the Quality of Life-Alzheimer’s Disease (QoL-AD) self-rating and proxy rating scale: Secondary analysis of Right Time Place Care data. Health Qual Life Outcome. 2018;16(1):131. doi: 10.1186/s12955-018-0959-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogel A, Mortensen EL, Hasselbalch SG, Andersen BB, Waldemar G. Patient versus informant reported quality of life in the earliest phases of Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21(12):1132-1138. doi: 10.1002/gps.1619 [DOI] [PubMed] [Google Scholar]
- 22.Hilari K, Owen S, Farrelly SJ. Proxy and self-report agreement on the stroke and aphasia quality of life scale-39. J Neurol Neurosurg Psychiatry. 2007;78(10):1072-1075. doi: 10.1136/jnnp.2006.111476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ignatiou M, Christaki V, Chelas EN, Efstratiadou EA, Hilari K. Agreement between people with aphasia and their proxies on health-related quality of life after stroke, using the Greek SAQOL-39g. Psychology. 2012;03(9):686-690. doi: 10.4236/psych.2012.39104 [DOI] [Google Scholar]
- 24.Sands LP, Ferreira P, Stewart AL, Brod M, Yaffe K. What explains differences between dementia patients’ and their caregivers’ ratings of patients’ quality of life? Am J Geriatr Psychiatry. 2004;12(3):272-280. doi: 10.1097/00019442-200405000-00006 [DOI] [PubMed] [Google Scholar]
- 25.Albrecht GL, Devlieger PJ. The disability paradox: high quality of life against all odds. Soc Sci Med. 1999;48(8):977-988. doi: 10.1016/S0277-9536(98)00411-0 [DOI] [PubMed] [Google Scholar]
- 26.Hsieh S, Schubert S, Hoon C, Mioshi E, Hodges JR. Validation of the Addenbrooke’s cognitive examination III in frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2013;36(3-4):242-250. doi: 10.1159/000351671 [DOI] [PubMed] [Google Scholar]
- 27.Wear HJ, Wedderburn CJ, Mioshi E, et al. The Cambridge Behavioural Inventory revised. Dement Neuropsychol. 2008;2(2):102-107. doi: 10.1590/s1980-57642009dn20200005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Logsdon RG, Gibbons LE, McCurry SM, Teri L. Quality of life in Alzheimer’s disease: Patient and caregiver reports. J Ment Heal ageing. 1999;5(1):21-32. [Google Scholar]
- 29.Cartwright J. Primary Progressive Aphasia: The Potential for Change; 2015. https://research-repository.uwa.edu.au/en/publications/primary-progressive-aphasia-the-potential-for-change [Google Scholar]
- 30.Rose TA, Worrall LE, Hickson LM, Hoffmann TC. Aphasia friendly written health information: content and design characteristics. Int J Speech Lang Pathol. 2011;13(4):335-347. doi: 10.3109/17549507.2011.560396 [DOI] [PubMed] [Google Scholar]
- 31.Rose TA, Worrall LE, Hickson LM, Hoffmann TC. Exploring the use of graphics in written health information for people with aphasia. Aphasiology. 2011;25(12):1579-1599. doi: 10.1080/02687038.2011.626845 [DOI] [Google Scholar]
- 32.Pearl G, Cruice M. Facilitating the involvement of people with aphasia in stroke research by developing communicatively accessible research resources. Top Lang Disord. 2017;37(1):67-84. doi: 10.1097/TLD.0000000000000112 [DOI] [Google Scholar]
- 33.Aleligay A, Worrall LE, Rose TA. Readability of written health information provided to people with aphasia. Aphasiology. 2008;22(4):383-407. doi: 10.1080/02687030701415872 [DOI] [Google Scholar]
- 34.Altman DG, Bland JM. Measurement in Medicine: The analysis of method comparison studies. Stat. 1983;32(3):307. doi: 10.2307/2987937 [DOI] [Google Scholar]
- 35.Stolarova M, Wolf C, Rinker T, Brielmann A. How to assess and compare inter-rater reliability, agreement and correlation of ratings: an exemplary analysis of mother-father and parent-teacher expressive vocabulary rating pairs. Front Psychol. 2014;5:509. doi: 10.3389/fpsyg.2014.00509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giavarina D. Understanding Bland Altman analysis. Biochem Med. 2015;25(2):141-151. doi: 10.11613/BM.2015.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McHugh ML. Interrater reliability: The kappa statistic. Biochem Med. 2012;22(3):276-282. doi: 10.11613/bm.2012.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65-70. [Google Scholar]
- 39.Microsoft Corporation . Microsoft Excel [Internet]; 2018. https://office.microsoft.com/excel [Google Scholar]
- 40.JASP Team . JASP; 2022. [Computer software] https://jasp-stats.org/ [Google Scholar]
- 41.IBM Corp . IBM SPSS Statistics for Windows. New York, NY: Armonk; 2021. Version 28.0 . [Google Scholar]
- 42.Prabhakar AT, Mathew V, Sivadasan A, Aaron S, George A, Alexander M. Clinical profile of primary progressive aphasias in a tertiary care centre from India. Int J Speech Lang Pathol. 2019;21(6):547-552. doi: 10.1080/17549507.2018.1545870 [DOI] [PubMed] [Google Scholar]
- 43.Gianattasio KZ, Prather C, Glymour MM, Ciarleglio A, Power MC. Racial disparities and temporal trends in dementia misdiagnosis risk in the United States. Alzheimers Dement. 2019;5:891-898. doi: 10.1016/j.trci.2019.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim S, Mohaimin S, Min D, et al. Alzheimer’s disease and its related dementias among asian americans, native hawaiians, and pacific islanders: A scoping review. J Alzheimers Dis. 2020;77(2):523-537. doi: 10.3233/JAD-200509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chao SZ, Rosen HJ, Azor V, et al. Frontotemporal dementia in eight Chinese individuals. Neurocase. 2013;19(1):76-84. doi: 10.1080/13554794.2011.654218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheikh JI, Yesavage JA. 9/Geriatric Depression Scale (GDS): Recent Evidence and Development of a Shorter Version. Clin Gerontol. 1986;5(1-2):165-173. doi: 10.1300/J018v05n01_09 [DOI] [Google Scholar]
- 47.Ulugut H, Stek S, Wagemans LEE, et al. The natural history of primary progressive aphasia: beyond aphasia. J Neurol. 2022;269(3):1375-1385. doi: 10.1007/s00415-021-10689-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banks SJ, Weintraub S. Generalized and Symptom-Specific Insight in Behavioral Variant Frontotemporal Dementia and Primary Progressive Aphasia. J Neuropsychiatry Clin Neurosci. 2009;21(3):299-306. doi: 10.1176/jnp.2009.21.3.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grohn B, Worrall LE, Simmons-Mackie N, Hudson K. Living successfully with aphasia during the first year post-stroke: A longitudinal qualitative study. Aphasiology. 2014;28(12):1405-1425. doi: 10.1080/02687038.2014.935118 [DOI] [Google Scholar]
- 50.Kim E, Figeys M, Hubbard H, Wilson C. The impact of aphasia camp participation on quality of life: A primary progressive aphasia perspective. Semin Speech Lang. 2018;39(3):270-283. doi: 10.1055/s-0038-1660785 [DOI] [PubMed] [Google Scholar]
- 51.Douglas JT. Adaptation to early-stage nonfluent/agrammatic variant primary progressive aphasia: A first-person account. Am J Alzheimers Dis Other Demen. 2014;29(4):289-292. doi: 10.1177/1533317514523669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fittipaldi S, Ibanez A, Baez S, Manes F, Sedeno L, Garcia AM. More than words: Social cognition across variants of primary progressive aphasia. Neurosci Biobehav Rev. 2019;100:263-284. doi: 10.1016/J.NEUBIOREV.2019.02.020 [DOI] [PubMed] [Google Scholar]
- 53.Bertoux M, Duclos H, Caillaud M, et al. When affect overlaps with concept: Emotion recognition in semantic variant of primary progressive aphasia. Brain. 2020;143(12):3850-3864. doi: 10.1093/brain/awaa313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruggero L. Quality of life and living positively in primary progressive aphasia. PhD Thesis. Sydney, Australia: Macquarie University; 2022. [Google Scholar]
- 55.Ruggero L, Nickels L, Croot K. Perspectives on Living Positively with Primary Progressive Aphasia. Front Hum Neurosci. 2019;13:11. doi: 10.3389/conf.fnhum.2019.01.0004330809136 [DOI] [Google Scholar]
- 56.Hilari K, Needle JJ, Harrison KL. What are the important factors in health-related quality of life for people with aphasia? A systematic review. Arch Phys Med Rehabil. 2012;93(1 SUPPL):S86-S95. doi: 10.1016/j.apmr.2011.05.028 [DOI] [PubMed] [Google Scholar]
- 57.Egan P. What family caregivers think and feel when proxy assessing from different perspectives. Innov Aging. 2019;3(Supplement_1):S977-S977. doi: 10.1093/geroni/igz038.3540 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Quality of Life Ratings and Proxy Bias in Primary Progressive Aphasia: Two Sides to the Story? by Leanne Ruggero, Karen Croot, Lyndsey Nickels in American Journal of Alzheimer's Disease & Other Dementias®
Supplemental Material for Quality of Life Ratings and Proxy Bias in Primary Progressive Aphasia: Two Sides to the Story? by Leanne Ruggero, Karen Croot, Lyndsey Nickels in American Journal of Alzheimer's Disease & Other Dementias®
Supplemental Material for Quality of Life Ratings and Proxy Bias in Primary Progressive Aphasia: Two Sides to the Story? by Leanne Ruggero, Karen Croot, Lyndsey Nickels in American Journal of Alzheimer's Disease & Other Dementias®