Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder for which there is no cure. Here, we test a dual GLP-1/GIP receptor agonist (DA4-JC) that has a cell penetrating sequence added to enhance blood-brain barrier penetration. We show in a receptor activity study that DA4-JC has balanced activity on both GLP-1 and GIP receptors but not on GLP-2 or Glucagon receptors. A dose-response study in the APP/PS1 mouse model of AD showed both a dose-dependent drug effect on the inflammation response and the reduction of amyloid plaques in the brain. When comparing DA4-JC with the GLP-1 analogue liraglutide at equal doses of 10nmol/kg bw ip. once-daily for 8 weeks, DA4-JC was more effective in reversing memory loss, enhancing synaptic plasticity (LTP) in the hippocampus, reducing amyloid plaques and lowering pro-inflammatory cytokine levels in the brain. The results suggest that DA4-JC may be a novel treatment for AD.
Keywords: insulin, growth factor, brain, inflammation, TNF-alpha, synaptic plasticity
Introduction
Alzheimer’s disease (AD) is a chronic neurodegenerative disorder for which there is no effective treatment. One of the risk factors for AD is type 2 diabetes mellitus (T2DM). 1 -3 Both diseases share underlying molecular mechanisms such as insulin desensitization. 4 -8 On the basis of this, a novel strategy on how to treat AD is to regulate and normalize insulin signaling in the brain. Interestingly, Glucagon-like peptide 1 (GLP-1), an incretin hormone used for the treatment of T2DM, 9 and its mimetics also have protective effects in animal models of AD. 10 -12 GLP-1 receptor agonists such as liraglutide or lixisenatide demonstrated significant neuroprotective effects in the APP/PS1 mouse model of AD, preserving memory formation, synaptic plasticity in the hippocampus, reducing chronic inflammation and amyloid plaque load in the brain, and protecting mitochondrial activity. 10,13 -15 A pilot clinical trial testing liraglutide has shown beneficial effects in AD patients in upregulating glucose utilization in the brain as shown in 18FDG-PET scans. 16 Glucose-dependent insulinotropic polypeptide (GIP), another incretin hormone, also shows neuroprotective effects in the APP/PS1 mouse model of AD by rescuing memory formation and synaptic plasticity (long-term potentiation, LTP) in the hippocampus, reducing inflammation and amyloid plaque load. 17 -19
Novel dual GLP-1/GIP receptor agonists have been developed to treat diabetes. 20 Some show superior effects to single GLP-1 receptor agonists in clinical trials. 21 We previously tested the dual agonist DA1-JC (NNC0090-2746) and it showed moderate neuroprotective effects that were not superior to GLP-1 analogues. 22,23 In a clinical trial in diabetic patients, this dual agonist did not show superiority to a GLP-1 analogue. 24 As this dual agonist is lipidated with a C16 fatty acid for enhanced survival time in the blood, the uptake in the brain is reduced. Removing this C16 fatty acid resulted in improved neuroprotective properties (DA3-CH). 25,26 When adding a cell-penetrating sequence (CPS) to the dual agonist, the protective effects were increased further (DA5-CH). 23,27 In the present study, we tested a novel dual GLP-1/GIP receptor agonist (DA4-JC) that has a poly-lys CPS sequence added to enhance the transport into the brain and that showed superior neuroprotective effects in a mouse model of Parkinson’s disease. 23 In the present study, we tested the receptor specificity for GLP-1 and GIP receptors. Furthermore, this novel dual agonist has never been tested in the APP/PS1 mouse model. Therefore, we tested several doses in this model to establish a dose-response relationship by measuring amyloid plaque load in the cortex and the chronic inflammation response. In a second study, we tested the neuroprotective effects of DA4-JC in comparison with liraglutide, a GLP-1 analogue that is on the market as a treatment for T2DM (Victoza), 28,29 in the APP/PS1 mouse model of AD.
Materials and Methods
Peptide and Chemicals
The peptides used in this study was synthesized by China Peptides Co, Ltd. (Shanghai, China) to 95% purity. The identity and purity of the peptide was confirmed by reversed-phase HPLC and characterized using matrix assisted laser resorption/ionization time of flight (MALDI–TOF) mass spectrometry. The peptide was stored in dry form and dissolved in double-distilled water containing 0.9% NaCl before experiments.
Sequence of DA4-JC. 30
YXEGTFTSDYSIYLDKQAAXEFVNWLLAGGPSSGAPPPSKKKKKK-NH2
X = aminoisobutyric acid
Receptor Activity cAMP Measurement Study
Transfection and tissue culture
COS-7 cells were cultured at 10% CO2 and 37°C in Dulbecco’s modified Eagles medium 1885 supplemented with 10% FBS, 2 mmol/L glutamine, 180 units/mL penicillin, and 45 g/mL streptomycin. Transient transfection was performed using the calcium phosphate precipitation method 31 with all receptors: GIP, GLP1, GLP2 and the glucagon receptor.
cAMP measurements
Transiently transfected COS-7 cells were seeded in 96-well plates 1 day after transfection at 35.000 cells/well in white plates and the experiments carried out the following day. At the assay day, the cells were washed twice with Hepes-buffered saline (HBS) buffer and incubated with HBS and 1 mmol/L 3-isobutyl-1-methylxanthine (IBMX) for 30 min at 37°C. 32 The agonists were added and incubated for 30 min at 37°C. The HitHunterTM cAMP XS assay (DiscoveRx, Herlev, Denmark) was carried out according to the manufacturer’s instructions. In vitro pharmacological analyzes were carried out with the GraphPad Prism. Sigmoid curves were fitted logistically with a Hillslope of 1.0. The calculations of Ki values were based on the Cheng Prussoffs formula. 33
Animals and Treatments
APPswe/PS1ΔE9 male mice with a C57 black 6 (C57BL/6) background were obtained from the Jackson Laboratory (Maine, US). Wild-type (WT) C57BL/6 female mice came from Charles River UK, Ltd (Kent, UK). Heterozygous APPswe/PS1ΔE9 males were bred with WT females C57BL/6 in the Lancaster University animal unit. Their offspring were genotyped for the APP sequence (Forward “GAATTCCGACATGACTCAGG,” Reverse: “GTTCTGCTGCATCTTGGACA”) by PCR with sequence specific primers as previously described [25]. Mice negative for the transgene served as WT controls. Animals of both genders used in our study and single-housed in individually ventilated cages (IVC’s) in a temperature-controlled colony room (21 ± 2°C). Animals were housed in an enriched environment with standard nesting material, bedding material, chewing sticks and polycarbonate play tunnels. They were under a 12 h light/dark cycle, with the lights on at 07.00 AM. Food and water were provided ad libitum. Dose-response study: APP/PS1 transgenic mice and wild-type (WT) mice that were 9-months of age were injected daily with DA-JC4 or saline. Three different doses of DA-JC4 were given ip. at 0.1nmol/kg, 1nmol/kg or 10nmol/kg body weight for 6 weeks, to establish a dose-response relationship. WT mice were given a dose of saline. Male and female APP/PS1 mice (50-58 g) and WT mice (50-53 g) were divided into 5 groups: 1) The WT mice that received an intraperitoneal (i.p.) injection of 10nmol/kg of saline; 2) The control group of APP/PS1 mice injected with saline; 3) The APP/PS1 group injected with 0.1nmol/kg of DA-JC4 dissolved in saline; 4) The APP/PS1 group treated with 1nmol/kg of DA-JC4 dissolved in saline; 5) The APP/PS1 group treated with 10nmol/kg of DA-JC4 dissolved in saline. Three mice per group were used for the histology.
For the behavioral study comparing DA4-JC to liraglutide, the dose was a once- daily injection of 10nmol/kg body weight ip. of either liraglutide or DA4-JC. When animals were 7 months of age, animals were injected for 8 weeks. Injections were given in the morning (8:00 am). Control mice received equal volumes of saline solution. All experimental procedures involving animals were approved by the UK home office (PPL70/8250/2015) in accordance with EU regulations. For the study comparing DA4-JC with liraglutide, animals were divided into 4 different groups (n = 12 per group). (i): control group treated with saline only; (ii): APP/PS1 group treated with saline only; (iii): APP/PS1 group treated with liraglutide, and (iv): APP/PS1 group treated with DA4-JC. We did not include a wild-type group, injected with drugs as we and others previously showed that wild type animals are not affected by GLP-1, GIP, or dual GLP-1/GIP receptor agonists, see. 13,18,22,23,34 -38
The Water Maze Test
The Morris water maze was employed to evaluate spatial learning and reference memory 8 weeks post initiation of the treatments. It conducted in a circular white plastic pool with a diameter of 150 cm and a depth of 60 cm. The pool was filled with water at 22 ± 1°C to avoid animal hypothermia and the escape platform (10 cm in diameter; made of clear acrylic glass) was submerged 1 cm below the water surface. The water was made opaque with a non-toxic white dye that rendered the escape platform invisible. The maze was illuminated by four 60-W light bulbs fixed on the floor of the pool perimeter. Extra-maze visual cues were positioned in the sidewalls of the apparatus. The pool was divided into 4 quadrants—North (N), South (S), East (E), and West (W). These points served as starting positions for the mice in apparatus. Swimming tracks of each mouse were monitored with a monochrome digital camera, mounted overhead (2.1 m above the center of the apparatus) and relayed to a water-maze tracking/analyser system software (Ethovision, Netherlands).
Acquisition phase
The acquisition trial phase consisted of 4 training days (Day1-4) and 4 trials per day with a 15-min inter-trial interval. Four points equally spaced along the circumference of the pool (North, South, East, West) served as the starting position, which was randomized across the 4 trials each day. If an animal did not reach the platform within 60 s, it was guided to the platform where it had to remain for 30 s, before being returned to its home cage. The escape latencies were recorded.
Probe trial
One day after finishing the acquisition task, a probe trial was performed in order to assess the spatial memory (after a 24 h delay). The platform was removed from the maze and animals were allowed to swim freely for 60 s. Time spent in the target quadrant was assessed, as was spatial acuity, which measured the amount of time spent in the exact area where the escape platform had been located.
Surgery and LTP Recordings in the Hippocampus Area CA1
Mice were anesthetized with urethane (ethyl carbamate, 1.8g/kg ip.) for the duration of all experiments. The skull was exposed and 3 holes with 0.8 mm diameter were drilled. Electrodes (tungsten with Teflon coating, Bilaney, Kent, UK) were inserted at 1.5 mm posterior and 1.0 mm lateral to the midline for the recording electrode, and 2.0 mm posterior to bregma and 1.5 mm lateral to the midline for the stimulating electrode. The electrodes were lowered down to the upper layers of the hippocampus and into the CA1 region until the appearance of a negative field excitatory postsynaptic potential (fEPSP) that had a latency of 10-12 msec. Recordings of fEPSPs were made from the stratum radiatum in the CA1 region in response to stimulation of the Schaffer collateral/commissural pathway. fEPSPs were recorded on a computerized stimulating and recording unit (PowerLab, ADI Instruments) in which the trigger threshold was adjustable. The triggered unit activated a constant current stimulus isolator (Neurolog, UK). fEPSPS were sampled at 20 kHz. The high frequency stimulation protocol for inducing LTP consisted of 4 trains of 100 stimuli, interstimulus interval 5 msec (200 Hz). This LTP induction protocol was chosen to prevent saturation of LTP and thus allow the possibility to detect improvements as well as impairments of the LTP in the APP/PS1 mice. The stimulation intensity was at 60% of the maximum fEPSP, as shown in the input-output stimulus-response correlation. LTP was measured as a percentage of baseline fEPSP slope recorded over a 30-minute period before application of high frequency stimulation. This value was taken as 100% of the excitatory postsynaptic potential slope and all recorded values were normalized to this baseline value. N = 6 per group.
Histology
The mice were anaesthetized with 5% chloral hydrate (0.007 ml/g, i.p.) and perfuse-fixed transcardially with 0.01 M phosphate buffer saline (PBS) followed by 4% paraformaldehyde. Brains were removed from skulls and fixed overnight in 4% paraformaldehyde before dehydrating in 30% sucrose in 0.1 M PB. Brains were sectioned coronally at a thickness of 30 µm on a cryostat. The sections were washed 3 times in tris-buffered saline (TBS) before being treated with heat mediated antigen retrieval, using 50nM sodium citrate solution (pH 8.5-9) for 30 minutes at 80°C. After endogenous peroxidase activity was blocked by 0.6% H2O2 for 30 minutes at room temperature, the tissues were washed in PBS for 5 minutes. The sections were incubated for 20 minutes with diluted normal blocking serum (Vectastain Elite ABC Kit), which was then pipetted from the wells. The sections were incubated for a further 30 minutes with a primary antibody diluted in PBS, and then washed for 5 minutes in PBS. The primary antibodies used were: GFAP (rabbit anti-GFAP, 1:1000), IBA1 (rabbit anti-IBA1, 1:2000) and Abeta (rabbit anti-Abeta, 1:500, Invitrogen). Sections were incubated for 30 minutes with diluted biotinylated goat anti-rabbit IgG secondary antibody (Vectastain Elite ABC Kit), and then washed for 5 minutes in PBS. The Vectastain Elite ABC Reagent (Avidin DH solution and biotinylated enzyme) was then used to immerse the sections for another 30 minutes, followed by a final wash for 5 minutes in PBS. The tissues were then incubated with a chromogen/substrate solution, using DAB (3, 3-diaminobenzidine, Vectastain kit) and hydrogen peroxide to block endogenous peroxidase activity, for approximately 8 minutes until desired stain intensity developed. All staining was visualized by Axio Scope 1 (Zeiss, Germany) and analysis of stained objects was conducted for plaque load, dense core plaques and inflammation. The number of stained cells or plaques of each image (2 images per section and mouse were analyzed, in total approximately 8-10 sections per mouse, 16-20 images total were taken) was quantified using a multi threshold plug in with Image J (NIH, USA).
Western Blot
Brain hemispheres were homogenized on ice in RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) containing protease blockers. After 30 min incubation, lysates were concentrated by centrifugation at 12000 rpm for 5 min at 4°C. The protein concentration of the samples then was quantified by BCA protein assay (Beyotime Institute of Biotechnology, Shanghai, China), using bovine serum albumin as standard. Equivalent amounts of protein were loaded on 12% SDS-polyacrylamide gel and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5%BSA in Tris-buffered saline (Boster Biotechnology Co., Ltd. Wuhan, China) and incubated overnight at 4°C with the rabbit antibody against IL-1ß (1:2000), rabbit anti-TNF-a (1:1000), followed by incubation for 2 h at room temperature with the HRP-labeled goat anti-rabbit immunoglobulin (1:1000; Abcam, Cambridge, UK). The bound antibodies were then visulalized by ECL-enhanced chemilluminescence (Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer’s instructions. In some cases, the blots were stripped and reprobed with a rabbit anti-mouseβ-actin (1:1000; Abcam, Cambridge, UK) to ensure equal sample loading. All western blots were repeated 3 times. Western blot images were captured with a chemiluminescent imaging system (Sage creation, Beijing, China). All bands were quantified using the image system of Quantity one (Bio-Rad, Hercules, CA, USA).
Statistics
Data were analyzed using the program Prism (Graphpad software Inc., USA), with the level of probability set at 95%. Results are expressed as means ± standard error of the mean (SEM). Data were analyzed by 1-way or 2-way ANOVA, followed by Tukey’s Multiple Comparison post-hoc tests.
Results
Receptor Activation Study
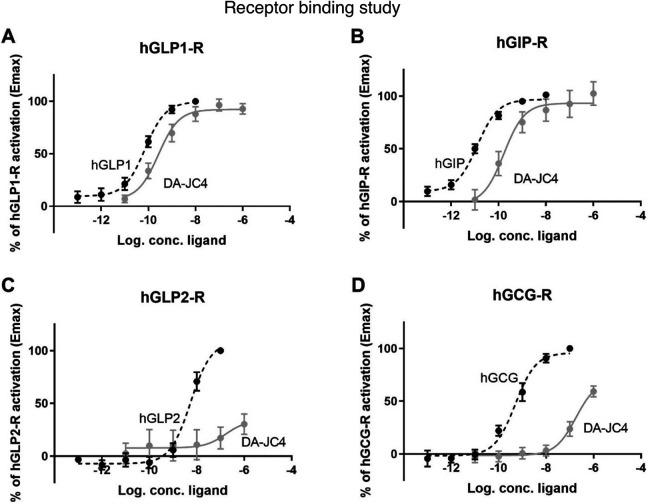
COS-7 cells were successfully transfected with the hGCG-R, hGIP-R, hGLP2-R and hGLP-1 R. The native peptide hormones glucagon, GIP, GLP2 and GLP1 activated their respective receptors with potencies similar to previously described .For GLP-1, we observed a potency (shown as LogEC50 ± SEM) of -10,1 ± -0,11, for GIP:-10,9 ± 0,09, for glucagon: -9,3 ± 0,12 and for GLP2:,-8,2 ± 0,03 (Figure 1A-D). The ability of DA4-JC to activate the receptors was determined on all 4 receptors. We observed that DA4-JC acted as a high potency agonist of the GLP-1 R and the GIP-R with the same efficacies (Emax) as the endogenous ligands, thus acting as a full agonist on both receptors. Moreover it acted with similar potencies on the 2 receptors with logEC50 ± SEM of -9,6 ± 0,18 for the GLP-1 R and -9,8 ± 0,27 for the GIP-R (Figure 1A and B). Compared to the respective endogenous agonists, DA4-JC thus acted with a decreased potency of 3-fold at the GLP-1 receptor and 13-fold at the GIP receptor. In contrast, no activation by DA4-JC was observed at the GLP2-R, (Figure 1C). Only minor and low potent activation was seen at the glucagon receptor with logEC50 ± SEM of -6,7 ± 0,22 without reaching an efficacy plateau even at a concentration of 1uM (Figure 1D).
Figure 1.
Activation of hGLP1-R, hGIP-R, hGLP2-R and hGCG-R by endogenous agonist and DA4-JC and cAMP accumulation in COS-7 cells transfected with (A) hGLP-1 receptor, treated with increasing concentrations of hGLP-1 and DA4-JC (B) hGIP-R treated with increasing concentrations of hGIP and DA4-JC (C) hGLP2-R treated with increasing concentrations of hGLP-2 and DA4-JC (C) hGCG-R treated with increasing concentrations of hGCG and DA4-JC.
Dose-Response Study
Amyloid plaque load in the cortex
A 1-way ANOVA found an overall difference in amyloid plaque load (F = 16.04, DF4,94, p < 0.0001). In Tukey’s multiple comparison post-hoc tests, differences between groups were found (see Figure 2A).
Figure 2.
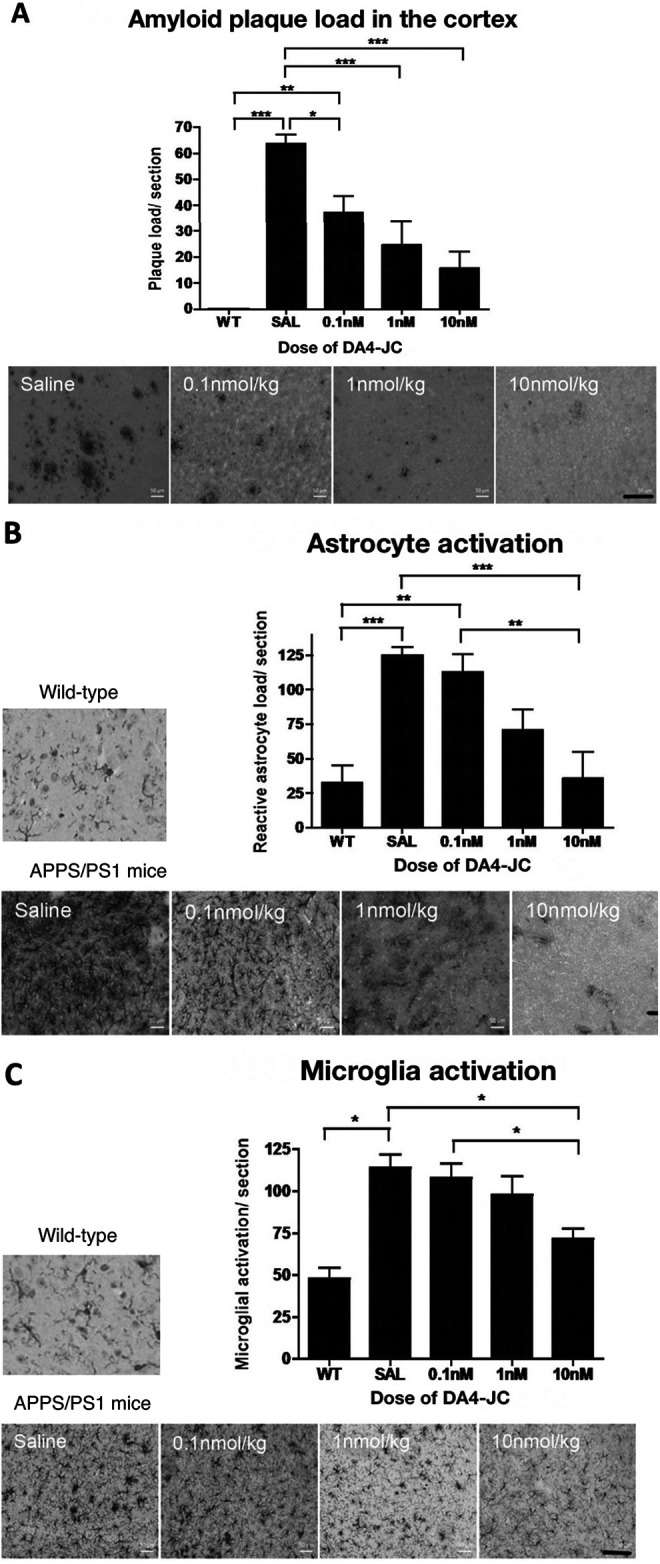
DA4-JC4 dose-response study in the APP/PS1 mouse model of AD. A: Immunohistochemical analysis of amyloid plaque load in the cortex of 9-month old APP/PS1 mice. The 4 different treatments given to the APP/PS1 mice, are shown in the images above, presenting a reduced number of amyloid plaques in the cortex (scale bar 100 µm). Data shown as mean ± S.E.M. (One-way ANOVA, Tukey’s Multiple Comparison post-hoc test, *=p < 0.05, **=p < 0.01, ***=p < 0.001). B: Immunohistochemical analysis of the number of reactive astrocytes in the cortex of 9-month old APP/PS1 mice. DA4-JC treatment reduced the number of activated astrocytes in the brain. The 5 images are illustrating the different treatments given to the APP/PS1 mice, showing the reduction in the number of reactive astrocytes per section within the cortex (scale bar 100 µm). Data shown as mean ± S.E.M. (*=p < 0.05, **=p < 0.01, ***=p < 0.001). C: Immunohistochemical analysis of the number of activated microglia in the cortex of 9-month old APP/PS1 mice. DA4-JC treatment reduced the number of activated microglia in the brain. The 5 images are illustrating the different treatments given to the wild-type and APP/PS1 mice, showing the reduction in activated microglia within the cortex (scale bar 100 µm). Data shown as mean ± S.E.M. (*=p < 0.05, **=p < 0.01, ***=p < 0.001).
Activated astrocyte numbers in the cortex
A 1-way ANOVA found an overall difference in activated astrocyte load using the GFAP staining method (F = 9.678, DF4,94, p < 0.0001). In Tukey’s multiple comparison post-hoc tests, differences between groups were found (see Figure 2B).
Activated microglia numbers in the cortex
A 1-way ANOVA found an overall difference in activated microglia load using the IBA-1 staining method (F = 9.678, DF4,94, p < 0.0001). In Tukey’s multiple comparison post-hoc tests, differences between groups were found (see Figure 2C).
Comparison of DA4-JC and Liraglutide in the APP/PS1 Mouse Model of AD
Water maze results
Acquisition phase
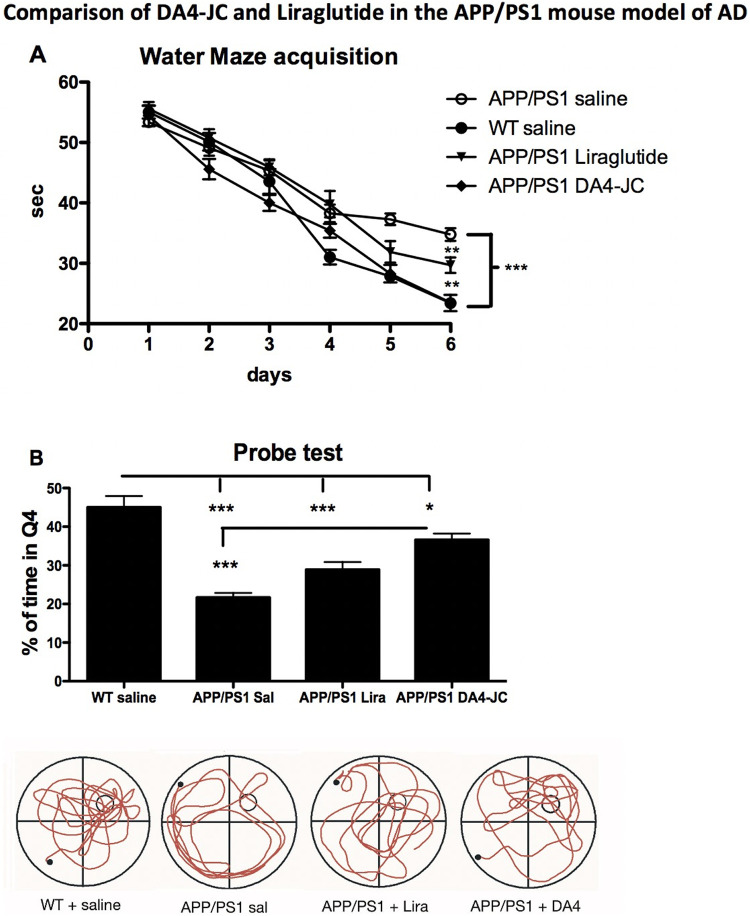
A 2-way repeated measures ANOVA found a difference between groups (F = 16.42, DF3,36, p < 0.0001) and over time (F = 237.9, DF5,36, p < 0.0001). The interaction was significant (F = 3.98, DF16,36, p < 0.0001). In post-hoc analyses, a difference was found between APP/PS1 saline group and the WT;APP/PS1-DA4-JC groups (both p < 0.001), and between the APP/PS1 saline group and the APP/PS1 liraglutide group (p < 0.01). A difference was found between the APP/PS1 liraglutide group and the WT;APP/PS1-DA4-JC groups (both p < 0.01). There was no difference between the WT and the APP/PS1-DA4-JC group. (see Figure 3A).
Figure 3.
(A): Acquisition times for water maze training. A 2-way ANOVA found an overall difference for drug treatment (p < 0.001) and over time (p < 0.001). Post-hoc tests showed differences between groups. (B): Probe test percentage of target quadrant swim times. A 1-way ANOVA found an overall difference between groups (p < 0.001). Post-hoc tests showed differences between groups. *=p < 0.05; **=p < 0.01; ***=p < 0.001. N = 12 per group.
Probe test
A difference between all groups was found in a 1-way ANOVA test (F = 24.3, p < 0.001). Tukey multiple comparison tests found a difference between groups. The WT group was different to APP/PS1 saline and APP/PS1 Liraglutide (p < 0.01). The WT group was different to APP/PS1 DA4-JC (p < 0.001). The APP/PS1 sal was different to the APP/PS1 DA4-JC group (p < 0.001). The APP/PS1 sal was not different to the APP/PS1 lira group (see Figure 3B).
Long-term potentiation in area CA1 of the hippocampus
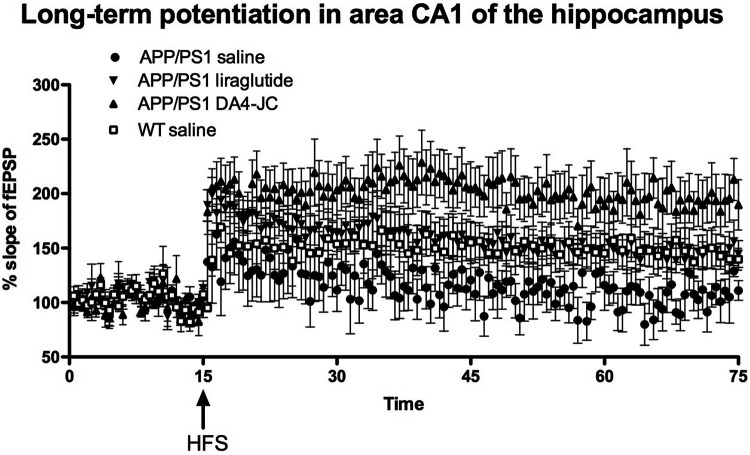
A 2-way repeated measures ANOVA found a difference between groups (F = 48.49, DF3,60, p < 0.0001)) and over time (F = 4.7, DF60,180, p < 0.001). In post-hoc comparisons, the APP/PS1 DA4-JC group is significantly different from the other groups (p < 0.001). DA4-JC is more effective than liraglutide. The APP/PS1 saline group (p < 0.001) is significantly different from the wild type and the APP/PS1 liraglutide group (p < 0.01), see Figure 4.
Figure 4.
Long-term potentiation of synaptic transmission in area CA1 of the hippocampus is much improved by DA4-JC. A 2-way ANOVA found an overall difference between groups (p < 0.001) and over time (P < 0.001). The APP/PS1 DA4-JC group is significantly different from the other 3 groups. The APP/PS1 saline group is significantly different from the wild type (p < 0.001) and the APP/PS1 liraglutide group (p < 0.01). N = 6 per group.
Amyloid plaque load in the cortex
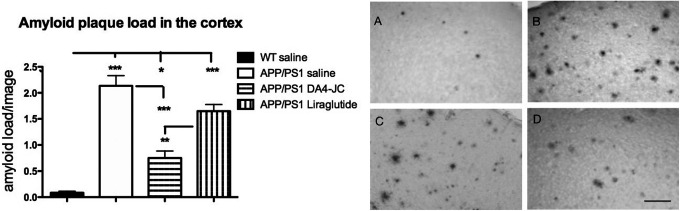
A 1-way ANOVA found an overall difference (F = 45.48; DF3,30, p < 0.0001). In Tukey’s multiple comparison post-hoc tests, differences between groups were found. The WT saline group was different from the APP/PS1 saline (p < 0.001), the APP/PS1 liraglutide group (p < 0.001) and the APP/PS1 DA4-JC group (p < 0.05). The APP/PS1 saline group was different from the APP/PS1 DA4-JC group (p < 0.001). The APP/PS1 liraglutide group was different from the APP/PS1 DA4-JC group (p < 0.001), see Figure 5.
Figure 5.
Quantification of beta-amyloid plaque loads in the neocortex. A 1-way ANOVA found an overall difference between groups (p < 0.001). DA4-JC was more effective in reducing the amyloid load compared to liraglutide in Tukey’s multiple comparison post-hoc tests. *=p < 0.05; **=p < 0.01; **=p < 0.001. N = 6-10 per group. Sample micrographs are shown. A = WT; B = APP/PS1 sal; C = APP/PS1 Lira; D = APP/PS1 DA4-JC. Scale bar = 50 µm.
Pro-inflammatory cytokines
The IL-1ß levels were significantly different over all groups as shown in a 1-way ANOVA (F = 49.42, DF3,30, p < 0.001). Tukey’s multiple comparison post-hoc tests showed a difference between WT and all other groups (p < 0.001). The APP/PS1 saline was different from the APP/PS1 DA4-JC group (p < 0.001).
The TNF-α levels were significantly different over all groups as shown in a 1-way ANOVA (F = 30.62, DF3,20, p < 0.001). Tukey’s multiple comparison post-hoc tests showed a difference between WT and APP/PS1 saline (p < 0.001) or APP/PS1 liraglutide groups (p < 0.001). The APP/PS1 saline was different from the APP/PS1 DA4-JC group (p < 0.001). The APP/PS1 liraglutide group was significantly different from the APP/PS1 DA4-JC group (p < 0.01), see Figure 6.
Figure 6.
Quantification of pro-inflammatory cytokines in the brain. A 1-way ANOVA found an overall difference between groups (p < 0.001). Western blots were repeated 3 times. DA4-JC was more effective in reducing the levels compared to liraglutide in Tukey’s multiple comparison post-hoc tests. *=p < 0.05; **=p < 0.01; **=p < 0.001. Sample bands are shown below.
Discussion
The results demonstrate for the first time that the novel GLP-1/GIP dual receptor agonist DA4-JC is specific for those receptors and has clear neuroprotective effects in the APP/PS1 mouse model of AD. The receptor activation tests showed a balanced activation of GIP and GLP-1 receptors while not activating GLP-2 or Glucagon receptors. This result is a confirmation of a previous study that tested dual agonist peptides with the same receptor binding sites. 20 The dose-response investigation shows a clear dose -effect relationship. The highest dose tested demonstrated significant anti-inflammatory effects and lowered the amyloid plaque load significantly. In the study that directly compared DA4-JC with liraglutide at equal doses, the dual receptor agonist was superior in most parameters tested. This result confirms our previous findings that single GLP-1 or GIP analogues can protect from memory loss in this mouse model of AD. 13,34,39,40 We have previously shown good effects of liraglutide at 25nmol/kg bw once-daily in the same mouse model of AD. 10,34 In a direct comparison with the GLP-1 receptor agonist lixisenatide we also tested the 10nmol/kg bw dose in this mouse model. The lower dose showed limited effects, which was also observed in the present study. 13 DA4-JC is more effective at this dose, most likely because the drug activates 2 receptor types. Another reason may be that DA4-JC can cross the blood-brain barrier (BBB) at a much higher rate than liraglutide. We tested BBB penetration of DA4-JC using radiolabeled peptides and found that it is superior to DA1-JC, exenatide or liraglutide in terms of uptake speed and percentage of blood vs. brain levels (Salameh et al., manuscript submitted). Memory formation in the spatial water maze task was improved by both drugs tested, but in the recall probe test, liraglutide did not show an improvement. In the synaptic plasticity (LTP) study, DA4-JC showed superior effects in improving synaptic plasticity in the hippocampus. We have previously shown that both GLP-1 receptor agonists 13 and GIP receptor agonists can rescue LTP in this animal model of AD. 39,40 It is therefore likely that the superior effect seen here in comparison to liraglutide is due to the combined activation of GLP-1 and GIP receptors. Furthermore, DA4-JC was more effective in lowering the amyloid plaque load in the cortex. As the gene expression and resulting protein synthesis in this transgenic mouse model which expresses 2 human mutated genes related to AD cannot be altered, this effect is most likely due to improved autophagy. We have previously shown that autophagy is impaired in this animal model of AD, and that a similar dual GLP-1/GIP receptor agonist can improve autophagy. 25 Finally, the study showed that the anti-inflammatory effects of DA4-JC are superior to liraglutide. The chronic inflammation response as seen in the activation of microglia and astrocytes and the release of the pro-inflammatory cytokines TNF-a and IL-1ß is much reduced by the novel Dual agonist, similar to single GLP-1 or GIP receptor agonists 19,34,41 but more effective. Dual GLP-1/GIP receptor agonists are therefore promising as a more effective treatment for AD. Several dual agonists are already in clinical trials and are safe to take. 21,24 One phase II clinical study in patients with diabetes showed improved effects of a dual GLP-1/GIP receptor agonist compared to a GLP-1 receptor agonist. 21 Some of the dual agonists developed for treating diabetes have functional groups attached to enhance the survival time in the blood stream. 20 However, those additions can reduce the transport across the BBB. 42,43 The GLP-1 receptor agonist exendin-4 has neuroprotective effects in animal models of AD, 12,44 but a pegylated form of exendin-4 does not cross the BBB. 45 We have shown that a lipidated dual agonist showed reduced neuroprotective effects. 23 Removing the C16 fatty acid enhanced the neuroprotective effect of the dual agonist, 26 and adding cell-penetrating sequences increased the transport across the BBB and the neuroprotective properties of those dual agonists in an AD rat model and in a Parkinson’s disease mouse model. 23,42,43 For a summary of these effects see. 46
DA4-JC is therefore a very promising new drug candidate to treat AD. As GLP-1 receptor agonists have already shown good protective effects in patients with AD or Parkinson’s disease, 16,47 -49 it is hoped that a dual GLP-1/GIP receptor agonist that can readily cross the BBB will show superior neuroprotective effects in patients with AD. 46
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study had been funded by a grant from the Alzheimer Society UK. Prof. Holscher is an inventor on a patent that covers the use of dual GLP-1/GIP receptor agonists as a novel treatment for AD. The patent is owned by Lancaster University, United Kingdom.
ORCID iD: Christian Hölscher  https://orcid.org/0000-0002-8159-3260
https://orcid.org/0000-0002-8159-3260
References
- 1. Cole A, Astell A, Green C, Sutherland C. Molecular connexions between dementia and diabetes. Neurosci Biobehav Rev. 2007;31:1046–1063. [DOI] [PubMed] [Google Scholar]
- 2. Strachan MW, Reynolds RM, Marioni RE, Price JF. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol. 2011;7:108–114. [DOI] [PubMed] [Google Scholar]
- 3. Schrijvers EMC, Witteman JCM, Sijbrands EJG, Hofman A, Koudstaal PJ, Breteler MMB. Insulin metabolism and the risk of Alzheimer disease: the Rotterdam study. Neurology. 2010;75:1982–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4:147–152. [DOI] [PubMed] [Google Scholar]
- 5. Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol. 2004;490:115. [DOI] [PubMed] [Google Scholar]
- 6. Li L, Hölscher C. Common pathological processes in Alzheimer disease and type 2 diabetes: a review. Brain Res Rev. 2007;56:384. [DOI] [PubMed] [Google Scholar]
- 7. Biessels GJ, Kappelle LJ. Increased risk of Alzheimer’s disease in type II diabetes: insulin resistance of the brain or insulin-induced amyloid pathology? Biochem Soc Trans. 2005;33:1041–1044. [DOI] [PubMed] [Google Scholar]
- 8. Talbot K, Wang HY. The nature, significance, and glucagon-like peptide-1 analog treatment of brain insulin resistance in Alzheimer’s disease. Alzheimers Dement. 2014;10:S12–S25. doi:10.1016/j.jalz.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262. [DOI] [PubMed] [Google Scholar]
- 10. McClean PL, Holscher C. Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology. 2014;76(Pt A):57–67. doi:10.1016/j.neuropharm.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 11. Hölscher C. GLP-1 and GIP analogues as novel treatments for Alzheimer’s and Parkinson’s disease. Cardiovasc Endocrinol. 2016;5:93–98. [Google Scholar]
- 12. Li Y, Duffy KB, Ottinger MA, et al. GLP-1 receptor stimulation reduces amyloid-β peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease. J Alzheimers Dis. 2010;19:1205–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McClean PL, Holscher C. Lixisenatide, a drug developed to treat type 2 diabetes, shows neuroprotective effects in a mouse model of Alzheimer’s disease. Neuropharmacology. 2014;86C:241–258. doi:10.1016/j.neuropharm.2014.07.015 [DOI] [PubMed] [Google Scholar]
- 14. Cai HY, Yang J-T, Wang Z-J, et al. Lixisenatide reduces amyloid plaques, neurofibrillary tangles and neuroinflammation in an APP/PS1/tau mouse model of Alzheimer’s disease. Biochem Biophys Res Commun. 2018;495:1034–1040. doi:10.1016/j.bbrc.2017.11.114 [DOI] [PubMed] [Google Scholar]
- 15. Cai HY, Hölscher C, Yue X-H, et al. Lixisenatide rescues spatial memory and synaptic plasticity from amyloid beta protein-induced impairments in rats. Neuroscience. 2014;277C:6–13. doi:10.1016/j.neuroscience.2014.02.022 [DOI] [PubMed] [Google Scholar]
- 16. Gejl M, Gjedde A, Egefjord L, et al. In Alzheimer’s disease, six-month treatment with GLP-1 analogue prevents decline of brain glucose metabolism: randomized, placebo-controlled, double-blind clinical trial. Front Aging Neurosci. 2016;8:1–10. doi:10.3389/fnagi.2016.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Faivre E, Gault VA, Thorens B, Holscher C. Glucose-dependent insulinotropic polypeptide receptor knockout mice are impaired in learning, synaptic plasticity, and neurogenesis. J Neurophysiol. 2011;105:1574–1580. doi:10.1152/jn.00866.2010 [DOI] [PubMed] [Google Scholar]
- 18. Faivre E, Hamilton A, Holscher C. Effects of acute and chronic administration of GIP analogues on cognition, synaptic plasticity and neurogenesis in mice. Eur J Pharmacol. 2012;674:294–306. [DOI] [PubMed] [Google Scholar]
- 19. Duffy AM, Holscher C. The incretin analogue D-Ala(2)GIP reduces plaque load, astrogliosis and oxidative stress in an APP/PS1 mouse model of Alzheimer’s disease. Neuroscience. 2013;228:294–300. doi:10.1016/j.neuroscience.2012.10.045 [DOI] [PubMed] [Google Scholar]
- 20. Finan B, Ma T, Ottaway N, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013;5:209ra151. doi:10.1126/scitranslmed.3007218 [DOI] [PubMed] [Google Scholar]
- 21. Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392:2180–2193. doi:10.1016/S0140-6736(18)32260-8 [DOI] [PubMed] [Google Scholar]
- 22. Jalewa J, Sharma M, Gengler S, Hölscher C. A novel GLP-1/GIP dual receptor agonist protects from 6-OHDA lesion in a rat model of Parkinson’s disease. Neuropharmacology. 2017;117:238–248. [DOI] [PubMed] [Google Scholar]
- 23. Feng P, Zhang X, Li D, et al. Two novel dual GLP-1/GIP receptor agonists are neuroprotective in the MPTP mouse model of Parkinson’s disease. Neuropharmacology. 2018;133:385–394. [DOI] [PubMed] [Google Scholar]
- 24. Frias JP, Bastyr EJ III, Vignati L, et al. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metab. 2017;26:343–352 e342. doi:10.1016/j.cmet.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 25. Panagaki T, Gengler S, Holscher C. The novel DA-CH3 dual incretin restores endoplasmic reticulum stress and autophagy impairments to attenuate Alzheimer-like pathology and cognitive decrements in the APPSWE/PS1DeltaE9 mouse model. J Alzheimers Dis. 2018;66:195–218. doi:10.3233/JAD-180584 [DOI] [PubMed] [Google Scholar]
- 26. Yuan Z, Li D, Feng P, et al. A novel GLP-1/GIP dual agonist is more effective than liraglutide in reducing inflammation and enhancing GDNF release in the MPTP mouse model of Parkinson’s disease. Eur J Pharmacol. 2017;812:82–90. doi:10.1016/j.ejphar.2017.06.029 [DOI] [PubMed] [Google Scholar]
- 27. Cao Y, Hölscher C, Hu M-M, et al. DA5-CH, a novel GLP-1/GIP dual agonist, effectively ameliorates the cognitive impairments and pathology in the APP/PS1 mouse model of Alzheimer’s disease. Eur J Pharmacol. 2018;827:215–226. doi:10.1016/j.ejphar.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 28. Feinglos MN, Saad MF, Pi-Sunyer FX, An B, Santiago O. Effects of liraglutide (NN2211), a long-acting GLP-1 analogue, on glycaemic control and bodyweight in subjects with Type 2 diabetes. Diabet Med. 2005;22:1016–1023. [DOI] [PubMed] [Google Scholar]
- 29. Vilsboll T. Liraglutide: a new treatment for type 2 diabetes. Drugs Today. 2009;45:101–113. [DOI] [PubMed] [Google Scholar]
- 30. Shi L, Zhang Z, Li L, Holscher C. A novel dual GLP-1/GIP receptor agonist alleviates cognitive decline by re-sensitizing insulin signaling in the Alzheimer icv. STZ rat model. Behav Brain Res. 2017;327:65–74. doi:10.1016/j.bbr.2017.03.032 [DOI] [PubMed] [Google Scholar]
- 31. Steen A, Thiele S, Guo D, Hansen LS, Frimurer TM, Rosenkilde MM. Biased and constitutive signaling in the CC-chemokine receptor CCR5 by manipulating the interface between transmembrane helices 6 and 7. J Biol Chem. 2013;288:12511–12521. doi:10.1074/jbc.M112.449587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hassing HA, Fares S, Larsen O, et al. Biased signaling of lipids and allosteric actions of synthetic molecules for GPR119. Biochem Pharmacol. 2016;119:66–75. doi:10.1016/j.bcp.2016.08.018 [DOI] [PubMed] [Google Scholar]
- 33. DeBlasi A, O’Reilly K, Motulsky HJ. Calculating receptor number from binding experiments using same compound as radioligand and competitor. Trends Pharmacol Sci. 1989;10:227–229. [DOI] [PubMed] [Google Scholar]
- 34. McClean P, Parthsarathy V, Faivre E, Hölscher C. The diabetes drug Liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J Neurosci. 2011;31:6587–6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu W, Jalewa J, Sharma M, Li G, Li L, Hölscher C. Neuroprotective effects of lixisenatide and liraglutide in the MPTP mouse model of Parkinson’s disease. Neuroscience. 2015;303:42–50. [DOI] [PubMed] [Google Scholar]
- 36. Parthsarathy V, Holscher C. The type 2 diabetes drug liraglutide reduces chronic inflammation induced by irradiation in the mouse brain. Eur J Pharmacol. 2013;700:42–50. doi:10.1016/j.ejphar.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 37. Tian MJ, Wang RF, Hölscher C, et al. The novel GLP-1/GIP dual receptor agonist DA3-CH is neuroprotective in the pilocarpine-induced epileptogenesis rat model. Epilepsy Res. 2019;154:97–106. doi:10.1016/j.eplepsyres.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 38. Li T, Tu L, Gu R, et al. Neuroprotection of GLP-1/GIP receptor agonist via inhibition of mitochondrial stress by AKT/JNK pathway in a Parkinson’s disease model. Life Sci. 2020. doi:10.1016/j.lfs.2020.117824 [DOI] [PubMed] [Google Scholar]
- 39. Faivre E, Holscher C. Neuroprotective effects of D-Ala2GIP on Alzheimer’s disease biomarkers in an APP/PS1 mouse model. Alzheimers Res Ther. 2013;5:20–28. doi:10.1186/alzrt174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Faivre E, Holscher C. D-Ala2GIP facilitated synaptic plasticity and reduces plaque load in aged wild type mice and in an Alzheimer’s disease mouse model. J Alzheimers Dis. 2013;35:267–283. doi:10.3233/JAD-121888 [DOI] [PubMed] [Google Scholar]
- 41. Ji C, Xue GF, Li G, Li D, Holscher C. Neuroprotective effects of glucose-dependent insulinotropic polypeptide in Alzheimer’s disease. Rev Neurosci. 2016;27:61–70. doi:10.1515/revneuro-2015-0021 [DOI] [PubMed] [Google Scholar]
- 42. Zhang L, Zhang L, Li Y, et al. The novel dual GLP-1/GIP receptor agonist DA-CH5 is superior to single GLP-1 receptor agonists in the MPTP model of Parkinson’s disease. J Parkinson Dis. 2020. doi:10.3233/JPD-191768 [DOI] [PubMed] [Google Scholar]
- 43. Li C, Liu W, Li X, et al. The novel GLP-1/GIP analogue DA5-CH reduces tau phosphorylation and normalizes theta rhythm in the icv. STZ rat model of AD. Brain Behav. 2020;10:e01505. doi:10.1002/brb3.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bomfim TR, Forny-Germano L, Sathler LB, et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Abeta oligomers. J Clin Invest. 2012;122:1339–1353. doi:10.1172/JCI57256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yun SP, Kam TI, Panicker N, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med. 2018;24:931–938. doi:10.1038/s41591-018-0051-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hölscher C. Brain insulin resistance: role in neurodegenerative disease and potential for targeting. Expert Opin Investig Drugs. 2020;29:333–348. doi:10.1080/13543784.2020.1738383 [DOI] [PubMed] [Google Scholar]
- 47. Athauda D, Maclagan K, Skene SS, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1664–1675. doi:10.1016/S0140-6736(17)31585-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aviles-Olmos I, Dickson J, Kefalopoulou Z, et al. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest. 2013;123:2730–2736. doi:10.1172/JCI68295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aviles-Olmos I, Dickson J, Kefalopoulou Z, et al. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J Parkinsons Dis. 2014;4:337–344. doi:10.3233/JPD-140364 [DOI] [PubMed] [Google Scholar]