Abstract
Stress responses are essential for survival, but become detrimental to health and cognition with chronic activation. Chronic hypothalamic-pituitary-adrenal axis release of glucocorticoids induces hypothalamic-pituitary-adrenal axis dysfunction and neuronal loss, decreases learning and memory, and modifies glucocorticoid receptor/mineralocorticoid receptor expression. Elderly who report increased stress are nearly 3 times more likely to develop Alzheimer’s disease, have decreased global cognition and faster cognitive decline than those reporting no stress. Patients with mild cognitive impairment are more sensitive to stress compared to healthy elderly and those with Alzheimer’s disease. Stress may also transduce neurodegeneration via the gut microbiome. Coping styles determine hippocampal mineralocorticoid receptor expression in mice, indicating that coping modifies cortisol’s effect on the brain. Identifying neuroprotective coping strategies that lessen the burden of stress may prevent or slow cognitive decline. Treatments and education designed to reduce stress should be recognized as neuroprotective.
Keywords: stress, cognition, coping, Alzheimer’s disease (AD), hypothalamic-pituitary-adrenal (HPA) axis, neurodegeneration
Significance Statement
Normal brain structure and cognitive functioning are compromised by stress which results in increased AD pathology. Choosing appropriate coping mechanisms towards stress may lessen its impact on our health and cognition, possibly preventing disease initiation or progression. This manuscript verifies previous research with novel concepts.
Introduction
Stress plays an integral role in disease, including neurodegenerative diseases such as Alzheimer’s disease (AD). 1,2 Over five million Americans suffer from AD, with an expected increase to 7.7 million by 2030. 3 Exposure to acute or chronic stress affects learning and memory function. Chronic stress accelerates aging, increases inflammation, cortisol, and catecholamines, 4,5 and induces changes in the gut microbiome. 6 In healthy male Wistar rats, maternal separation stress increases Amyloid Beta (Aβ)40 and Aβ42 levels, β secretase enzyme (BACE) 1, hyperphosphorylation of tau, and decreases hippocampal cell survival and proliferation. 7 Cell death mediated by tau requires tau hyperphosphorylation and determines Aβ-induced cell death. 8 Nineteen stress-responsive genes involved in neuroinflammation and accompanying tau pathology have been identified in rats. 9 Chronic stress-induced hippocampal and cortical neuronal atrophy and associated cognitive deficits are not seen in tau knock-out mice compared to controls. 10,11
The gut-brain axis influences neural development, cognition, and behavior 12 with chronic stress reducing the gut microbiome’s diversity and richness in mice. 13 -15 Germ free (GF) mice have a substantially higher response to restraint stress compared to pathogen free mice with a normal microbiome and mice raised with a selected microbiome. This effect is in part corrected by reconstitution of the microbiome, but only when corrected at an early age. 16 Changes in microbiome components influences amyloid deposition in mice with AD. 17,18 Microbiome alterations increase gut permeability, possibly promoting translocation of pathogens through the epithelial lining. This triggers an immunological response inducing proinflammatory cytokine production causing neuroinflammation. 19 Trimethylamine N-oxide (TMAO), a microbiome derived metabolite, was recently found in the cerebral spinal fluid (CSF) of MCI and AD individuals in significantly higher concentrations respectively compared to the unimpaired. Increased TMAO in CSF associates with AD pathology and neuronal degeneration. 20 Stress increases circulating TMAO and choline, a precursor to TMAO, in the blood of healthy mice 21 and healthy humans. 22 Stress exposure results in varied systemic effects on the body, inducing neurodegeneration and dysfunction in addition to AD pathogenesis. 1,2,4 -6,13 -15
Chronically elevated glucocorticoid levels cause hippocampal neuronal loss and cognitive impairment in rats, 23 non-human primates, 24 and humans. 25 Hippocampal volume negatively correlates with plasma cortisol levels in humans. 25,26 Chronic exposure to cortisol is neurotoxic in humans. 27 The hypothalamic-pituitary-adrenal (HPA) axis responds to stress by releasing glucocorticoids. 28 Hypothalamic production of corticotropin-releasing hormone (CRH) stimulates the pituitary gland to secrete adrenocorticotropin hormone (ACTH), activating adrenal glucocorticoid release. Neuroendocrine overstimulation or dysfunction, via this HPA axis, may contribute to AD development and neuronal loss. 29 Stress-induced cortisol elevation is associated with increased Aβ-peptide production, hyperphosphorylation of tau in AD mouse models 30,31 and healthy rats, 32 -34 and synapse loss in healthy rats. 35
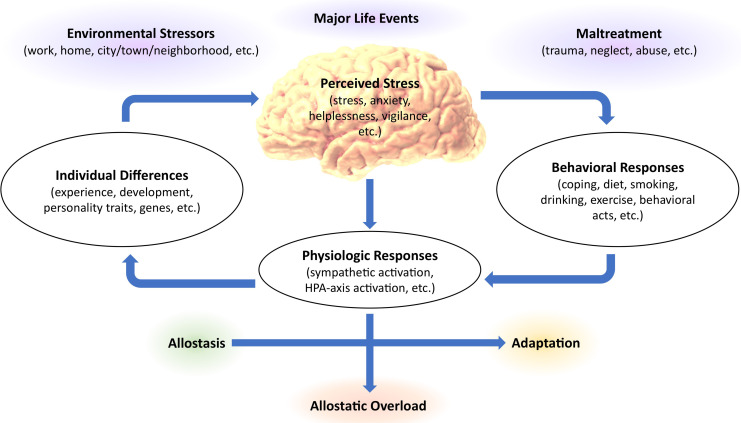
The HPA axis and autonomic nervous system are responsible for our adaptive stress response. 36 The autonomic nervous system is comprised of the sympathetic pathway, responsible for “fight or flight.” and the parasympathetic pathway, responsible for “resting and digesting.” Each acts in concert maintaining homeostasis. Hypersympathetic stimulation, or too much stress is fatal. 37 Yet, an adequate stress response is integral for survival, allowing us to adapt and respond to immediate threats. 38 Stressful stimuli activate HPA axis production of glucocorticoids and other hormones, 28 and initiate sympathetic stimulation with catecholamine production. 39 During stress, attention is enhanced, cardiac and respiratory output accelerates, and catabolism increases. 40 Allostasis is the appropriate stress level needed to actively maintain stability; awakening and rising from bed in the morning, or running from a predator. Allostatic overload is the detrimental wear and tear on the body from stress dysregulation. 36 Primates experience more stress-related diseases than any other animal, likely from our increased intellect, social ties, and emotional complexities. 41 Fish, birds, and reptiles secrete the same stress hormones as primates, however, our stress is predominantly socially generated and ambiguous. Many stressors are psychological, 42 such as economic worry or social rejection (see Figure 1). In animal models, allosteric overload induces hippocampal and prefrontal atrophy and amygdala hypertrophy. 43
Figure 1.
The response to stress in relation to allostasis or allostatic overload. The brain, our main controller of stress, regulates the autonomic nervous system, sympathetic nervous system, and neuroendocrine system. The status of these systems in conjunction with individual behaviors and differences determines the effect that stress has on health. (Adapted from. 44 ).
In mammals, cortisol and corticosterone are the main glucocorticoids found in the brain, circulating at 100-1000-fold total the concentration of aldosterone. 45 Cortisol, key in our stress response, is transduced by 2 ligand-dependent receptors 46 : a mineralocorticoid receptor (MR) and a glucocorticoid receptor (GR). Both are found in the amygdala responsible for emotional processing, the prefrontal cortex responsible for executive function, and the hippocampus responsible for memory and learning; they are also involved in HPA axis feedback regulation. 46,47 Glucocorticoids have a high-affinity for MR, nearing saturation at basal cortisol levels, whereas GRs activate with high cortisol levels. 48 In the brain, GRs cortisol affinity is one tenth that of MR, and aldosterone’s GR affinity is approximately one tenth that of cortisol. 45 MRs are important in initializing appraisal and choosing the most appropriate behavior to follow. GRs are responsible for memory storage and behavioral adaptations. 49 Although these receptors result in differing effects, both act to regulate synaptic plasticity during stress. 48 High circulating cortisol impairs spatial memory in aged rats 50,51 implicating GR stimulation in age-related cognitive decline. Yet, no consistent associations exist between either receptor and declining cognition. 52 -54 Cortisol bound GR or MR instigates a conformational change with the receptor/ligand complex entering the nucleus and binding directly to DNA or physically interacting with other transcription factors regulating gene expression. 55 -57 The receptor/ligand complex also induces non-genomic affects mediated by membrane receptors. 58
Over 80% of cortisol responsive genes are regulated by either GR or MR, or less commonly both. Activated MR or GR differentially influences calcium channel protein encoded mitochondrial RNA expression and calcium signaling. 59 MR activation in CA1 hippocampal neurons elicits small, ion-gated calcium currents 60 that significantly increase calcium output when GR is also activated, 61 suggesting that both receptors modulate calcium-dependent signal transduction. GR stimulation leads to oxidative damage as found in the mitochondrial DNA of aged brain models with high cortisol levels. 62 More research is needed to clarify the unique mechanisms involved in specific GR and MR gene transcription to better understand how a shift in the ratio of these chronically activated receptors allows cortisol’s role to switch from protective to harmful.
Self-Reported Stress Assessments
Elderly who report increased stress are 2.7 times more likely to develop AD than those who do not. 63 In elderly, less stress correlates with better health, followed by social interaction, socioeconomic factors, marital status, and living situation; but age, sex, race, and employment show no relation. 64 Education inversely relates to the frequency and severity of anxiety and stress in AD patients. 65 Increased self-reported stress, per the Life Events and Difficulties Schedule (LEDS), correlates with dementia diagnosis, but not cortisol levels. 66,67 Cortisol-awakening response (CAR) reflects peak cortisol after waking and inversely relates to progression from normal to mild cognitive impairment (MCI), but has no relationship with self-reported stress. 67 Higher perceived stress scale (PSS) scores correlate with lower global cognitive function and increased rates of cognitive decline in elderly. 68 Young, healthy individuals with at least one major life difficulty have poorer memory than those without negative life events within the past year. 69 Higher stress scores correlate with increased morning cortisol levels in amnestic MCI patients. 63 Yet episodic memory performance is not predicted by self-reported negative life events in the elderly. 70 These varied findings highlight our lack of understanding of how self-reported stress measures relate to cortisol levels and its effect on cognition in health and disease. MCI and AD patients may have difficulty assessing or recalling stress. 65 A simple checklist assessing stress is imprecise given the multiple factors linking stress to HPA axis activation. 71
Cortisol as a Potential Biomarker of Stress
Numerous studies assessed cortisol levels as a biomarker for stress in normal controls, patients with MCI, and AD. Large variations exist in measuring healthy elderly basal cortisol levels. Results show cortisol levels steadily maintaining with age, 72,73 subtly increasing, 74,75 or lowering. 76,77 Compared to controls, patients with MCI and AD can have higher salivary CAR levels, 78 with a decreased daily cortisol ratio compared to their CAR, with higher evening cortisol levels. 79 Alternatively, Wolf, et al. 80 found no significant differences in cortisol levels between healthy elderly, MCI, or AD patients when measured at 6 different points throughout the day, including the CAR. Peavy and colleagues 66 found an association between increased cortisol levels and a decreased rate of memory decline in individuals with MCI when followed for 3 years. This may reflect increased cortisol from vigilance and attention, compensating for memory loss. 81,82 Additionally, a stressful effort may be put forth by those who know they are impaired, resulting in higher cortisol. A better understanding of cortisol’s regulation in individuals with MCI or AD who have HPA axis dysfunction could clarify previous cortisol measurement inconsistencies. These varied findings highlight the inter-individual cortisol measurement variability among the elderly. 47 Additionally, timing and methodological differences in sampling cortisol may contribute to contradictory findings, such as location of sampling, time of day of sampling, and salivary versus plasma sampling.
The variability of results using cortisol levels could be explained by existing hippocampal damage in MCI and AD that affects cortisol’s impact on hippocampal function and memory. Arbel, et al. 83 utilized year-old Fischer-344 rats to represent normal age-related cognitive impairment and 3-month-old rats to represent controls and further divided them randomly into cortisol pellet implanted and placebo groups. Cognitively unimpaired rats with sustained-release cortisol pellet implants had significantly poorer learning and memory performance than placebo matched animals. The young unimpaired and older impaired pellet treated animals showed no significant differences on the Morris Water Maze (MWM). 83 Older rats with hippocampal loss may have poorer cortisol sensitivity compared to young unimpaired rats with intact hippocampi. Pellet treated young impaired and placebo treated old impaired groups show no behavioral differences in MWM attempts, suggesting that chronic cortisol exposure while young produces early cognitive decline. Histopathological analysis showed a 450% increase in damaged CA1 hippocampal cells in young pellet treated rats compared to baseline. Although present, treated old rats hippocampal cell damage was to a lesser degree, possibly from age-related hippocampal damage already being present at baseline measurements. 83 Thus, cognitive vulnerability to cortisol appears to be regulated by the magnitude of cortisol-induced hippocampal damage, with increased damage resulting in decreased cortisol sensitivity. Hippocampal cell loss in MCI could impair the efficient negative feedback system for the HPA axis, resulting in hypercortisolemia. This would further impair neuronal functioning and contribute to continued neurodegeneration, 84 but only to a certain extent, since too much stress-related hippocampal damage induces varied and decreased MR/GR ratio expression, 85 lessening the individual’s stress sensitivity in more progressed AD cases. In AD patients, dexamethasone administration, suppressing cortisol release, results in decreased suppression of cortisol compared to healthy elderly individuals, indicating impaired HPA axis regulation. 86 In very young mice that overexpress amyloid precursor protein (APP), elevated cortisol appears to be from APP overexpression rather than amyloid plaque deposition and hippocampal neurodegeneration, given that the plaques and neurodegeneration are absent at this early age. 87 Additionally, oligomeric, soluble Aβ, not precipitated amyloid plaques, are toxic inducing neurodegeneration. 88,89 A hyperactive HPA axis may reflect a more advanced disease state with hypercortisolemia due to HPA axis dysfunction continuing to contribute to, and potentially accelerate, cognitive decline. Individuals with below normal cortisol levels may be at greater risk for MCI or AD since both too little and too much cortisol results in deleterious health effects. 90
Mental illness is associated with hyper- and hyposecretion of glucocorticoids, 91 which may also contribute to the high instance of anxiety 92 and depression 92,93 in AD. The prefrontal cortex and hippocampus are involved in HPA axis inhibition, with the amygdala activating it. 46,94 Since both glucocorticoid receptors are found in these regions and help to regulate the HPA axis, 46,47 future studies should assess if stress-induced GR or MR expression influences mental disorder initiation. Melancholic patients have a glucocorticoid feedback resistance, 95 whereas post-traumatic stress disorder (PTSD) patients have a decreased continual stress responses 96 with lower basal corticosteroid levels 97 indicating HPA axis dysfunction. Insult to these HPA axis regulatory regions, by allosteric overload, may induce behavioral dysfunction in AD patients further impairing their ability to properly regulate cortisol.
Corticotropin releasing-hormone (CRH) may be a better biomarker than cortisol for stress. Like cortisol, CRH is also regulated based on neuroendocrine stress responses and its receptors are expressed in numerous brain regions, including the hippocampus. CRH neurons activate sympathetic stimulation 98,99 and further regulate adrenal production of catecholamines. 100 CRH elicits stress-associated behavioral responses and sympathetic stimulation, like anxiety, and decreased appetite. 101 Although CRH is a more general mediator of stress adaptation than cortisol, it adversely impacts hippocampal structures in response to stress 102,103 similar to cortisol. Early-life stress increases the number of hippocampal neurons expressing CRH. 104 CRH mutant animals display reduced tau and Aβ related pathologies, 105,106 and mutations in the main CRH receptor (Crfr1) reduced stress-induced hyperphosphorylation of tau and Aβ deposition. 102 CRH neurons can also be activated by Aβ, 87 further increasing toxic Aβ release. 107 Although neurotoxic at high concentrations, modest hippocampal release of CRH is essential for long-term potentiation (LTP) priming. 108 Blocking CRH signaling abolishes stress-induced hippocampal spine density reductions and resultant memory defects in stressed mice. 109 This suggests that, like cortisol, CRH increases contribute to neurodegeneration. Glucocorticoids, like cortisol, are less involved than CRH in HPA axis control and cognitive health since healthy adrenalectomized Sprague-Dawley rats show no difference in tau phosphorylation compared to controls, 110 supporting CRH as the protein kinase inducer. Furthermore, stress-induced increases in phosphorylated tau is lower in mice deficient in CRH than controls, suggesting a critical role for CRH in AD pathogenesis. 105 Variability in measuring glucocorticoids is problematic, 111 underscoring the difficulty in using cortisol as a direct representation of stress. Therefore, alternative markers of stress should be validated.
Assessing the Effects of Stress on Cognitive Performance
In AD mouse models, stress accelerates cognitive loss. 31,112,113 Stress in healthy elderly people results in decreased word recall. 114 MCI patient’s cortisol levels negatively correlate with immediate memory and learning. 115 No correlation between cognitive scores and cortisol was found in AD, 115,116 suggesting an association between cognitive decline and increased cortisol in healthy elderly individuals and MCI, but not full blown AD. MCI individuals are most sensitive cognitively to glucocorticoids compared to those with AD and the healthy elderly, with sensitivity declining with disease progression. 115 A decrease in MR/GR in MCI, perhaps resultant of hippocampal atrophy, may affect cortisol sensitivity. 117 Initial hippocampal loss of glucocorticoid receptors in MCI could result in impaired cortisol transduction, whereas healthy individuals with similar cortisol concentrations maintain the beneficial to detrimental threshold of cortisol signal transduction. As the disease progresses, continued hippocampal loss of MR/GR receptors substantially diminishes cortisol’s effect on cognition.
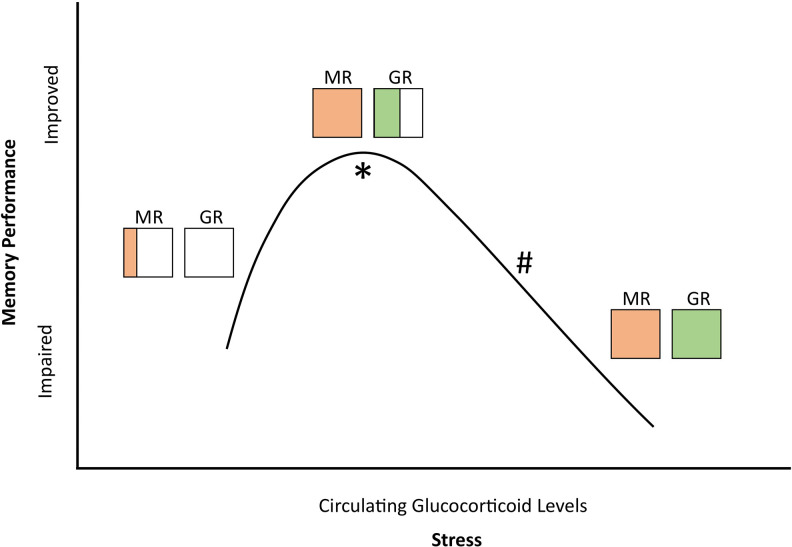
Cognitive performance varies depending on the memory process being analyzed. Consolidation is the encoding of new information from short to long-term memory. 118 Memory retrieval refers to accessing already consolidated traces. 119 -121 A rise in glucocorticoids enhances consolidation and impairs it with exogenous administration of the cortisol synthesis inhibitor, metyrapone. 122 Based on binding affinity, low glucocorticoid levels result in high occupation of the more sensitive MRs but low occupation of GRs, enhancing consolidation. When the ratio is reversed, retrieval is impaired. 123,124 A “fight or flight” experience is seldom forgotten, but during it, little else is recalled 119 -121 (see Figure 2).
Figure 2.
Stress-induced glucocorticoid levels correlate with changing MR and GR occupancy ratios. Diagonal lines and horizontal lines indicate relative bound MR or GR, respectively. * indicates when consolidation is improved. # indicates when retrieval is impaired. (Adapted from. 124 ).
Stress downregulates both MR and GR expression in an uneven distribution, with some local MR upregulations. 125 In rats, stress differentially downregulates MR and GR expression, 85,126 which is also mirrored in primates. 127 Differing degrees of downregulated MR and GR alter the MR/GR ratio, 85 indicating a pathological process rather than tachyphlaxis from cortisol saturation. Given that GR saturation is linked to age-related cognitive decline, its downregulation could play a neuroprotective role with chronically elevated cortisol levels.
Mice with a global GR deletion rapidly die postnatally from impaired lung function, preventing the endocrine effects from being analyzed 128 and indicating that some GR activity is required for development and survival. GR is overexpressed in GRov mice, resulting in a prolonged stress response, decreased mRNA levels of glutamate signaling genes,129 reduced excitatory transmission, 130 and long-term memory impairment. 129,130 Glucocorticoid receptor antagonism is effective in enhancing cognition. In middle aged mice chronic GR antagonism, by mifepristone, abolishes the electrophysiological disturbances seen in aged control hippocampi, 131 but in aged rats, chronic stress-induced morphological changes of pyramidal neurons were not reversed after stress recovery. 132 This suggests that aged animals can’t reverse all negative effects of glucocorticoids. 11β-hydroxysteroid dehydrogenases (11β-HSD) are pre-receptor regulators working to modify our cortisol response, with 11β-HSD1 activating cortisol and 11β-HSD2 inactivating cortisol. 45 Aged mice, with a 11β-HSD type 1 knockout (11β-HSD1-/-) preventing conversion of cortisone to active cortisol, given a GR antagonist during stress show no significant reductions in spatial memory 48 ; likely resultant of insufficient cortisol levels occupying GR initially. 11β-HSD1 deficient young mice increased GR expression to increase cellular responsiveness in a low cortisol environment, an effect not seen in aged 11β-HSD1-/- mice 133 suggesting reduced plasticity of GR with aging. In 11β-HSD1-/- mice given a MR antagonist, like spironolactone, spatial memory is impaired 134,135 due to below threshold cortisol levels occupying fewer MR. Although cortisol stimulates both hippocampal MR and GR few genes are responsive to both receptors 59 supporting separate transduction pathways for the MR and GR cascades. These combined findings suggest that increasing MR activation and decreasing GR activation, by managing cortisol levels or using GR antagonists, may amend memory deficits. Unfortunately, the chronic use of a GR antagonist in humans induces generalized glucocorticoid resistance with hypercortisolemia, 136 indicating possible impaired HPA axis regulation, as GRs play a role in shutting the axis off through negative feedback. 137 A more selective 11β-HSD1 inhibitor, aimed at reducing cortisol levels in the aged brain, restoring the receptor activation balance back to MR from GR, is needed. Cognitive function improved in healthy elderly men using a selective 11β-HSD1 inhibitor, without increasing HPA axis activation. 138 Alternatively, short-term intermittent GR antagonist treatments may prove effective.
Difficulties in Assessing Stress
Individual differences in our stress responses depend on how we perceive a situation and the quality of our health when we encounter stress. 139 Stress responses can be modified by many variables: growth factors, bacterial ligands, cytokines, opioids, and adipokines—all modulate glucocorticoid release independent of pituitary ACTH. 140 Michaud, et al. 141 asserts that cortisol/stress associations may depend mainly on the types of stressors and stress management mechanisms. In healthy young mice, early stress caused increased BACE and Aβ levels that persisted for the life of the animal. 142 Thus, stress not only has immediate effects but the specific type or the age at which stress occurs has long-lasting effects. Stressed non-human primate adults downregulate hippocampal GR expression and prefrontal cortical MR expression, whereas early life stress downregulates only prefrontal cortical GR expression. 127 The testing environment where cognitive assessments are given will also produce varied results. Older adults have a heightened test environment sensitivity compared to younger adults. 143 When the assessment directions are modified, decreasing emphasis on memory, the elderly performed comparable to the young adults. 143,144 Perhaps assessing the aging population’s cognitive capacities is stressful, stemming from the fear of underlying dementia.
Individualized Modifiable Covariates
When assessing stress and its severity, humans, unlike other animals, have more behavioral complexity and flexibility in our social support and our utilization of art and music as stress reducers. 41 We all experience some stress in our lifetime. How we manage and cope with stress will determine the magnitude it effects our health. Coping is the ever changing behavioral and cognitive effort used to manage stress. 145 Prior to coping, primary and secondary appraisals occur. Primary appraisal assesses what, if anything, is at stake in the specific encounter. Secondary appraisal evaluates what, if anything, could be done to overcome, prevent, or improve the outcome. After appraising 2 types of coping can be utilized: 1) regulating stressful emotions and 2) altering the person(s) or environment causing the stress; referred to as emotion-based coping and problem-focused coping respectively. 146 Successful coping is associated with decreased adrenocortical stress responses. Coping unsuccessfully relates to feelings of helplessness or hopelessness, further increasing cortisol levels in a vicious cycle. 147
Mild to moderate AD patients use a range of coping strategies, with problem-focused coping being the most frequent. Effort put forth by the patient was the leading coping skill observed, such as self-prompting or looking up the answer themselves, followed by attempts to obtain caregiver help. 148 More impaired patients sought more caregiver help indicating that coping with AD may be assessed as a relational experience, especially with continued progression. 149 Involving a loved one for support protected patients from emotional detachment. 150 Coping by isolation or denial may increase stress, highlighting the importance of a support system for newly diagnosed individuals. Avoidance, concealment, minimization, self-blame, and vagueness have also been implemented by individuals with AD. 151,152 Denial and other defense mechanisms in coping with stress induces increased adrenocortical activity in AD. 153 However, these strategies may minimize ones difficulties, maintaining self-esteem, which can lessen stress. 152
In healthy individuals following an acute stressor reduced autobiographical memory is a cognitive avoidant coping mechanism, reducing the memory linked with a stressor, preventing the re-experience of associated negative emotions. As cognitive-avoidant coping increases, memory detail decreases from pre to post stressor. 154 Although healthy individuals use this avoidance strategy in a flexible way, 155,156 more research is needed to assess its use in individuals with an altered cognition. Assessing whether cognitive avoidant coping lessens the chronic effects of stress by preventing the re-experience of stressful events should be analyzed.
Changes in ways of coping may indicate further cognitive decline. In amnestic MCI patients, increased self-reports of stress and cortisol levels correlated with higher emotion-based coping, rather than problem-focused coping. 63 In AD patients, those with higher Mini Mental Status Examination (MMSE) scores used problem-focused coping more frequently than emotion-based. In the lesser impaired, executive functioning remains intact, allowing for problem-focused coping. Decreased cognition compromises executive function, resulting in difficulty selecting strategies to control the stressor. An adaptive shift to control emotions may induce fear and emotional fatigue compared to elderly controls. 65 Thus, with worsening cognition a shift from problem-focused to emotion-based coping occurs as an increasing number of problems becomes harder to negotiate.
Individual differences in rodent aggression reflect valuable coping strategies to stress. Aggressive male mice with short attack latencies (SAL) manipulate situations, removing themselves or the source of stress, whereas non-aggressive mice with long attack latencies (LAL) reduce their emotional impact by reacting with immobility or withdrawal. LAL mice with a nonaggressive opponent passively cope, whereas SAL mice actively cope. 157 -160 When chronically exposed to an inescapable stressor, LAL mice suffer chronic decreased body weights with elevated corticosterone concentrations compared to SAL mice. Stress reduces hippocampal MR mRNA and the MR/GR ratio in LAL mice, but not in SAL mice. 161 Thus, active coping may be neuroprotective. It’s unknown if increased GR expression in stressed LAL mice changes over time with stress removal. If a healthy coping mechanism can accommodate cognitive decline, patients may be taught to reduce their stress sensitivity. Coping with stress in relation to aging and dementia has yet to be explored along with assessing the caregiver-patient relationship, and their coping strategies. Although coping greatly affects our stress management, personality characteristics do as well. Calmer, more mature, and more energetic teenagers were less likely to be diagnosed with dementia later in life compared to their peers. 162 Neurotic individuals experience more stressful events 163,164 and are predisposed to experience distress and negative emotions, regardless of stress level. 164
Providing MCI and AD patients and families with resources will lessen the stress of living with a neurodegenerative disease. When we believe we are capable of managing a negative event, we are less likely to regard it as threatening or stressful. 145 MCI or AD families benefit from services like home health care, educational materials, and caregiver support. 165 Without such resources the uncertainty of their condition could induce additional stress hastening cognitive decline.
Possible AD Treatments Aimed at Regulating Stress
Current AD treatments using acetylcholinesterase inhibitors have side effects and a limited window of efficacy in mild to moderate AD. 166 Lifestyle adaptations reducing or better managing stress may prove protective against neurodegeneration. Memory training including stress management improves activities of daily living (ADL), but not memory performance, for community retirement residents. 167 Pharmacological therapies, selectively lowering stress hormone levels, have been tested on animals in hopes of their use in humans with neurodegenerative diseases. A Corticotropin-releasing factor receptor-1 (CRFR1) antagonist reduced amyloid pathology and increased synaptic and cognitive function in AD-Tg mice, 168 however, in humans, elevated liver enzymes induced study termination. 109 High throughput screening assays have recently been developed to pursue new CRFR1 antagonists. 168
Cortisol availability and action depends on tissue-specific intracellular metabolism by 11β-HSDs 169 that catalyze intracellular regeneration of corticosterone and cortisol. Stress suppresses hippocampal LTP 170 and is prevented by the administration of the 11β-HSD1 inhibitor UE2316. 171 The low hippocampal corticosterone environment maintains even with a hyperactive HPA axis and high circulating cortisol levels. 172 Although reduced glucocorticoid regeneration in the brain improves cognition, preventing memory decline in an AD mouse model (Tg2576), chronic treatments with UE2316 didn’t prevent Aβ plaque formation; Thus, 11β-HSD1 inhibitors have a potential as a cognitive enhancer in AD. 173
A homeostatically stable microbiome appears to be neuroprotective. Six GR receptor pathways are upregulated in germ free mice compared to only 2 being upregulated in specific pathogen free mice treated with E. coli lipopolysaccharide treatment. 174 Gut dysbiosis inducing gut microbiome alterations may be ameliorated by probiotics, given that they attenuate stress, enhance memory and reduce anxiety-like behavior. 175,176 Probiotics improve hippocampal dependent spatial memory and synaptic plasticity in rats administered with Aβ injections. 177 In rats, the probiotic strain Lactobacillus paracasei NCC2461 amended stress-induced gut alterations, completely restoring gut paracellular permeability compared to Bifidobacterium lactis NCC362 and Lactobacillus johnsonii NCC533, emphasizing the importance of specific strains. 178 Balanced diets high in omega-3 polyunsaturated fatty acids normalize stress-induced microbiome alterations 179 and resultant memory deficits in mice. 180 Further research is needed to elucidate the role of the microbiome on cognition in healthy and AD affected individuals and how its modification may correct or prevent AD pathogenesis in mice. Although some positive trends have emerged from natural therapies for AD the underlying mechanisms behind some nootropics modes of action remain unclear. Clinical trials with larger sample sizes and long term efficacy tested with longitudinal studies are needed.
Summary
The brain, our stress response controller and interpreter, suffers physical and functional damage from stress. 2,85,117,125 -127 Damage depends on the age we experience stress, 127 whether chronic or acute, and how we cope with it. This damage can persist throughout life. 181 Stress accelerates aging, cognitive decline, and neurodegenerative disease. 4,5 Individuals with MCI, compared to healthy controls and AD, have the highest risk of stress-related cognitive decline, 115 likely due to the changing glucocorticoid sensitivity induced by hippocampal atrophy. Stress and glucocorticoids alter GR/MR expression, 85,125 -127 but so can differing coping styles. 161 Coping style affects stress-induced hippocampal changes in mice, 161 however, it is currently unknown if coping affects cognition, and if certain styles of coping could prevent or slow progression of cognitive decline in humans. Investigating whether stress-induced changes in the brain can be reversed with beneficial coping mechanisms needs further study.
Abbreviations
- 11β-HSD
11β-hydroxysteroid dehydrogenase
- Aβ
amyloid beta
- ACTH
adrenocorticotropin-releasing hormone
- AD
Alzheimer’s disease
- ADL
activities of daily living
- APP
amyloid precursor protein
- BACE
β secretase enzyme
- CA1
cornu ammonis 1
- CAR
cortisol-awakening response
- Crfr1
corticotropin-releasing factor receptor-1
- CRH
corticotropin-releasing hormone
- CSF
cerebral spinal fluid
- DNA
deoxyribonucleic acid
- GF
germ free
- GR
glucocorticoid receptor
- Grov
glucocorticoid receptor overexpression
- HPA
hypothalamic-pituitary-adrenal
- LAL
long attack latency
- LEDS
Life Events and Difficulties Schedule
- LTP
long-term potentiation
- MCI
mild cognitive impairment
- MMSE
Mini Mental State Examination
- MR
mineralocorticoid receptor
- mRNA
messenger ribonucleic acid
- MWM
Morris Water Maze
- PTSD
post-traumatic stress disorder
- PSS
perceived stress scale
- SAL
short attack latency
- Tg
transgenic
- Tg2576
Alzheimer’s disease mouse model
- TMAO
trimethylamine N-oxide
- UE2316
11β-hydroxysteroid dehydrogenase1 inhibitor.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Shelby A. Kline  https://orcid.org/0000-0002-6718-5236
https://orcid.org/0000-0002-6718-5236
Michael S. Mega  https://orcid.org/0000-0002-2155-864X
https://orcid.org/0000-0002-2155-864X
References
- 1. Wilson RS, Barnes LL, Bennett DA, et al. Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurology. 2005;64(2):380–382. [DOI] [PubMed] [Google Scholar]
- 2. Selye H. Stress and the general adaptation syndrome. Br Med J. 1950;1(4667):1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akram M, Nawaz A. Effects of medicinal plants on Alzheimer’s disease and memory deficits. Neural Regen Res. 2017;12(4):660–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens). 2009;8(1):7–22. [DOI] [PubMed] [Google Scholar]
- 5. Ranabir S, Reetu K. Stress and hormones. Indian J Endocrinol Metab. 2011;15(1):18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maltz RM, Keirsey J, Kim SC, et al. Social stress affects colonic inflammation, the gut microbiome, and short-chain fatty acid levels and receptors. J Pediatr Gastroenterol Nutr. 2019;68(4):533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martisova E, Aisa B, Guerenu G, Ramirez MJ. Effects of early maternal separation on biobehavioral and neuropathological markers of Alzheimer’s disease in adult male rats. Curr Alzheimer Res. 2013;10(4):420–432. [DOI] [PubMed] [Google Scholar]
- 8. Bakota L, Brandt R. Tau biology and tau-directed therapies for Alzheimer’s disease. Drugs. 2016;76(3):301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Novak P, Cente M, Kosikova N, et al. Stress-induced alterations of immune profile in animals suffering by tau protein-driven neurodegeneration. Cell Mol Neurobiol. 2018;38(1):243–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lopes S, Teplytska L, Vaz-Silva J, et al. Tau deletion prevents stress-induced dendritic atrophy in prefrontal cortex: role of synaptic mitochondria. Cereb Cortex. 2017;27(4):2580–2591. [DOI] [PubMed] [Google Scholar]
- 11. Lopes S, Vaz-Silva J, Pinto V, et al. Tau protein is essential for stress-induced brain pathology. Proc Natl Acad Sci U S A. 2016;113(26):E3755–E3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21(6):738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25(3):397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bailey MT, Dowd SE, Parry NM, Galley JD, Schauer DB, Lyte M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect Immun. 2010;78(4):1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress. 2017;7:124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558(Pt 1):263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Minter MR, Zhang C, Leone V, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep. 2016;6:30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harach T, Marungruang N, Duthilleul N, et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep. 2017;7:41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giau VV, Wu SY, Jamerlan A, An SSA, Kim SY, Hulme J. Gut microbiota and their neuroinflammatory implications in Alzheimer’s disease. Nutrients. 2018;10(11):1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vogt NM, Romano KA, Darst BF, et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimers Res Ther. 2018;10(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kinross JM, Alkhamesi N, Barton RH, et al. Global metabolic phenotyping in an experimental laparotomy model of surgical trauma. J Proteome Res. 2011;10(1):277–287. [DOI] [PubMed] [Google Scholar]
- 22. Rezzi S, Martin FP, Alonso C, et al. Metabotyping of biofluids reveals stress-based differences in gut permeability in healthy individuals. J Proteome Res. 2009;8(10):4799–4809. [DOI] [PubMed] [Google Scholar]
- 23. Landfield PW, Baskin RK, Pitler TA. Brain aging correlates: retardation by hormonal-pharmacological treatments. Science. 1981;214(4520):581–584. [DOI] [PubMed] [Google Scholar]
- 24. Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10(9):2897–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lupien SJ, de Leon M, de Santi S, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1(1):69–73. [DOI] [PubMed] [Google Scholar]
- 26. Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biol Psychiatry. 1992;32(9):756–765. [DOI] [PubMed] [Google Scholar]
- 27. Lupien S, Lecours AR, Lussier I, Schwartz G, Nair NP, Meaney MJ. Basal cortisol levels and cognitive deficits in human aging. J Neurosci. 1994;14(5 Pt 1):2893–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McEwen BS, Weiss JM, Schwartz LS. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220(5170):911–912. [DOI] [PubMed] [Google Scholar]
- 29. Stein-Behrens B, Mattson MP, Chang I, Yeh M, Sapolsky R. Stress exacerbates neuron loss and cytoskeletal pathology in the hippocampus. J Neurosci. 1994;14(9):5373–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2006;26(35):9047–9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jeong YH, Park CH, Yoo J, et al. Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APPV717I-CT100 transgenic mice, an Alzheimer’s disease model. FASEB J. 2006;20(6):729–731. [DOI] [PubMed] [Google Scholar]
- 32. Sayer R, Robertson D, Balfour DJ, Breen KC, Stewart CA. The effect of stress on the expression of the amyloid precursor protein in rat brain. Neurosci Lett. 2008;431(3):197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rosa ML, Guimaraes FS, de Oliveira RM, Padovan CM, Pearson RC, Del Bel EA. Restraint stress induces beta-amyloid precursor protein mRNA expression in the rat basolateral amygdala. Brain Res Bull. 2005;65(1):69–75. [DOI] [PubMed] [Google Scholar]
- 34. Solas M, Aisa B, Mugueta MC, Del Rio J, Tordera RM, Ramirez MJ. Interactions between age, stress and insulin on cognition: implications for Alzheimer’s disease. Neuropsychopharmacology. 2010;35(8):1664–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tata DA, Marciano VA, Anderson BJ. Synapse loss from chronically elevated glucocorticoids: relationship to neuropil volume and cell number in hippocampal area CA3. J Comp Neurol. 2006;498(3):363–374. [DOI] [PubMed] [Google Scholar]
- 36. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. [DOI] [PubMed] [Google Scholar]
- 37. Japundzic-Zigon N, Sarenac O, Lozic M, et al. Sudden death: neurogenic causes, prediction and prevention. Eur J Prev Cardiol. 2018;25(1):29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Finsterwald C, Alberini CM. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies. Neurobiol Learn Mem. 2014;112:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leonard BE. Stress, norepinephrine and depression. J Psychiatry Neurosci. 2001;26(Suppl):S11–S16. [PMC free article] [PubMed] [Google Scholar]
- 40. Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- 41. Shwartz M. Robert Sapolsky Discusses Physiological Effects of Stress. Stanford Report; 2007. [Google Scholar]
- 42. Sapolsky RM. Stress in the wild. Sci Am. 1990;262(1):116–123. [DOI] [PubMed] [Google Scholar]
- 43. McEwen BS, Chattarji S. Molecular mechanisms of neuroplasticity and pharmacological implications: the example of tianeptine. Eur Neuropsychopharmacol. 2004;14(Suppl 5):S497–S502. [DOI] [PubMed] [Google Scholar]
- 44. McEwen BS. Protective and damaging effects of stress mediators. New England J Med. 1998;338(1):171–179. [DOI] [PubMed] [Google Scholar]
- 45. Gomez-Sanchez E, Gomez-Sanchez CE. The multifaceted mineralocorticoid receptor. Compr Physiol. 2014;4(3):965–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12(2):118–134. [DOI] [PubMed] [Google Scholar]
- 47. de Souza-Talarico JN, Marin MF, Sindi S, Lupien SJ. Effects of stress hormones on the brain and cognition: evidence from normal to pathological aging. Dement Neuropsychol. 2011;5(1):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yau JL, Noble J, Seckl JR. 11beta-hydroxysteroid dehydrogenase type 1 deficiency prevents memory deficits with aging by switching from glucocorticoid receptor to mineralocorticoid receptor-mediated cognitive control. J Neurosci. 2011;31(11):4188–4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22(10):422–426. [DOI] [PubMed] [Google Scholar]
- 50. Issa AM, Rowe W, Gauthier S, Meaney MJ. Hypothalamic-pituitary-adrenal activity in aged, cognitively impaired and cognitively unimpaired rats. J Neurosci. 1990;10(10):3247–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yau JL, Olsson T, Morris RG, Meaney MJ, Seckl JR. Glucocorticoids, hippocampal corticosteroid receptor gene expression and antidepressant treatment: relationship with spatial learning in young and aged rats. Neuroscience. 1995;66(3):571–581. [DOI] [PubMed] [Google Scholar]
- 52. Lund PK, Hoyt EC, Bizon J, et al. Transcriptional mechanisms of hippocampal aging. Exp Gerontol. 2004;39(11-12):1613–1622. [DOI] [PubMed] [Google Scholar]
- 53. Kuningas M, de Rijk RH, Westendorp RG, Jolles J, Slagboom PE, van Heemst D. Mental performance in old age dependent on cortisol and genetic variance in the mineralocorticoid and glucocorticoid receptors. Neuropsychopharmacology. 2007;32(6):1295–1301. [DOI] [PubMed] [Google Scholar]
- 54. Soontornniyomkij V, Risbrough VB, Young JW, et al. Short-term recognition memory impairment is associated with decreased expression of FK506 binding protein 51 in the aged mouse brain. Age (Dordr). 2010;32(3):309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Beato M. Gene regulation by steroid hormones. Cell. 1989;56(3):335–344. [DOI] [PubMed] [Google Scholar]
- 56. Rogatsky I, Ivashkiv LB. Glucocorticoid modulation of cytokine signaling. Tissue Antigens. 2006;68(1):1–12. [DOI] [PubMed] [Google Scholar]
- 57. Yamamoto KR. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. [DOI] [PubMed] [Google Scholar]
- 58. Haller J, Mikics E, Makara GB. The effects of non-genomic glucocorticoid mechanisms on bodily functions and the central neural system. A critical evaluation of findings. Front Neuro Endocrinol. 2008;29(2):273–291. [DOI] [PubMed] [Google Scholar]
- 59. Datson NA, van der Perk J, de Kloet ER, Vreugdenhil E. Identification of corticosteroid-responsive genes in rat hippocampus using serial analysis of gene expression. Eur J Neurosci. 2001;14(4):675–689. [DOI] [PubMed] [Google Scholar]
- 60. Karst H, Wadman WJ, Joels M. Corticosteroid receptor-dependent modulation of calcium currents in rat hippocampal CA1 neurons. Brain Res. 1994;649(1-2):234–242. [DOI] [PubMed] [Google Scholar]
- 61. Kerr DS, Campbell LW, Thibault O, Landfield PW. Hippocampal glucocorticoid receptor activation enhances voltage-dependent Ca2+ conductances: relevance to brain aging. Proc Natl Acad Sci U S A. 1992;89(18):8527–8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hamilton ML, Van Remmen H, Drake JA, et al. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci U S A. 2001;98(18):10469–10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Souza-Talarico JN, Chaves EC, Nitrini R, Caramelli P. Chronic stress is associated with high cortisol levels and emotional coping mechanisms in amnestic mild cognitive impairment. Dement Geriatr Cogn Disord. 2009;28(5):465–470. [DOI] [PubMed] [Google Scholar]
- 64. Larson R. Thirty years of research on the subjective well-being of older Americans. J Gerontol. 1978;33(1):109–125. [DOI] [PubMed] [Google Scholar]
- 65. de Souza-Talarico JN, Chaves EC, Nitrini R, Caramelli P. Stress and coping in older people with Alzheimer’s disease. J Clin Nurs. 2009;18(3):457–465. [DOI] [PubMed] [Google Scholar]
- 66. Peavy GM, Salmon DP, Jacobson MW, et al. Effects of chronic stress on memory decline in cognitively normal and mildly impaired older adults. Am J Psychiatry. 2009;166(12):1384–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Peavy GM, Jacobson MW, Salmon DP, et al. The influence of chronic stress on dementia-related diagnostic change in older adults. Alzheimer Dis Assoc Disord. 2012;26(3):260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aggarwal NT, Wilson RS, Beck TL, et al. Perceived stress and change in cognitive function among adults 65 years and older. Psychosom Med. 2014;76(1):80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wilding J, Andrews B, Hejdenberg J. Relations between life difficulties, measures of working memory operation, and examination performance in a student sample. Memory. 2007;15(1):57–62. [DOI] [PubMed] [Google Scholar]
- 70. Rosnick CB, Small BJ, McEvoy CL, Borenstein AR, Mortimer JA. Negative life events and cognitive performance in a population of older adults. J Aging Health. 2007;19(4):612–629. [DOI] [PubMed] [Google Scholar]
- 71. Hellhammer DH, Wust S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34(2):163–171. [DOI] [PubMed] [Google Scholar]
- 72. Waltman C, Blackman MR, Chrousos GP, Riemann C, Harman SM. Spontaneous and glucocorticoid-inhibited adrenocorticotropic hormone and cortisol secretion are similar in healthy young and old men. J Clin Endocrinol Metab. 1991;73(3):495–502. [DOI] [PubMed] [Google Scholar]
- 73. Lupien S, Lecours AR, Schwartz G, et al. Longitudinal study of basal cortisol levels in healthy elderly subjects: evidence for subgroups. Neurobiol Aging. 1996;17(1):95–105. [DOI] [PubMed] [Google Scholar]
- 74. Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996;81(7):2468–2473. [DOI] [PubMed] [Google Scholar]
- 75. Dodt C, Theine KJ, Uthgenannt D, Born J, Fehm HL. Basal secretory activity of the hypothalamo-pituitary-adrenocortical axis is enhanced in healthy elderly. An assessment during undisturbed night-time sleep. Eur J Endocrinol. 1994;131(5):443–450. [DOI] [PubMed] [Google Scholar]
- 76. Drafta D, Schindler AE, Stroe E, Neacsu E. Age-related changes of plasma steroids in normal adult males. J Steroid Biochem. 1982;17(6):683–687. [DOI] [PubMed] [Google Scholar]
- 77. Sharma M, Palacios-Bois J, Schwartz G, et al. Circadian rhythms of melatonin and cortisol in aging. Biol Psychiatry. 1989;25(3):305–319. [DOI] [PubMed] [Google Scholar]
- 78. Arsenault-Lapierre G, Chertkow H, Lupien S. Seasonal effects on cortisol secretion in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2010;31(6):1051–1054. [DOI] [PubMed] [Google Scholar]
- 79. Johar H, Emeny RT, Bidlingmaier M, et al. Lower morning to evening cortisol ratio is associated with cognitive impairment in men but not women: an analysis of 733 older subjects of the cross-sectional KORA-Age study. Psychoneuroendocrinology. 2015;51:296–306. [DOI] [PubMed] [Google Scholar]
- 80. Wolf OT, Convit A, Thorn E, de Leon MJ. Salivary cortisol day profiles in elderly with mild cognitive impairment. Psychoneuroendocrinology. 2002;27(7):777–789. [DOI] [PubMed] [Google Scholar]
- 81. Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn. 2007;65(3):209–237. [DOI] [PubMed] [Google Scholar]
- 82. Carlson LE, Sherwin BB. Relationships among cortisol (CRT), dehydroepiandrosterone-sulfate (DHEAS), and memory in a longitudinal study of healthy elderly men and women. Neurobiol Aging. 1999;20(3):315–324. [DOI] [PubMed] [Google Scholar]
- 83. Arbel I, Kadar T, Silbermann M, Levy A. The effects of long-term corticosterone administration on hippocampal morphology and cognitive performance of middle-aged rats. Brain Res. 1994;657(1-2):227–235. [DOI] [PubMed] [Google Scholar]
- 84. Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7(3):284–301. [DOI] [PubMed] [Google Scholar]
- 85. Zhe D, Fang H, Yuxiu S. Expressions of hippocampal mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) in the single-prolonged stress-rats. Acta Histochem Cytochem. 2008;41(4):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Murialdo G, Barreca A, Nobili F, et al. Dexamethasone effects on cortisol secretion in Alzheimer’s disease: some clinical and hormonal features in suppressor and non suppressor patients. J Endocrinol Invest. 2000;23(3):178–186. [DOI] [PubMed] [Google Scholar]
- 87. Justice NJ, Huang L, Tian JB, et al. Posttraumatic stress disorder-like induction elevates beta-amyloid levels, which directly activates corticotropin-releasing factor neurons to exacerbate stress responses. J Neurosci. 2015;35(6):2612–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tackenberg C, Ghori A, Brandt R. Thin, stubby or mushroom: spine pathology in Alzheimer’s disease. Curr Alzheimer Res. 2009;6(3):261–268. [DOI] [PubMed] [Google Scholar]
- 89. Lacor PN, Buniel MC, Furlow PW, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27(4):796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Justice NJ. The relationship between stress and Alzheimer’s disease. Neurobiol Stress. 2018;8:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1201–1213. [DOI] [PubMed] [Google Scholar]
- 92. Ferretti L, McCurry SM, Logsdon R, Gibbons L, Teri L. Anxiety and Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2001;14(1):52–58. [DOI] [PubMed] [Google Scholar]
- 93. Rovner BW, Broadhead J, Spencer M, Carson K, Folstein MF. Depression and Alzheimer’s disease. Am J Psychiatry. 1989;146(3):350–353. [DOI] [PubMed] [Google Scholar]
- 94. Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20(2):78–84. [DOI] [PubMed] [Google Scholar]
- 95. Kathol RG, Jaeckle RS, Lopez JF, Meller WH. Pathophysiology of HPA axis abnormalities in patients with major depression: an update. Am J Psychiatry. 1989;146(3):311–317. [DOI] [PubMed] [Google Scholar]
- 96. Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. [DOI] [PubMed] [Google Scholar]
- 97. Yehuda R, Giller EL, Southwick SM, Lowy MT, Mason JW. Hypothalamic-pituitary-adrenal dysfunction in posttraumatic stress disorder. Biol Psychiatry. 1991;30(10):1031–1048. [DOI] [PubMed] [Google Scholar]
- 98. Brown MR, Fisher LA. Corticotropin-releasing factor: effects on the autonomic nervous system and visceral systems. Fed Proc. 1985;44(1 Pt 2):243–248. [PubMed] [Google Scholar]
- 99. Valentino RJ, Foote SL, Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 1983;270(2):363–367. [DOI] [PubMed] [Google Scholar]
- 100. Tsatsanis C, Dermitzaki E, Venihaki M, et al. The corticotropin-releasing factor (CRF) family of peptides as local modulators of adrenal function. Cell Mol Life Sci. 2007;64(13):1638–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15(2):71–100. [DOI] [PubMed] [Google Scholar]
- 102. Rissman RA, Lee KF, Vale W, Sawchenko PE. Corticotropin-releasing factor receptors differentially regulate stress-induced tau phosphorylation. J Neurosci. 2007;27(24):6552–6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gesing A, Bilang-Bleuel A, Droste SK, Linthorst AC, Holsboer F, Reul JM. Psychological stress increases hippocampal mineralocorticoid receptor levels: involvement of corticotropin-releasing hormone. J Neurosci. 2001;21(13):4822–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ivy AS, Rex CS, Chen Y, et al. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J Neurosci. 2010;30(39):13005–13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Filipcik P, Novak P, Mravec B, et al. Tau protein phosphorylation in diverse brain areas of normal and CRH deficient mice: up-regulation by stress. Cell Mol Neurobiol. 2012;32(5):837–845. [DOI] [PubMed] [Google Scholar]
- 106. Kvetnansky R, Novak P, Vargovic P, et al. Exaggerated phosphorylation of brain tau protein in CRH KO mice exposed to repeated immobilization stress. Stress. 2016;19(4):395–405. [DOI] [PubMed] [Google Scholar]
- 107. Penke B, Bogar F, Fulop L. Beta-amyloid and the patho mechanisms of Alzheimer’s disease: a comprehensive view. Molecules. 2017;22(10):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J Neurosci. 2002;22(9):3788–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chen C, Grigoriadis DE. NBI 30775 (R121919), an orally active antagonist of the corticotropin-releasing factor (CRF) type-1 receptor for the treatment of anxiety and depression. Drug Development Res. 2005;65(4):216–226. [Google Scholar]
- 110. Korneyev A, Binder L, Bernardis J. Rapid reversible phosphorylation of rat brain tau proteins in response to cold water stress. Neurosci Lett. 1995;191(1-2):19–22. [DOI] [PubMed] [Google Scholar]
- 111. Baxter JD, Rousseau GG. Glucocorticoid hormone action: an overview. Monogr Endocrinol. 1979;12:1–24. [DOI] [PubMed] [Google Scholar]
- 112. Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127(3):601–609. [DOI] [PubMed] [Google Scholar]
- 113. Han B, Wang JH, Geng Y, et al. Chronic stress contributes to cognitive dysfunction and hippocampal metabolic abnormalities in APP/PS1 mice. Cell Physiol Biochem. 2017;41(5):1766–1776. [DOI] [PubMed] [Google Scholar]
- 114. Lupien SJ, Gaudreau S, Tchiteya BM, et al. Stress-induced declarative memory impairment in healthy elderly subjects: relationship to cortisol reactivity. J Clin Endocrinol Metab. 1997;82(7):2070–2075. [DOI] [PubMed] [Google Scholar]
- 115. Souza-Talarico JN, Chaves EC, Lupien SJ, Nitrini R, Caramelli P. Relationship between cortisol levels and memory performance may be modulated by the presence or absence of cognitive impairment: evidence from healthy elderly, mild cognitive impairment and Alzheimer’s disease subjects. J Alzheimers Dis. 2010;19(3):839–848. [DOI] [PubMed] [Google Scholar]
- 116. Souza-Talarico JN, Caramelli P, Nitrini R, Chaves EC. Effect of cortisol levels on working memory performance in elderly subjects with Alzheimer’s disease. Arq Neuropsiquiatr. 2008;66(3B):619–624. [DOI] [PubMed] [Google Scholar]
- 117. Whitwell JL, Przybelski SA, Weigand SD, et al. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain. 2007;130(Pt 7):1777–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. McGaugh JL. Memory—a century of consolidation. Science. 2000;287(5451):248–251. [DOI] [PubMed] [Google Scholar]
- 119. Buchanan TW, Tranel D, Adolphs R. Impaired memory retrieval correlates with individual differences in cortisol response but not autonomic response. Learn Mem. 2006;13(3):382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Buchanan TW, Tranel D. Stress and emotional memory retrieval: effects of sex and cortisol response. Neuro Biol Learn Mem. 2008;89(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Het S, Ramlow G, Wolf OT. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 2005;30(8):771–784. [DOI] [PubMed] [Google Scholar]
- 122. Maheu FS, Joober R, Beaulieu S, Lupien SJ. Differential effects of adrenergic and corticosteroid hormonal systems on human short- and long-term declarative memory for emotionally arousing material. Behav Neurosci. 2004;118(2):420–428. [DOI] [PubMed] [Google Scholar]
- 123. Rimmele U, Meier F, Lange T, Born J. Suppressing the morning rise in cortisol impairs free recall. Learn Mem. 2010;17(4):186–190. [DOI] [PubMed] [Google Scholar]
- 124. Lupien SJ, Buss C, Schramek TE, Maheu F, Pruessner J. Hormetic influence of glucocorticoids on human memory. Nonlinearity Biol Toxicol Med. 2005;3(1):23–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Meyer U, van Kampen M, Isovich E, Flugge G, Fuchs E. Chronic psychosocial stress regulates the expression of both GR and MR mRNA in the hippocampal formation of tree shrews. Hippocampus. 2001;11(3):329–336. [DOI] [PubMed] [Google Scholar]
- 126. Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuro Psychopharmacol Biol Psychiatry. 2012;39(1):112–119. [DOI] [PubMed] [Google Scholar]
- 127. Patel PD, Katz M, Karssen AM, Lyons DM. Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology. 2008;33(3):360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Cole TJ, Blendy JA, Monaghan AP, et al. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9(13):1608–1621. [DOI] [PubMed] [Google Scholar]
- 129. Wei Q, Hebda-Bauer EK, Pletsch A, et al. Overexpressing the glucocorticoid receptor in forebrain causes an aging-like neuroendocrine phenotype and mild cognitive dysfunction. J Neurosci. 2007;27(33):8836–8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Dauvermann MR, Lee G, Dawson N. Glutamatergic regulation of cognition and functional brain connectivity: insights from pharmacological, genetic and translational schizophrenia research. Br J Pharmacol. 2017;174(19):3136–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Talmi M, Carlier E, Bengelloun W, Soumireu-Mourat B. Chronic RU486 treatment reduces age-related alterations of mouse hippocampal function. Neurobiol Aging. 1996;17(1):9–14. [DOI] [PubMed] [Google Scholar]
- 132. Bloss EB, Janssen WG, McEwen BS, Morrison JH. Interactive effects of stress and aging on structural plasticity in the prefrontal cortex. J Neurosci. 2010;30(19):6726–6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Eldridge JC, Brodish A, Kute TE, Landfield PW. Apparent age-related resistance of type II hippocampal corticosteroid receptors to down-regulation during chronic escape training. J Neurosci. 1989;9(9):3237–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav Neurosci. 1992;106(1):62–71. [DOI] [PubMed] [Google Scholar]
- 135. Yau JL, Noble J, Seckl JR. Continuous blockade of brain mineralocorticoid receptors impairs spatial learning in rats. Neurosci Lett. 1999;277(1):45–48. [DOI] [PubMed] [Google Scholar]
- 136. Bamberger CM, Chrousos GP. The glucocorticoid receptor and RU 486 in man. Ann N Y Acad Sci. 1995;761:296–310. [DOI] [PubMed] [Google Scholar]
- 137. Akil H. Stressed and depressed. Nat Med. 2005;11(2):116–118. [DOI] [PubMed] [Google Scholar]
- 138. Sandeep TC, Yau JL, Maclullich AM, et al. 11Beta-hydroxysteroid dehydrogenase inhibition improves cognitive function in healthy elderly men and type 2 diabetics. Proc Natl Acad Sci U S A. 2004;101(17):6734–6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. [DOI] [PubMed] [Google Scholar]
- 140. Bornstein SR, Engeland WC, Ehrhart-Bornstein M, Herman JP. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Meta. 2008;19(5):175–180. [DOI] [PubMed] [Google Scholar]
- 141. Michaud K, Matheson K, Kelly O, Anisman H. Impact of stressors in a natural context on release of cortisol in healthy adult humans: a meta-analysis. Stress. 2008;11(3):177–197. [DOI] [PubMed] [Google Scholar]
- 142. Martisova E, Solas M, Gerenu G, Milagro FI, Campion J, Ramirez MJ. Mechanisms involved in BACE upregulation associated to stress. Curr Alzheimer Res. 2012;9(7):822–829. [DOI] [PubMed] [Google Scholar]
- 143. Rahhal TA, Colcombe SJ, Hasher L. Instructional manipulations and age differences in memory: now you see them, now you don’t. Psychol Aging. 2001;16(4):697–706. [DOI] [PubMed] [Google Scholar]
- 144. Hasher L, Zacks RT, Rahhal TA. Timing, instructions, and inhibitory control: some missing factors in the age and memory debate. Gerontology. 1999;45(6):355–357. [DOI] [PubMed] [Google Scholar]
- 145. Lazarus RS, Folkman S. Stress, Appraisal, and Coping. Springer; 1984. [Google Scholar]
- 146. Folkman S, Lazarus RS, Dunkel-Schetter C, Delongis A, Gruen RJ. Dynamics of a stressful encounter: cognitive appraisal, coping, and encounter outcomes. J Pers Soc Psychol. 1986;50(5):992–1003. [DOI] [PubMed] [Google Scholar]
- 147. Henry JP. Biological basis of the stress response. Integr Physiol Behav Sci. 1992;27(1):66–83. [DOI] [PubMed] [Google Scholar]
- 148. Oyebode JR, Motala JR, Hardy RM, Oliver C. Coping with challenges to memory in people with mild to moderate Alzheimer’s disease: observation of behaviour in response to analogues of everyday situations. Aging Ment Health. 2009;13(1):46–53. [DOI] [PubMed] [Google Scholar]
- 149. Nolan K. The Dynamics of Dementia: Working Together, Working Separately or Working Alone? McGraw-Hill; 2003. [Google Scholar]
- 150. Sarnoff I, Zimbardo PG. Anxiety, fear, and social affiliation. J Abnorm Soc Psychol. 1961;62:356–363. [DOI] [PubMed] [Google Scholar]
- 151. Bahro M, Silber E, Sunderland T. How do patients with Alzheimer’s disease cope with their illness? A clinical experience report. J Am Geriatr Soc. 1995;43(1):41–46. [DOI] [PubMed] [Google Scholar]
- 152. Gillies BA. A memory like clockwork: accounts of living through dementia. Aging Mental Health. 2000;4(4):366–374. [Google Scholar]
- 153. Vaernes R, Ursin H, Darragh A, Lambe R. Endocrine response patterns and psychological correlates. J Psychosom Res. 1982;26(2):123–131. [DOI] [PubMed] [Google Scholar]
- 154. Debeer E, Raes F, Claes S, Vrieze E, Williams JM, Hermans D. Relationship between cognitive avoidant coping and changes in over general autobiographical memory retrieval following an acute stressor. J Behav Ther Exp Psychiatry. 2012;43(Suppl 1):S37–S42. [DOI] [PubMed] [Google Scholar]
- 155. Hermans D, de Decker A, de Peuter S, Raes F, Eelen P, Williams JM. Autobiographical memory specificity and affect regulation: coping with a negative life event. Depress Anxiety. 2008;25(9):787–792. [DOI] [PubMed] [Google Scholar]
- 156. Raes F, Hermans D, de Decker A, Eelen P, Williams JM. Autobiographical memory specificity and affect regulation: an experimental approach. Emotion. 2003;3(2):201–206. [DOI] [PubMed] [Google Scholar]
- 157. Benus RF, Bohus B, Koolhaas JM, van Oortmerssen GA. Behavioural strategies of aggressive and non-aggressive male mice in active shock avoidance. Behav Processes. 1989;20(1-3):1–12. [DOI] [PubMed] [Google Scholar]
- 158. Benus RF, Bohus B, Koolhaas JM, van Oortmerssen GA. Behavioural differences between artificially selected aggressive and non-aggressive mice: response to apomorphine. Behav Brain Res. 1991;43(2):203–208. [DOI] [PubMed] [Google Scholar]
- 159. Benus RF, Bohus B, Koolhaas JM, van Oortmerssen GA. Heritable variation for aggression as a reflection of individual coping strategies. Experientia. 1991;47(10):1008–1019. [DOI] [PubMed] [Google Scholar]
- 160. Sluyter F, Korte SM, Bohus B, Van Oortmerssen GA. Behavioral stress response of genetically selected aggressive and nonaggressive wild house mice in the shock-probe/defensive burying test. Pharmacol Biochem Behav. 1996;54(1):113–116. [DOI] [PubMed] [Google Scholar]
- 161. Veenema AH, Meijer OC, de Kloet ER, Koolhaas JM. Genetic selection for coping style predicts stressor susceptibility. J Neuro Endocrinol. 2003;15(3):256–267. [DOI] [PubMed] [Google Scholar]
- 162. Lehmann C. Why personality traits in the teen years may protect against dementia decades later. Neurology Today. 2019;19(24):10–11. [Google Scholar]
- 163. Bolger N, Zuckerman A. A framework for studying personality in the stress process. J Pers Soc Psychol. 1995;69(5):890–902. [DOI] [PubMed] [Google Scholar]
- 164. Watson D, Clark LA. Negative affectivity: the disposition to experience aversive emotional states. Psychol Bull. 1984;96(3):465–490. [PubMed] [Google Scholar]
- 165. McIlvane JM, Popa MA, Robinson B, Houseweart K, Haley WE. Perceptions of illness, coping, and well-being in persons with mild cognitive impairment and their care partners. Alzheimer Dis Assoc Disord. 2008;22(3):284–292. [DOI] [PubMed] [Google Scholar]
- 166. Houghton PJ, Howes MJ. Natural products and derivatives affecting neurotransmission relevant to Alzheimer’s and Parkinson’s disease. Neuro Signals. 2005;14(1-2):6–22. [DOI] [PubMed] [Google Scholar]
- 167. McDougall GJ. Memory improvement in assisted living elders. Issues Ment Health Nurs. 2000;21(2):217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Zhang C, Kuo CC, Moghadam SH, et al. Corticotropin-releasing factor receptor-1 antagonism mitigates beta amyloid pathology and cognitive and synaptic deficits in a mouse model of Alzheimer’s disease. Alzheimers Dement. 2016;12(5):527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Morgan SA, McCabe EL, Gathercole LL, et al. 11beta-HSD1 is the major regulator of the tissue-specific effects of circulating glucocorticoid excess. Proc Natl Acad Sci U S A. 2014;111(24):E2482–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Kim EY, Mahmoud GS, Grover LM. REM sleep deprivation inhibits LTP in vivo in area CA1 of rat hippocampus. Neurosci Lett. 2005;388(3):163–167. [DOI] [PubMed] [Google Scholar]
- 171. Sarabdjitsingh RA, Zhou M, Yau JL, et al. Inhibiting 11beta-hydroxysteroid dehydrogenase type 1 prevents stress effects on hippocampal synaptic plasticity and impairs contextual fear conditioning. Neuropharmacology. 2014;81:231–236. [DOI] [PubMed] [Google Scholar]
- 172. Holmes MC, Seckl JR. The role of 11beta-hydroxysteroid dehydrogenases in the brain. Mol Cell Endocrinol. 2006;248(1-2):9–14. [DOI] [PubMed] [Google Scholar]
- 173. Sooy K, Noble J, McBride A, et al. Cognitive and disease-modifying effects of 11beta-hydroxysteroid dehydrogenase type 1 inhibition in male Tg2576 mice, a model of Alzheimer’s disease. Endocrinology. 2015;156(12):4592–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Luo Y, Zeng B, Zeng L, et al. Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the hippocampus. Transl Psychiatry. 2018;8(1):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574–581. [DOI] [PubMed] [Google Scholar]
- 176. Misra S, Medhi B. Role of probiotics as memory enhancer. Indian J Pharmacol. 2013;45(3):311–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Rezaei Asl Z, Sepehri G, Salami M. Probiotic treatment improves the impaired spatial cognitive performance and restores synaptic plasticity in an animal model of Alzheimer’s disease. Behav Brain Res. 2019;376:112183. [DOI] [PubMed] [Google Scholar]
- 178. Eutamene H, Lamine F, Chabo C, et al. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J Nutr. 2007;137(8):1901–1907. [DOI] [PubMed] [Google Scholar]
- 179. Pusceddu MM, Kelly P, Ariffin N, Cryan JF, Clarke G, Dinan TG. N-3 PUFAs have beneficial effects on anxiety and cognition in female rats: effects of early life stress. Psychoneuroendocrinology. 2015;58:79–90. [DOI] [PubMed] [Google Scholar]
- 180. Provensi G, Schmidt SD, Boehme M, et al. Preventing adolescent stress-induced cognitive and microbiome changes by diet. Proc Natl Acad Sci U S A. 2019;116(19):9644–9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Hoeijmakers L, Ruigrok SR, Amelianchik A, et al. Early-life stress lastingly alters the neuroinflammatory response to amyloid pathology in an Alzheimer’s disease mouse model. Brain Behav Immun. 2017;63:160–175. [DOI] [PubMed] [Google Scholar]