Abstract
Lifestyle factors may individually protect against the development of mild cognitive impairment. We investigate the relationships between both self-reported physical activity and measured physical function with cognition in a population of elderly adults, more than half of whom follow vegetarian dietary patterns. Otherwise healthy adults (n = 127, mean age 74.9 ± 7.9 years, 61.3% current vegetarians) were assessed using a comprehensive neuropsychological battery. A principal components analysis derived processing speed, executive function, and memory/language factors. Participants reported current levels of vigorous physical activity on questionnaires, and physical function and mobility were measured with the Physical Performance Test (PPT) and Timed Up and Go (TUG) Test. Generalized linear models estimated β coefficients for cross-sectional associations between cognitive factors and indicators of physical abilities and self-reported physical activity. Better physical function indicated by PPT was associated with higher scores on the processing speed factor (β = 0.21 SDs for each 4.4-point increase in PPT score; p = 0.02). Faster TUG times were also associated with higher processing speed factor scores (β = 0.21 SDs increase for each 2.8 second less TUG time; p = 0.02). Self-reported levels of vigorous physical activity were not associated with any area of cognitive function; the association between PPT, TUG and processing speed was independent of physical activity. Associations between PPT and TUG and processing speed were stronger among participants who followed vegetarian dietary patterns. Better physical function may have an effect on cognition in a context of healthy lifestyles.
Keywords: MCI, physical performance test, timed up and go test, physical activity, memory, physical function
Significance
Among elderly adults who adhere to vegetarian diets, better physical functioning is related to better cognitive function. The notion that a “comprehensive” healthy lifestyle, encompassing more than one domain of health-promoting behaviors, should be further investigated.
Introduction
Mild cognitive impairment (MCI), a heterogeneous intermediary spectrum of cognitive dysfunction between normal cognition and dementia, 1,2 affects 16-20% of US adults aged 60 and older, with prevalence estimates varying based on diagnostic criteria used and how criteria are operationalized. 1,3 -6 Individuals with MCI experience deficits in memory and or other cognitive domains beyond normal age-associated cognitive changes. While those deficits are not severe enough to significantly impact their ability to function independently as with dementia, 2,7,8 individuals with MCI are at a higher risk for Alzheimer’s disease (AD), 9 and progress to dementia at a rate of 10-15% per year. 1,4,10 As such, cognitively impaired persons require nursing home care at twice the rate of cognitively intact persons and incur significantly greater healthcare costs. 11,12 Despite the fact that age increases the risk of MCI, substantial cognitive decline is not believed to be an inevitable consequence of aging, but instead reflects underlying diseases or conditions. 4,13 While substantial investment in drug development has been made, no medication is currently available to effectively treat the pathogenic substrates thought to underlie MCI. Thus, identifying alternative approaches to prevent or delay the onset of cognitive impairment (and dementia) is of significant interest.
A number of population-based observational and experimental studies suggest that in addition to greater education, lifestyle factors may individually protect against the development of MCI, including participating in cognitively stimulating activities, being physically active, consuming a diet high in mono- and poly-unsaturated fatty acids and following the Mediterranean diet. 14 -23 Diet is strongly associated with cardiovascular disease (CVD), 24 -27 and CVD in turn is associated with cognitive dysfunction. 28 Having a physically active lifestyle which could lead to greater physical fitness, may protect the aging brain by improving cerebral blood flow, increasing cortical and hippocampal volumes, and improving synaptic plasticity and neurogenesis. 29 -31 Few studies have examined the effect of more than one “healthy” lifestyle factors on cognitive function in the elderly, such as the combination of a healthy diet and physical activity. 32,33 Furthermore, studies have utilized self-reports of physical activity and exercise, 17 -19,34 and others have included evaluations of physical function, 35,36 attributes that indicate the ability to perform everyday physical tasks. 37 While these two factors (frequency of activity/exercise and functional abilities) are related, they nevertheless represent distinct constructs that may be associated with cognitive function through different mechanisms. Physical activity has been shown to improve cerebrovascular health which, in turn, can lead to improved cognitive function, 29 -31 whereas performance on tasks of physical function may, at least in part, reflect efficiency of central nervous system networks involved in completing activities of daily living. 38 Thus, we examine cross-sectional associations between both self-reported physical activity and measured physical function and different areas of cognition and memory impairment in a population of elderly adults for whom more than half follow vegetarian dietary patterns.
Materials and Methods
Study Population: Adventist Health Study-2 (AHS-2) Cohort
The AHS-2 is a prospective cohort study of over 96,000 Seventh-day Adventists in the US and Canada, which was originally established in 2002 to investigate the role of various foods and nutrients, lifestyle factors and metabolic risk indicators in cancer causation. During 2002-2007, Caucasian (65.3%) and African-American (26.9%) adult men and women with a mean age of 59 years (range 30-110 years) were enrolled in the AHS-2 cohort, and completed a 50-page baseline questionnaire which included sections on demographic, dietary, anthropometric and lifestyle factors. 39 Participants are mailed annual study newsletters and followed-up with biennial questionnaires. The cohort is healthy: at baseline, high proportions reported being in excellent health; 45% of cohort members follow vegetarian diets, 40 the non-vegetarians consume relatively lower amounts of meat compared to the general population, 40,41 only 1.1% are current smokers and 6.6% currently drink alcohol. 39 Since the cohort has aged and the majority of the cohort is elderly, the AHS-2 presents an opportunity to study additional age-related (non-cancer) chronic diseases.
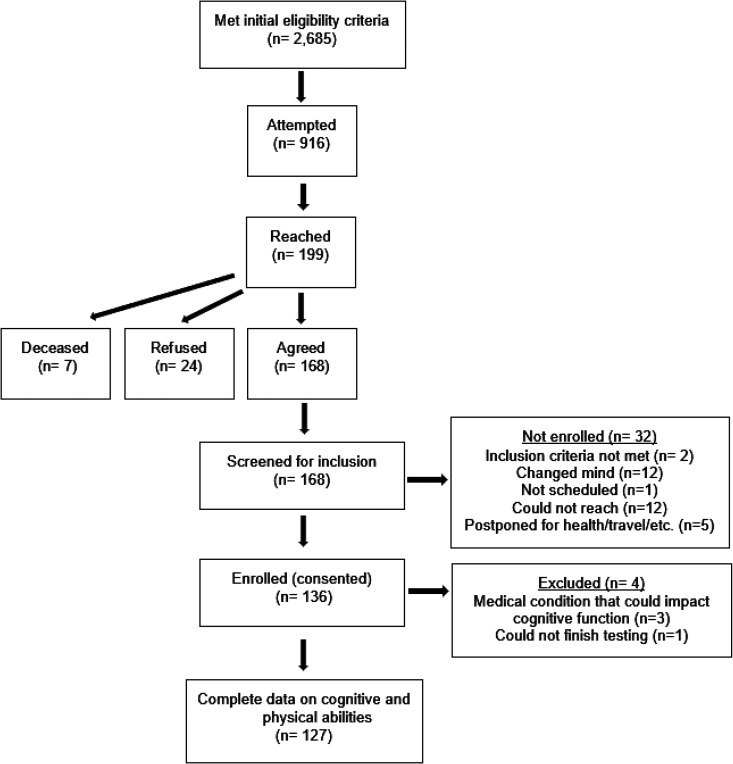
In 2016, we identified 2,685 members of the cohort for whom study records indicated were 60 years or older, community-dwelling and living within 75 miles of Loma Linda University (LLU). During 2016-2018, 199 were reached by telephone and invited to participate in the study. Of those, 168 (84%) agreed to participate and were screened for eligibility. Two did not meet inclusion criteria for being proficient in writing, speaking and understanding English. Twelve changed their mind, one could not be scheduled, 12 could not be reached again, and 5 postponed participation due to travel or health-related reasons. One hundred and thirty-five otherwise healthy adults were enrolled in the study and attended an in-person visit at our study clinic. Four were later excluded for having a medical condition that could adversely impact cognitive function. One hundred and twenty-seven participants had complete data on cognitive and physical abilities and were included in analyses (Figure 1).
Figure 1.
Flow of participants in the AHS-2 CAN GRASP study.
Data Collection
Participants completed a brief questionnaire designed to be consistent with previous AHS-2 follow-up surveys which included items on current medications, recent hospitalizations and regular levels of vigorous physical activity 42 over the past 12 months (“self-reported physical activity”). Physical activity items asked about the number of times per week and average minutes per time engaged in activities such as brisk walking, jogging and bicycling long enough or with enough intensity to work up a sweat, get the heart thumping or get out of breath, and were adopted from Paffenbarger Physical Activity Questionnaire (PPAQ) which has been previously validated in the AHS-2 and other populations. 43 -45 Usual levels of vigorous physical activity in metabolic equivalents (METs)-hours per week (continuous) were calculating multiplying the self-reported frequency and duration in these activities by intensity, following previous work. 46,47
Current level of physical function and mobility were measured during the clinic visit using the 9-item version of the Physical Performance Test (PPT) and Timed Up and Go (TUG) Test. The PPT assesses multiple domains of physical function including upper fine motor function, upper coarse motor function, balance, mobility, coordination and endurance using observed performance of tasks that simulate activities of daily living of various degrees of difficulty in elderly persons. 48 Participants are instructed to perform various tasks including writing a sentence, simulated eating, lifting a book onto a shelf above shoulder height, simulated dressing, picking up a penny from the floor, walking 50 feet, turning in a circle, climbing one flight of stairs and number of flights of stairs climbed. The time to complete each task is measured in seconds, and scores ranging from 0-4 are assigned based on completion times. A total score (maximum possible of 36) is calculated as the sum of individual scores from each task, with a higher score indicating better physical performance. Participants have 2 trials to complete each task, if needed, in which case the better of the 2 scores is used to calculate the total score. Assistive devices are permitted for the last 5 tasks. The Timed Up and Go (TUG) Test assesses a patient’s ability to rise from a seated position, walk 10 feet, turn and return to their seat. 49 Participants are allowed 2 trials, with only the second trial time recorded (if used). For the TUG, scoring is based on the time in seconds to complete the task, with a higher score indicative of poorer physical performance.
Baseline dietary pattern was determined using responses to a validated food frequency questionnaire (FFQ) that was self-administered at cohort enrollment. 50 Dietary habits were queried at the time of cognitive assessment through recall of frequency of consumption of 5 foods (meat, poultry, fish, eggs and dairy) for each decade from 10 years of age to present. Using participant’s age, current dietary habit (vegetarian or non-vegetarian) was determined from questionnaire responses. Vegetarians were defined as either (a) consuming meats, fish, and dairy <1 time/month (vegans), (b) consuming dairy ≥1 time/month and meats, and fish <1 time/month (lacto-ovo vegetarians), or (c) consuming fish ≥1 time/month, no limits on dairy, and meats <1 time/month (pesco-vegetarians).
At the in-person visit, participants were administered a 2-hour comprehensive neuropsychological battery, which included tests of verbal learning and memory [Rey Auditory Verbal Learning Test (RAVLT), and Logical Memory subtest of the Wechsler Memory Scale, 4th edition], attention (Digit Span subtest of the Wechsler Adult Intelligence Scale, 4th edition, WAIS-IV), processing speed (Coding subtest of the WAIS-IV, Stroop, Trail-Making Test, Cogstate), executive function (Stroop, Trail-Making Test, FAS), visuospatial abilities (Rey-O Complex Figure), language (Boston Naming Test, FAS, Animals), and global cognitive functioning [Montreal Cognitive Assessment (MoCA), Mini-Mental State Examination (MMSE) 51 ] by trained psychometrists. The American National Adult Reading Test (AMNART) 52 was administered as a measure of estimated premorbid verbal intelligence (VIQ), and the Geriatric Depression Scale (GDS) 53 to assess mood. Height was measured with a portable stadiometer and weight with a Tanita® scale. Body mass index (BMI) was calculated [weight (kg)/height (m2)] and categorized into normal or underweight (BMI ≤ 24.9), overweight (BMI = 25-29.9) or obese (BMI > 30).
Biospecimen Collection and Genotyping for ApoE
Approximately 8 ml of peripheral blood from each participant was drawn into BD Vacutainer® Mononuclear Cell Preparation Tubes (CPTs) with sodium citrate (REF 362761; Becton Dickinson, Franklin Lakes, NJ) by a phlebotomist following standard venipuncture procedures and manufacturer’s instruction. CPTs were delivered to and processed within 2 hours of the blood draw at the Center for Genomics at LLU following manufacturer’s protocols. Briefly, the CPTs were centrifuged at 1,300 RCF for 30 minutes at room temperature in a swing-bucket centrifuge. The bottom layer containing white blood cells (WBCs) was transferred into 15 ml conical tubes and washed with PBS by centrifugation at 300 RCF for 15 minutes. Cell pellets were treated with RBC Lysis Solution (Cat. 158902; Qiagen, Hilden, Germany) and washed again with PBS. WBCs were collected and examined on a TC10 automated cell counter (BioRad, Hercules, CA) for cell numbers and viabilities. WBC pellets were stored in −80°C freezers before DNA extraction.
Genomic DNA were extracted using the Qiagen All prep DNA/RNA/miRNA Universal kit (Qiagen, Hilden, Germany) from frozen human WBCs. DNA was quantified using Qubit 3.0 with dsDNA high sensitivity reagent (Life Technologies, Pleasanton, CA). Genotyping was carried out in 96-well plates using TaqMan Genotyping Master Mix and SNP probes (Life Technologies, Pleasanton, CA). qPCR was performed on the Applied Biosystems QuantStudio 7 Real Time PCR System according to manufacturer’s specifications and data was analyzed using the SDS2.4 software. Genotyping for 2 single nucleotide polymorphisms (rs429358 and rs7412) was used to determine the apolipoprotein E (ApoE) genotype (2/3; 3/3; 3/4; 4/4). For analyses, ApoE genotype was collapsed into 3 categories (2/3; 3/3; 2/4, 3/4 or 4/4).
The study was approved by the Institutional Review Board at Loma Linda University (Protocol# 5150240) and each participant provided written informed consent.
Statistical Analysis
For data reduction purposes, a principal component analysis was performed on the individual neuropsychological test scores using ones as prior communality estimates. The principal axis method was used to extract components employing a varimax (orthogonal) rotation. Nine eigenvalues were >1 which would have resulted in more components than was desirable in relation to our sample size. Visual examination of a scree plot led to a decision to retain the first 3 components, which accounted for 49% of the total variance. An item was considered to have loaded on a given component if the factor loading was greater than |0.40|. Using these criteria, 13 items loaded on the first factor which was named “processing speed”; 8 items loaded on the second factor “executive function” and 10 items loaded on the third factor “memory/language” (Table 1).
Table 1.
Rotated Factor Pattern From Principal Components Analysis.
| Neuropsychological battery item | Factor | ||
|---|---|---|---|
| Processing speed | Executive function | Memory/Language | |
| RAVLT—List A Trial 1 recall | −7 | 29 | 55* |
| RAVLT—Total recall Trials 1-5 | 13 | 18 | 78* |
| RAVLT—List A, short-delay recall | 19 | 6 | 79* |
| RAVLT—List A, long-delay recall | 8 | 9 | 75* |
| Logical Memory I, Total Recall | 3 | 18 | 78* |
| Logical Memory II, Total Recall | 3 | 12 | 82* |
| Logical Memory II, Recognition Total | 3 | 13 | 74* |
| Rey—Osterrieth, Copy | 15 | 27 | 11 |
| Rey—Osterrieth, 3-minute recall | 15 | 33 | 41* |
| Boston Naming Test | 9 | 37 | 42* |
| FAS, Total | 18 | 49* | 16 |
| Animals, Total | 33 | 18 | 56* |
| Stroop Word, Total Correct | 57* | 35 | 7 |
| Stroop Color, Total Correct | 70* | 26 | 10 |
| Stroop Color-Word, Total Correct | 64* | 31 | 12 |
| Trail Making Test A, completion time | 60* | 10 | 14 |
| Trail Making Test B, completion time | 56* | 33 | 35 |
| Coding, Total | 67* | 14 | 13 |
| Digit Span—Longest sequence forward | −5 | 79* | 8 |
| Digit Span—Longest sequence backward | 14 | 73* | 25 |
| Digit Span—Longest sequence sequencing | 11 | 68* | 16 |
| Digit Span Forward (raw score) | −2 | 83* | 10 |
| Digit Span Backward (raw score) | 15 | 78* | 18 |
| Digit Span Sequencing (raw score) | 17 | 77* | 17 |
| Digit Span Total Raw Score | 12 | 96* | 18 |
| CogState—Detection, reaction time (mean) | 54* | −10 | −9 |
| CogState—Identification, reaction time (mean) | 78* | 6 | −4 |
| CogState—One-Back, reaction time (mean) | 81* | 9 | −3 |
| CogState—Two-Back, reaction time (mean) | 71* | 5 | 5 |
| CogState—Set-Shifting, reaction time (mean) | 59* | −11 | 6 |
| CogState—Detection, accuracy | 46* | 6 | 30 |
| CogState—Identification, accuracy | 23 | 24 | 26 |
| CogState—One-Back, accuracy | 40 | 15 | 25 |
| CogState—Two-Back, accuracy | 48* | 23 | 39 |
| CogState—Set-Shifting, accuracy | 32 | 10 | 27 |
* Indicates a factor loading >|0.40|.
In order to examine diagnostic risk associated with physical function, participants were classified as having mild memory impairment if at least one of their scores on tests of memory (RAVLT and Logical Memory immediate and long delayed recalls) were less than 1.5 standard deviations below population-based normative means (n = 25, 19.7%). 54,55 We focused specifically on memory impairment, and did not attempt to classify participants as impaired along other cognitive domains because amnestic MCI in particular (as opposed to non-amnestic forms of MCI) has been identified as a precursor to AD. 5
Descriptive statistics for participants were summarized and correlation coefficients were calculated to assess correlations between variables. Generalized linear models were used to estimate β coefficients for cross-sectional associations between cognitive factors and measures of physical function and mobility (PPT and TUG), and of self-reported vigorous physical activity (questionnaire-based). For models of cognitive factors, β coefficients were expressed in standard deviation (SD) units above or below the mean score. If we found an association with a cognitive factor, we further examined whether individual tests that contributed to the factor were driving the association. For models of individual cognitive tests, β coefficients were unit increases or decreases in test scores. Logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (95% CI) for mild memory impairment. We considered whether covariates including age (years), sex, race (white, non-white), education (years), BMI, mood (GDS score), and ApoE genotype were confounders of these associations, initially by examining associations of these variables with the cognitive factors and then by comparing β coefficients from models of cognition and physical functioning and activity with and without the inclusion of these variables. We retained those variables in multivariable models that were either univariately associated with cognition and or that led to changes in β coefficient by > 10%. We also examined whether the association between physical function and cognition was independent of physical activity by comparing models with and without adjustment for self-reported vigorous physical activity. If there was an association between individual cognitive factors and physical function and activity, we further investigated effect modification by age, sex, physical activity (for models with physical function) and dietary pattern testing interaction terms in regression models. If there was evidence of a statistically significant interaction (p < 0.10), we conducted stratified analyses by categories of the effect modifying variable. For interpretation, continuous variables of BMI, GDS, PPT, TUG, and self-reported vigorous physical activity in MET-hours per week were centered on their standard deviation.
All analyses used SAS version 9.4 (SAS Institute Inc., Cary, N.C., USA).
Results
Study participants ranged in age from 60-92 years (mean age 74.8 ± 7.9 years), were predominantly white and the majority were female (Table 2). At baseline/enrollment in the cohort, 53.5% of participants followed vegetarian dietary patterns; of those who completed questionnaire items on dietary habits, 60.9% were vegetarian at the time of cognitive assessment. Vegetarians were less likely to be overweight or obese and had higher scores on the GDS than non-vegetarians, otherwise were comparable in characteristics (Supplemental Table 1). The average BMI was 27.1 (± 5.5), and 40.2% of participants were of normal weight or were underweight. Mean scores on the PPT and TUG were comparable with published values for populations of other community-dwelling adults. 56 -58 Participants were well-educated and their estimated VIQ indicated high level of premorbid intelligence; 19.7% were categorized as having mild memory impairment, based on impairment in at least 1 of 4 memory scores. Average GDS scores (3.0 ± 3.1) indicated that participants did not exhibit significant depressive symptoms. 53,59
Table 2.
Characteristics of Study Participants (n = 127).
| Characteristic | Mean ± SD or n (%) |
|---|---|
| Age, years | 74.9 ± 7.9, range 60-92 |
| Sex | |
| Male | 54 (42.5) |
| Female | 73 (57.5) |
| Race | |
| White | 101 (79.5) |
| Non-White | 26 (20.5) |
| Education, years | 16.7 ± 2.5 |
| MMSE | 29.1 ± 1.2 |
| MOCA | 25.2 ± 3.2 |
| GDS | 3.0 ± 3.1 |
| AMNART IQ | 118.6 ± 7.4 |
| Mild memory impairment, yes | 25 (19.7) |
| Vegetarian diet | |
| at baseline | 68 (53.5) |
| at cognitive assessment† | 67 (60.9) |
| BMI | 27.1 ± 5.5 |
| normal, underweight (BMI ≤24.9) | 51 (40.2) |
| overweight (BMI 25-29.9) | 45 (35.4) |
| obese (BMI > 30) | 31 (24.4) |
| ApoE genotype* | |
| 2/3 | 14 (12.6) |
| 3/3 | 66 (59.5) |
| 2/4, 3/4 or 4/4 | 31 (27.9) |
| PPT total (range) | 26.6 ± 4.4 (13-34) |
| TUG time (sec) (range) | 10.4 ± 2.8 (5.3-22.4) |
| Vigorous activity, MET-hours per week (range)‡ | 9.6 ± 9.4 (0-31.7) |
† n = 113.
* n = 100.
‡Metabolic equivalents.
Scores on the PPT were strongly inversely correlated with TUG time (r = −0.82; p < 0.0001). Self-reported levels of vigorous physical activity were weakly correlated with PPT scores (r = 0.14; p = 0.11) and modestly with TUG time (r = −0.29; p = 0.001). Age, BMI and GDS fit criteria as confounding variables and were therefore retained in multivariable models of measured physical abilities.
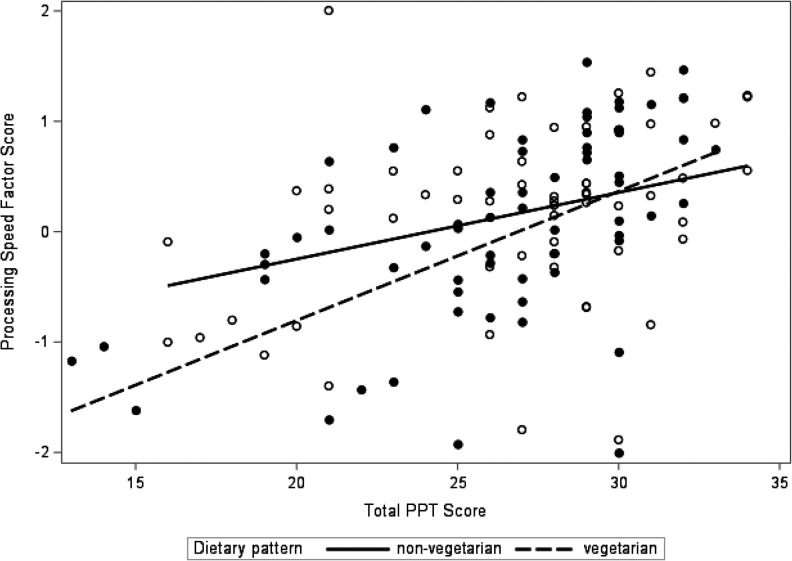
Better physical performance indicated by the PPT was associated with higher scores on the cognitive processing speed factor (β = 0.21 SDs for each 4.4-point increase in PPT; adjusted for age, BMI and GDS); p = 0.02) (Model 2, Table 3A). This association appeared to be driven by individual tests that loaded heavily on this factor, with better scores observed for Stroop Word (β = 0.64, [SE(β) = 0.33]; p = 0.05), and Coding (β = 0.65, [SE(β) = 0.31]; p = 0.04). Higher PPT scores were also associated with faster reaction times on the Cogstate Identification task (IDN) (β = 6.56, [SE(β) = 2.36]; p = 0.006). The relationship between PPT and processing speed was independent of self-reported level of vigorous physical activity (Model 3, Table 3A). Tests for interaction terms for PPT by age, sex and self-reported vigorous physical activity were not statistically significant (all p-values > 0.10), however the interaction with vegetarian dietary pattern was (p-value = 0.03). Models stratified by dietary pattern suggested that the association between PPT and cognitive processing speed was stronger among vegetarians (β = 0.38 [SE(β) = 0.14]; p = 0.008) than non-vegetarians (β = 0.10 [SE(β) = 0.12]; p = 0.44) with adjustment for age, BMI, GDS and self-reported vigorous physical activity (Model 4; Table 3A; Figure 2). Compared to non-vegetarians with PPT scores at or below the mean, vegetarians with PPT scores above the mean did better on cognitive processing speed tasks but these results did not achieve statistical significance (β = 0.18 [SE(β) = 0.26]; p = 0.49).
Table 3.
A and B, Results From Regression Models of Associations (β [SE (β)]; p-Value) Between Measured Physical Abilities, Self-Reported Vigorous Physical Activity, and Processing Speed Factor.
| Variable | Model 1: Crude | Model 2: Adjusted for age, BMI, GDS | Model 3: Model 2 + Vigorous activity | Model 4: Stratified by dietary pattern | |
|---|---|---|---|---|---|
| Vegetarian | Non-Vegetarian | ||||
| Physical abilities and self-reported vigorous physical activity | |||||
| PPT (each 4.4-point increase in total score) | 0.40 (0.08); p < 0.0001 | 0.21 (0.09); p = 0.02 | 0.25 (0.09); p = 0.009 | 0.38 (0.14); p = 0.008 | 0.10 (0.12) p = 0.44 |
| Vigorous activity (per SD MET-hours per week)* | −0.04 (0.09); p = 0.63 | −0.06 (0.08); p = 0.45 | 0.02 (0.13); p = 0.86 | −0.16 (0.10) p = 0.12 | |
| Covariates | |||||
| Age, years | −0.07 (0.01); p < 0.0001 | −0.05 (0.01); p < 0.0001 | −0.05 (0.01); p < 0.0001 | −0.06 (0.02); p = 0.001 | −0.04 (0.01); p = 0.01 |
| Sex | |||||
| Female | ref | ||||
| Male | 0.08 (0.18); p = 0.64 | - | - | - | - |
| Race | |||||
| White | ref | ||||
| Non-White | −0.17 (0.22); p = 0.44 | - | - | - | - |
| Education, years | 0.02 (0.04); p = 0.57 | - | - | - | - |
| GDS† (per SD) | −0.17 (0.09); p = 0.06 | −0.14 (0.08); p = 0.07 | -0.13 (0.08); p = 0.09 | −0.11 (0.10); p = 0.30 | −0.27 (0.14); p = 0.05 |
| Vegetarian diet at baseline | |||||
| No | ref | ||||
| Yes | −0.23 (0.18); p = 0.20 | - | - | - | - |
| BMI (per SD) | −0.04 (0.09); p = 0.69 | 0.05 (0.08); p = 0.55 | 0.05 (0.09); p = 0.56 | 0.06 (0.13); p = 0.62 | 0.02 (0.11); p = 0.87 |
| ApoE genotype‡ | |||||
| 2/3 | ref | ||||
| 3/3 | −0.03 (0.29); p = 0.92 | - | - | - | - |
| 2/4, 3/4 or 4/4 | −0.23 (0.32); p = 0.47 | - | - | - | - |
| Variable | Model 1: Crude | Model 2: Adjusted for age, BMI, GDS | Model 3: Model 2 + Vigorous activity | Model 4: Stratified by dietary pattern | |
| Vegetarian | Non-Vegetarian | ||||
| Physical abilities and self-reported vigorous physical activity | |||||
| TUG time (each 2.8 secs lower) | 0.39 (0.08); p < 0.0001 | 0.21 (0.09); p = 0.02 | 0.27 (0.09); p = 0.006 | 0.35 (0.15); p = 0.02 | 0.14 (0.11); p = 0.23 |
|
Vigorous activity (per SD MET-hours per week)* |
−0.04 (0.09); p = 0.63 | −0.11 (0.08); p = 0.20 | −0.08 (0.14); p = 0.58 | −0.17 (0.10); p = 0.10 | |
| Covariates | |||||
| Age, years | −0.07 (0.01); p < 0.0001 | −0.06 (0.01); p < 0.0001 | −0.05 (0.01); p < 0.0001 | −0.06 (0.02); p = 0.0009 | −0.04 (0.01); p = 0.005 |
| Sex | |||||
| Female | ref | ||||
| Male | 0.08 (0.18); p = 0.64 | ||||
| Race | |||||
| White | ref | ||||
| Non-White | −0.17 (0.22); p = 0.44 | ||||
| Education, years | 0.02 (0.04); p = 0.57 | ||||
| GDS† (per SD) | −0.17 (0.09); p = 0.06 | −0.14 (0.08); p = 0.06 | −0.15 (0.08); p = 0.06 | −0.12 (0.11); p = 0.26 | −0.28 (0.13); p = 0.04 |
| Vegetarian diet at baseline | |||||
| No | ref | ||||
| Yes | −0.23 (0.18); p = 0.20 | ||||
| BMI (per SD) | −0.04 (0.09); p = 0.69 | 0.04 (0.08); p = 0.66 | 0.03 (0.08); p = 0.75 | 0.03 (0.13); p = 0.81 | 0.01 (0.10); p = 0.91 |
| ApoE genotype‡ | |||||
| 2/3 | ref | ||||
| 3/3 | −0.03 (0.29); p = 0.92 | ||||
| 2/4, 3/4 or 4/4 | −0.23 (0.32); p = 0.47 | ||||
* Metabolic equivalents; †GDS: Geriatric Depression Scale; ‡n = 100.
Figure 2.
Association between PPT and processing speed abilities by dietary pattern.
Faster times on the TUG test were also associated with higher scores on the cognitive processing speed factor (β = 0.21 SDs increase for each 2.8 seconds less time on the TUG; p = 0.02 adjusted for age, BMI and GDS) (Model 2, Table 3B). Faster reaction times on the Cogstate IDN (β = 26.8; [SE(β) = 10.2]; p = 0.01) appeared to be driving the association. Similar to PPT, the relationship between TUG and cognitive processing speed was independent of self-reported level of vigorous physical activity (Model 3, Table 3B). Tests for interaction terms for TUG by age, sex and self-reported vigorous physical activity were not statistically significant (all p-values > 0.10), however the p-value for interaction with vegetarian dietary pattern was 0.08. Stratified models also suggested that the association between TUG and cognitive processing speed was stronger among vegetarians (Table 3; Model 4). Compared to non-vegetarians with TUG times above the mean, vegetarians with TUG times at or below the mean did better on processing speed tasks but these results did not achieve statistical significance (β = 0.24 [SE(β) = 0.24]; p = 0.33).
Better physical functioning measured by the PPT and TUG was not associated with the memory/language or executive function factors (Supplementary Table 2).
Self-reported levels of vigorous physical activity were not associated with cognitive processing speed (β = −0.04 [SE(β) = 0.09] per SD MET-hours per week; p = 0.63) or the other 2 cognitive factors (Supplementary Table 2). Neither measure of physical function or self-reported physical activity was associated with a statistically significant higher odds of mild memory impairment (Supplementary Table 2).
Discussion
In this study of elderly community-dwelling adults who predominantly follow vegetarian dietary patterns, greater observed physical abilities, as measured by the PPT and TUG, were associated with better performance on cognitive tasks associated with cognitive processing speed. Self-reported vigorous physical activity, on the other hand, was not individually associated with cognitive function, and the relationship with measured physical function and processing speed was independent of physical activity. Our results also suggest that a vegetarian diet modified the association between physical functioning and better cognitive processing speed abilities.
Prior research supports relationships between measured physical abilities and cognitive function 14,60 and results from some studies are similar to our own. A study of 125 predominantly white non-cognitively impaired community-dwelling adults aged 75 years and older who did not engage in regular exercise, found that a cognitive speed factor derived from a factor analysis was independently associated with total modified PPT score. 36 The 6 individual tests that loaded on the cognitive speed factor were measures involving cognitive processing and psychomotor speed but requiring visual scanning, attention and learning. In that study, performance on memory tasks was not associated with PPT score. A large multi-center study of Canadian adults with a mean age of 63 years and negative histories of neurological diseases reported that 3 measures of mobility (walking speed, balance and chair stands) were each associated with processing speed, verbal learning and executive function. 61 In that study, age modified the mobility-cognition relationships, with associations generally being stronger with increasing age. Performance on the TUG was cross-sectionally associated with Stroop scores among community-dwelling middle-aged and older adults in China. 62 A small cross-sectional study of community-dwelling adults 55 years and older also found higher physical activity, measured with a wrist-worn accelerometer, was associated with better global cognitive function among individuals without MCI but not among participants with probable MCI. 15
Our study was small, thus not detecting associations with some of the cognitive measures, self-reported physical activity, or with mild memory impairment may have been a result of being underpowered. Moreover, our cohort was exceptionally healthy with a relatively low rate of overall cognitive impairment; even among those with mild memory impairment, more than half had an impaired score on only 1 of the 4 memory scores. Thus, a more heterogeneous sample with a wider range of impairments would be helpful for future studies. With cross-sectional data, we cannot infer the directionality or temporality of the associations that we detected. It could be that declines in cognitive function affect or precede physical declines, but we did not have baseline cognitive function with which to compare. We examined demographic, metabolic, psychological, genetic and lifestyle factors as potential confounders and adjusted for age, BMI and mood in our analyses. Other factors which we did not take into consideration such as comorbidities or medication use could confound the associations. Our sample size limited the number of variables we could include in models as confounders as well as our power to test for interactions. Our small sample size could have limited the detection of differences in associations by sex, since biological sex has been suggested as a possible mediator of relationships between exercise and brain health in aging. 60
Physical activity improves both cardiovascular and metabolic function, 35 and could potentially improve cognitive function through mechanistic changes at molecular, cellular and systemic levels. 30 Increases in cerebral blood flow and metabolism as would result from physical activity, could lead to the formation of neural networks 16 as well as more efficient neural activity 14 associated with frontal-executive abilities such as processing speed, verbal encoding and retrieval which we observed in our study. Physical activity may also increase brain neurotrophins and growth factors which could lead to greater synaptic plasticity and neurogenesis. 14,29 Physical activity may reduce against oxidative stress by upregulating endogenous antioxidant enzymes. 63,64 Physical activity may also help maintain brain volume and reduce atrophy. 65 In retrospect, given that our study population consisted of elderly adults, it may not have been appropriate to query vigorous physical activity, and we may have been better able to examine study hypotheses had we collected data on the frequency and duration of moderate activities as well.
The PPT is scored using timed measurements so that it may be administered with minimal interpretation or judgment by the observer. 48 Many of the cognitive measures that loaded heavily on the processing speed factor in our study are also timed measures. It is possible that the correlation between PPT and cognitive processing relates to the pace with which participants are able to complete both physical and cognitive tasks. However, despite the timed basis for scoring, the PPT was designed to measure several domains of functioning including upper body strength and dexterity and mobility so as to capture a range of abilities and abilities associated with activities of daily living. Moreover, some of the cognitive processing speed tasks did not involve motor, i.e. physical speed, but relied on mental speed, mainly perceptual and verbal speed. Thus, we would argue that performance on the cognitive processing speed tasks represents a different construct from the physical abilities measured by PPT, and that our findings demonstrate that better physical functioning is associated with faster cognitive processing speed.
The PPT and TUG are direct observations of physical function, and therefore an advantage of our study was that we were able to objectively quantify functional capabilities of our participants. While we asked participants about their physical activity, we did not solely rely on self-reports in our analyses. 48 Self-reports of physical activity in older adults may have low to moderate validity compared with objective assessments, 15,66 with studies suggesting that older adults may overestimate higher activity levels 67 and underestimate lower activity levels. 68 Furthermore, physical function represents another aspect of health distinct from level of physical activity, as it reflects the efficiency with which one can complete simple everyday tasks, and may be more closely tied to preservation of functional independence in older adults. 69 Among elderly, the PPT has been shown to have excellent reliability 48,70 and good to excellent validity with other tests of physical performance 71 but moderate correlations with self-reports of physical abilities on questionnaires. 72
Several studies generally find some protective effect of a Mediterranean diet on MCI incidence and prevalence, and on MCI conversion to AD. 72 -79 Similar to the “Mediterranean diet,” vegetarian dietary patterns are plant-based, yet typically incorporate even larger quantities of fruits and vegetables, and exclude consumption of some or all animal products. 80,81 As such, vegetarian diets are rich in phytochemicals and antioxidant micronutrients, 82 many of which may exert anti-inflammatory and antioxidant effects. 83,84 At enrollment in the AHS-2 cohort and 10 years prior to this study, 53.9% of study participants followed vegetarian dietary patterns. At the time of cognitive assessment, 61.3% of study participants were vegetarian. Our results thus support associations between physical function and cognition with additional modification by vegetarian dietary pattern as a lifestyle factor. The notion that a “comprehensive” healthy lifestyle, encompassing more than one domain of health-promoting behaviors, may prevent cognitive decline has been proposed. 32 The joint effect of diet and physical function and activity on preventing the risk of cognitive decline has received some support. 33,85 Additional studies should investigate potential common mechanisms underlying the relationship of different lifestyle-related behaviors with cognition. 86 The results of this study with others could support recommendations of behaviors which clinicians could encourage in their elderly patients.
Supplemental Material
Supplemental Material, Figure_Legends for Observed Physical Function Is Associated With Better Cognition Among Elderly Adults: The Adventist Health Study-2 by Nicole M. Gatto, Jennifer Garcia-Cano, Crissy Irani, Tiantian Liu, Cameron Arakaki, Gary Fraser, Charles Wang and Grace J. Lee in American Journal of Alzheimer"s Disease & Other Dementias
Supplemental Material, Supplemental_Material for Observed Physical Function Is Associated With Better Cognition Among Elderly Adults: The Adventist Health Study-2 by Nicole M. Gatto, Jennifer Garcia-Cano, Crissy Irani, Tiantian Liu, Cameron Arakaki, Gary Fraser, Charles Wang and Grace J. Lee in American Journal of Alzheimer"s Disease & Other Dementias
Abbreviations
- MCI
mild cognitive impairment
- PPT
Physical Performance Test
- TUG
Timed Up and Go Test
- AD
Alzheimer’s disease
- CVD
cardiovascular disease
- AHS-2
Adventist Health Study-2
- RAVLT
Rey Auditory Verbal Learning Test
- MoCA
Montreal Cognitive Assessment
- MMSE
Mini-Mental State Examination
- AMNART
American National Adult Reading Test
- VIQ
verbal intelligence
- GDS
Geriatric Depression Scale
- CPT
Cell Preparation Tubes
- WBC
white blood cell
- ApoE
apolipoprotein E
- BMI
body-mass index
- SD
standard deviation
- METs
metabolic equivalents
- OR
odds ratio
- 95% CI
95% confidence interval.
Footnotes
Authors’ Note: Authors contributed to this work as follows: formulating the research question(s): NG & GJL; designing the study: NMG, GJL, GF, CW; carrying it out: NMG, GJL, GF, CW, CA, CI, TL; analyzing the data: NMG, JGC, GJL; writing the article: NMG, GJL, GF, CW, JGC, CI, TL, CA. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the Institutional Review Board at Loma Linda University. Written informed consent was obtained from all subjects.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by GRASP Award 2140309 from Loma Linda University, Office of Research Affairs.
ORCID iD: Nicole M. Gatto  https://orcid.org/0000-0001-7873-8310
https://orcid.org/0000-0001-7873-8310
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Ger Med. 2013;29(4):753–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sachdev PS, Lipnicki DM, Kochan NA, et al. The prevalence of mild cognitive impairment in diverse geographical and ethnocultural regions: the COSMIC collaboration. PLoS One. 2015;10(11):e0142388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Larrea FA, Fisk JD, Graham JE, Stadnyk K. Prevalence of cognitive impairment and dementia as defined by neuropsychological test performance. Neuroepidemiology. 2000;19(3):121–129. [DOI] [PubMed] [Google Scholar]
- 4. DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol. 2003;2(1):15–21. [DOI] [PubMed] [Google Scholar]
- 5. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. [DOI] [PubMed] [Google Scholar]
- 6. Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ. Epidemiology of dementias and Alzheimer’s disease. Arch Med Res. 2012;43(8):600–608. [DOI] [PubMed] [Google Scholar]
- 7. Mufson EJ, Chen EY, Cochran EJ, Beckett LA, Bennett DA, Kordower JH. Entorhinal cortex beta-amyloid load in individuals with mild cognitive impairment. Exp Neurol. 1999;158(2):469–490. [DOI] [PubMed] [Google Scholar]
- 8. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59(10):1594–1599. [DOI] [PubMed] [Google Scholar]
- 10. Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75(10):889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coughlin TA, Liu K. Health care costs of older persons with cognitive impairments. Gerontologist. 1989;29(2):173–182. [DOI] [PubMed] [Google Scholar]
- 12. Mackin RS, Delucchi KL, Bennett RW, Arean PA. The effect of cognitive impairment on mental healthcare costs for individuals with severe psychiatric illness. Am J Geriatr Psychiatry. 2011;19(2):176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Evans DA, Funkenstein HH, Albert MS, et al. Prevalence of Alzheimer’s disease in a community population of older persons. Higher than previously reported. JAMA. 1989;262(18):2551–2556. [PubMed] [Google Scholar]
- 14. Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clinic Proc. 2011;86(9):876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Falck RS, Landry GJ, Best JR, Davis JC, Chiu BK, Liu-Ambrose T. Cross-sectional relationships of physical activity and sedentary behavior with cognitive function in older adults with probable mild cognitive impairment. Physical Therapy. 2017;97(10):975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frith E, Loprinzi PD. Physical activity is associated with higher cognitive function among adults at risk for Alzheimer’s disease. Compl Therap Med. 2018;36:46–49. [DOI] [PubMed] [Google Scholar]
- 17. Gagliardi C, Papa R, Postacchini D, Giuli C. Association between cognitive status and physical activity: study profile on baseline survey of the my mind project. Int J Environ Res Public Health. 2016;13(6):585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Howard EP, Morris JN, Steel K, et al. Short-term lifestyle strategies for sustaining cognitive status. BioMed Res Int. 2016;2016:7405748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lam LC, Ong PA, Dikot Y, et al. Intellectual and physical activities, but not social activities, are associated with better global cognition: a multi-site evaluation of the cognition and lifestyle activity study for seniors in Asia (CLASSA). Age Ageing. 2015;44(5):835–840. [DOI] [PubMed] [Google Scholar]
- 20. Sink KM, Espeland MA, Castro CM, et al. Effect of a 24-month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: the life randomized trial. JAMA. 2015;314(8):781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vidoni ED, Johnson DK, Morris JK, et al. Dose-response of aerobic exercise on cognition: a community-based, pilot randomized controlled trial. PLoS One. 2015;10(7):e0131647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Willey JZ, Gardener H, Caunca MR, et al. Leisure-time physical activity associates with cognitive decline: The Northern Manhattan study. Neurology. 2016;86(20):1897–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psych Med. 2010;72(3):239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heimburger DC. Nutrition’s interface with health and disease. In: Goldman L, Ausiello D, eds. Cecil Medicine. 24th ed. Saunders Elsevier; 2011. [Google Scholar]
- 25. Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82–96. [DOI] [PubMed] [Google Scholar]
- 26. Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115(11):1481–1501. [DOI] [PubMed] [Google Scholar]
- 27. Mozaffarian D. Nutrition and cardiovascular disease. In: Bonow RO, Mann DL, Zipes DP, Libby P, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Saunders Elsevier; 2011. [Google Scholar]
- 28. Rosamond W, Flegal K, Friday G, et al. Heart Disease and Stroke Statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–e171. [DOI] [PubMed] [Google Scholar]
- 29. Cass SP. Alzheimer’s disease and exercise: a literature review. Curr Sports Med Rep. 2017;16(1):19–22. [DOI] [PubMed] [Google Scholar]
- 30. Loprinzi PD, Herod SM, Cardinal BJ, Noakes TD. Physical activity and the brain: a review of this dynamic, bi-directional relationship. Brain Research. 2013;1539:95–104. [DOI] [PubMed] [Google Scholar]
- 31. Varma VR, Tang X, Carlson MC. Hippocampal sub-regional shape and physical activity in older adults. Hippocampus. 2016;26(8):1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu T, Luo H, Tang JY, Wong GH. Does lifestyle matter? Individual lifestyle factors and their additive effects associated with cognitive function in older men and women. Aging Ment Health. 2020;24(3):405–412. [DOI] [PubMed] [Google Scholar]
- 33. Shakersain B, Rizzuto D, Wang HX, et al. An active lifestyle reinforces the effect of a healthy diet on cognitive function: a population-based longitudinal study. Nutrients. 2018;10(9):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lerche S, Gutfreund A, Brockmann K, et al. Effect of physical activity on cognitive flexibility, depression and RBD in healthy elderly. Clin Neurol Neurosurg. 2018;165:88–93. [DOI] [PubMed] [Google Scholar]
- 35. Barnes JN, Corkery AT. Exercise improves vascular function, but does this translate to the brain? Brain Plasticity (Amsterdam, Netherlands). 2018;4(1):65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Binder EF, Storandt M, Birge SJ. The relation between psychometric test performance and physical performance in older adults. J Gerontol A Biol Sci Med Sci. 1999;54(8):M428–M432. [DOI] [PubMed] [Google Scholar]
- 37. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep (Washington, DC: 1974). 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 38. Rozzini R, Frisoni GB, Bianchetti A, Zanetti O, Trabucchi M. Physical Performance Test and Activities of Daily Living scales in the assessment of health status in elderly people. J Am Geriatr Soc. 1993;41(10):1109–1113. [DOI] [PubMed] [Google Scholar]
- 39. Butler TL, Fraser GE, Beeson WL, et al. Cohort profile: The Adventist Health Study-2 (AHS-2). Int J Epidemiol. 2008;37(2):260–265. [DOI] [PubMed] [Google Scholar]
- 40. Rizzo NS, Jaceldo-Siegl K, Sabate J, Fraser GE. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J Acad Nutr Diet. 2013;113(12):1610–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tantamango-Bartley Y, Jaceldo-Siegl K, Fan J, Fraser G. Vegetarian diets and the incidence of cancer in a low-risk population. Cancer Epidemiol Biomarkers Prev. 2013;22(2):286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tonstad S, Herring P, Lee J, Johnson JD. Two physical activity measures: Paffenbarger Physical Activity Questionnaire versus Aerobics Center Longitudinal Study as predictors of adult-onset type 2 diabetes in a follow-up study. AJHP. 2018;32(4):1070–1077. [DOI] [PubMed] [Google Scholar]
- 43. Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108(3):161–175. [DOI] [PubMed] [Google Scholar]
- 44. Singh PN, Fraser GE, Knutsen SF, Lindsted KD, Bennett HW. Validity of a physical activity questionnaire among African-American Seventh-Day Adventists. Med Sci Sport Exer. 2001;33(3):468–475. [DOI] [PubMed] [Google Scholar]
- 45. Singh PN, Tonstad S, Abbey DE, Fraser GE. Validity of selected physical activity questions in white Seventh-Day Adventists and non-Adventists. Med Sci Sport Exer. 1996;28(8):1026–1037. [DOI] [PubMed] [Google Scholar]
- 46. Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80. [DOI] [PubMed] [Google Scholar]
- 47. Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–1093. [DOI] [PubMed] [Google Scholar]
- 48. Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. The physical performance test. J Am Geriatr Soc. 1990;38(10):1105–1112. [DOI] [PubMed] [Google Scholar]
- 49. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. [DOI] [PubMed] [Google Scholar]
- 50. Jaceldo-Siegl K, Fan J, Sabate J, et al. Race-specific validation of food intake obtained from a comprehensive FFQ: the Adventist Health Study-2. Public Health Nut. 2011;14(11):1988–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psych Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 52. Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuro. 1991;13(6):933–949. [DOI] [PubMed] [Google Scholar]
- 53. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psych Res. 1982;17(1):37–49. [DOI] [PubMed] [Google Scholar]
- 54. Schmidt M. Rey Auditory and Verbal Learning Test: A Handbook. Western Psychological Services; 1996. [Google Scholar]
- 55. Wechsler D. Wechsler Memory Scale. 4th ed. Psychological Corporation; 2009. [Google Scholar]
- 56. Steffen TM, Mollinger LA. Age- and gender-related test performance in community-dwelling adults. JNPT. 2005;29(4):181–188. [DOI] [PubMed] [Google Scholar]
- 57. Papadakis MA, Grady D, Tierney MJ, Black D, Wells L, Grunfeld C. Insulin-like growth factor 1 and functional status in healthy older men. J Am Geriat Soci. 1995;43(12):1350–1355. [DOI] [PubMed] [Google Scholar]
- 58. Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Physical Therapy. 2002;82(2):128–137. [DOI] [PubMed] [Google Scholar]
- 59. Smarr KL, Keefer AL. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arth Care Res. 2011;63(Suppl 11):S454–S466. [DOI] [PubMed] [Google Scholar]
- 60. Barha CK, Liu-Ambrose T. Exercise and the aging brain: considerations for sex differences. Brain Plasticity (Amsterdam, Netherlands). 2018;4(1):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Demnitz N, Hogan DB, Dawes H, et al. Cognition and mobility show a global association in middle- and late-adulthood: analyses from the Canadian Longitudinal Study on Aging. Gait Posture. 2018;64:238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen HY, Tang PF. Factors contributing to single- and dual-task timed “up & go” test performance in middle-aged and older adults who are active and dwell in the community. Physical Therapy. 2016;96(3):284–292. [DOI] [PubMed] [Google Scholar]
- 63. Donato AJ, Eskurza I, Silver AE, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100(11):1659–1666. [DOI] [PubMed] [Google Scholar]
- 64. Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell. 2011;10(6):1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Weinstein AM, Voss MW, Prakash RS, et al. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav Immun. 2012;26(5):811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ahmad S, Harris T, Limb E, et al. Evaluation of reliability and validity of the General Practice Physical Activity Questionnaire (GPPAQ) in 60-74 year old primary care patients. BMC Family Practice. 2015;16:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Van Holle V, De Bourdeaudhuij I, Deforche B, Van Cauwenberg J, Van Dyck D. Assessment of physical activity in older Belgian adults: validity and reliability of an adapted interview version of the long international Physical Activity Questionnaire (IPAQ-L). BMC Public Health. 2015;15:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chu AH, Ng SH, Koh D, Muller-Riemenschneider F. Reliability and validity of the self- and interviewer-administered versions of the Global Physical Activity Questionnaire (GPAQ). PLoS One. 2015;10(9):e0136944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zanetti O, Frisoni GB, Rozzini L, Bianchetti A, Trabucchi M. Validity of direct assessment of functional status as a tool for measuring Alzheimer’s disease severity. Age Ageing. 1998;27(5):615–622. [DOI] [PubMed] [Google Scholar]
- 70. King MB, Judge JO, Whipple R, Wolfson L. Reliability and responsiveness of two physical performance measures examined in the context of a functional training intervention. Physical Therapy. 2000;80(1):8–16. [PubMed] [Google Scholar]
- 71. Sherman SE, Reuben D. Measures of functional status in community-dwelling elders. J Gene Int Med. 1998;13(12):817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Etgen T, Sander D, Bickel H, Forstl H. Mild cognitive impairment and dementia: the importance of modifiable risk factors. Deutsches Arzteblatt Int. 2011;108(44):743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Feart C, Samieri C, Barberger-Gateau P. Mediterranean diet and cognitive function in older adults. Curr Opinion Clinical Nut Metabolic Care. 2010;13(1):14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Feart C, Samieri C, Rondeau V, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302(6):638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gu Y, Scarmeas N. Dietary patterns in Alzheimer’s disease and cognitive aging. Curr Alzheimer Res. 2011;8(5):510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet and stroke, cognitive impairment, depression: a meta-analysis. Ann Neurol. 2013;74(4):580–591. [DOI] [PubMed] [Google Scholar]
- 77. Samieri C, Okereke OI, ED E, Grodstein F. Long-term adherence to the Mediterranean diet is associated with overall cognitive status, but not cognitive decline, in women. J Nut. 2013;143(4):493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66(2):216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr. 2011;93(3):601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fraser GE. Vegetarian diets: what do we know of their effects on common chronic diseases? Am J Clin Nutr. 2009;89(5):1607s–1612s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Satija A, Hu FB. Plant-based diets and cardiovascular health. Trends Cardiovasc Med. 2018;28(7): 437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. McEvoy CT, Temple N, Woodside JV. Vegetarian diets, low-meat diets and health: a review. Public Health Nut. 2012;15(12):2287–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Godos J, Bella F, Sciacca S, Galvano F, Grosso G. Vegetarianism and breast, colorectal and prostate cancer risk: an overview and meta-analysis of cohort studies. J Hum Nutr Diet. 2017;30(3):349–359. [DOI] [PubMed] [Google Scholar]
- 84. Haghighatdoost F, Bellissimo N, Totosy de Zepetnek JO, Rouhani MH. Association of vegetarian diet with inflammatory biomarkers: a systematic review and meta-analysis of observational studies. Public Health Nut. 2017;20(15):2713–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Anastasiou CA, Yannakoulia M, Kontogianni MD, et al. Mediterranean lifestyle in relation to cognitive health: results from the HELIAD study. Nutrients. 2018;10(10): 1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Norton Maria CM. Lifestyle behavior pattern is associated with different levels of risk for incident dementia and Alzheimer’s disease: the Cache County Study. J Am Geriatr Soc. 2012;60(3):405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Figure_Legends for Observed Physical Function Is Associated With Better Cognition Among Elderly Adults: The Adventist Health Study-2 by Nicole M. Gatto, Jennifer Garcia-Cano, Crissy Irani, Tiantian Liu, Cameron Arakaki, Gary Fraser, Charles Wang and Grace J. Lee in American Journal of Alzheimer"s Disease & Other Dementias
Supplemental Material, Supplemental_Material for Observed Physical Function Is Associated With Better Cognition Among Elderly Adults: The Adventist Health Study-2 by Nicole M. Gatto, Jennifer Garcia-Cano, Crissy Irani, Tiantian Liu, Cameron Arakaki, Gary Fraser, Charles Wang and Grace J. Lee in American Journal of Alzheimer"s Disease & Other Dementias