Abstract
Background
Nab‐paclitaxel plus gemcitabine is a standard treatment for metastatic/locally advanced pancreatic cancer. The effectiveness of neoadjuvant therapy with nab‐paclitaxel plus gemcitabine (GnP‐NAT) in patients with borderline resectable pancreatic cancer (BRPC) remains unclear.
Patients and Methods
This single‐arm phase II trial included 61 patients with BRPC that were treated with two cycles of GnP‐NAT, (nab‐paclitaxel 125 mg/m2 and gemcitabine 1000 mg/m2), on days 1, 8, and 15 over a 4‐week period, which comprised one cycle. The primary endpoint was overall survival time. In the absence of disease progression, patients underwent planned pancreatectomy.
Results
Median overall survival, the primary endpoint, was 25.2 months, and the median recurrence‐free survival was 12.3 months. The overall rate of grade 3/4 events was 73.8%. One patient, who had a history of radiation therapy for past esophageal cancer, died from exacerbation via pneumonia. The overall resection rate was 73.8% (n = 45), and the R0 resection rate was 63.9% (n = 39). Overall, postoperative complications were found in 19 patients (42%) with 24 events, and nine patients (20%) with nine events ≥ grade IIIa, based on Dindo's classification.
Conclusions
This protocol treatment is thought to be a feasible, safe, and promising treatment regimen, but we caution against its use in patients with a history of interstitial lung disease and/or prior pulmonary irradiation. The survival data from this study suggest the need for further investigations of GnP‐NAT efficacy in patients with BRPC, as well as prospective evaluation of adverse events.
Clinical Trial Registration
UMIN Clinical Trials Registry, UMIN000024154 and ClinicalTrials.gov, NCT02926183.
Keywords: borderline resectable, gemcitabine, nab‐paclitaxel, pancreatic cancer, phase II clinical trial
Neoadjuvant nab‐paclitaxel/gemcitabine brings median 25 months overall survival in borderline resectable pancreatic cancer. The study cautions against neoadjuvant nab‐paclitaxel/gemcitabine therapy in patients with pulmonary risk. The prognosis in the borderline resectable‐arterial group was worse than that of patients in venous group.
1. INTRODUCTION
Upfront surgery for pancreatic ductal adenocarcinoma (PDAC) involving major vessels potentially results in a high incidence of residual cancer at the surgical margin and in rapid recurrence, even after radical resection. 1 Patients with a high risk of R1 status at the surgical margin have therefore been categorized into the borderline resectable pancreatic cancer (BRPC) group. Neoadjuvant therapy aims for a higher R0 resection rate. 2 The survival benefits of neoadjuvant therapy for patients with BRPC remain controversial, however, because of its systemically enhanced malignant character. 3 The overall survival of patients with BRPC who underwent neoadjuvant therapy followed by surgical resection has tended to be better than that of patients who underwent upfront surgery. 2 , 3 , 4 , 5
Recently, the introduction of chemotherapies with stronger regimen and promising rapid response have led to their higher adoption rate as a neoadjuvant therapy for BRPC. 6 , 7 In restrictively‐selected patients, FOLFIRINOX (leucovorin and fluorouracil plus irinotecan and oxaliplatin) was applied and modified as a neoadjuvant therapy to intensify the local response and to reduce myelosuppression, such as severe neutropenia. 2 , 8 However, irinotecan used within the FOLFIRINOX regimen has significant side effects, including neutropenia and delayed‐type diarrhea. The risk of life‐threatening neutropenia of irinotecan has been consistently associated with genetic variations in UGT1A1. 9 Meta‐analyses have shown that individuals with reduced UGT1A1 activity, as detected by the presence of the UGT1A1*28 allele, have increased risk of the two major adverse effects of irinotecan: neutropenia and diarrhea. 10 Meanwhile, nab‐paclitaxel plus gemcitabine has been used for metastatic/locally advanced unresectable PDAC with safety and efficacy; a previous study reported rapid and higher response rates not only for metastatic lesions, but also for the primary tumor. 11 Several studies, including prospective intention‐to‐treat‐based or retrospective analyses, have indicated considerable benefits of neoadjuvant therapy with gemcitabine and nab‐paclitaxel (GnP‐NAT) in BRPCs for long‐term survival. This contributes to improved tumor suppression, patient selection, and facilitation of surgical procedures. 12 , 13 , 14 However, the effectiveness of GnP‐NAT in patients with BRPC remains insufficiently understood.
Attention should be paid to the balance of benefits and harm for the stronger regimen of neoadjuvant therapy. Recently, we reported on the safety of GnP‐NAT for BRPC in a phase I study. 15 Despite the proven safety, the survival benefit of GnP‐NAT remains unclear, and the primary concern is still the adverse effects of the protocol treatment shown in a prospective multicenter clinical trial. 16
We therefore conducted a prospective multicenter single‐arm phase II trial of patients with BRPC to investigate the safety of the protocol treatment and the efficacy of GnP‐NAT on overall survival. 16 This study was conducted as a specified clinical study using off‐label pharmaceuticals specified by the Ministry of Health, Labor, and Welfare of Japan (jRCTs051180104).
2. PATIENTS AND METHODS
2.1. Protocol digest of the study
2.1.1. Objective
Overall survival after neoadjuvant therapy and surgery for BRPC is recognized as a goal of a multidisciplinary approach. 1 The NAC‐GA trial was a prospective, multicenter, single‐arm, open‐label, phase II trial. Its implementation was planned for patients with BRPC defined as “borderline” by the National Comprehensive Cancer Network (NCCN Clinical Practice Guidelines in Oncology, pancreatic adenocarcinoma‐Version 2.2016) to investigate improvement of overall survival after the first day of initial therapy. 17 Staging laparoscopy, when possible, is included in routine preoperative examinations.
2.1.2. Study oversight
The NAC‐GA trial was conducted at 15 leading high‐volume centers for pancreatic surgery certified by the Japanese Society of Hepato‐Biliary‐Pancreatic Surgery. The trial was approved by the institutional review board of each participating institution. The protocol was approved by the institutional review boards of Wakayama Medical University (No. 1881) and all participating institutions. This trial is registered on the UMIN Clinical Trials Registry (UMIN000024154) and on ClinicalTrials.gov (NCT02926183) and was carried out in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies of the Ministry of Health, Labor, and Welfare of Japan. The study was designed and initiated by the academic investigators and was funded by Taiho Pharmaceutical Co. Ltd. Employees of Taiho Pharmaceutical Co. Ltd. had no access to the data during the trial, they did not participate in the data analysis, and did not participate in the preparation of the manuscript other than to review it. An independent data and safety monitoring board provided regulatory oversight by annually reviewing concealed patient data. The data and safety monitoring board did not conduct a prespecified interim analysis after the enrollment of the 61 patients with confirmed eligibility for the study.
2.1.3. Endpoints
This clinical trial focused upon evaluation of overall survival time from the first day of protocol therapy to death from any cause. It was censored on the final confirmation date of survival, or on the date of the final confirmation of patient's survival before being lost to follow‐up. Secondary endpoints were recurrence‐free survival (first day of protocol therapy to the date of relapse or death from any cause), safety of the protocol therapy (adverse effects), morbidity (≥grade I of Dindo's classification), 18 response rate (the proportion of patients with complete response or partial response; determined per RECIST 1.1 19 ), disease control rate (the proportion of patients with complete response, partial response, or stable disease, 19 ) preoperative/postoperative tumor marker (carbohydrate antigen [CA] 19‐9, carcinoembryonic antigen [CEA]), rate of normalization, reduction rate of maximum standardized uptake (SUV‐max) value on PET‐CT (institutions with PET‐CT only), chemotherapeutic effect (grade based on Evans classification), resection rate, R0 resection rate, surgical data, overall morbidity rates, rate of patients undergoing postoperative adjuvant therapy, dose intensity, quality of life (QOL) shown by fatigue and malaise assessed by the questionnaire of FACIT‐F (Japanese version), 20 and peripheral sensory neuropathy (PSN) assessed by the questionnaire of FACT/GOG‐NTX subscale (Version 4; Japanese version) 21 and Patient Neurotoxicity Questionnaire (PNQ).
2.1.4. Eligibility criteria
Inclusion and exclusion criteria were described previously. 16
2.1.5. Registration
Eligibility report forms were sent for registration at the Clinical Study Support Center at Wakayama Medical University. Information regarding the necessary follow‐up tests was then sent from there to all participating institutions.
2.1.6. Treatment
Enrolled patients were administered a 30‐min intravenous infusion of nab‐paclitaxel (125 mg/m2), followed by a 30‐min intravenous infusion of gemcitabine (1000 mg/m2), on days 1, 8, and 15 over a 4‐week period as one cycle of regimen. 9 All patients were given 1 week of rest between cycles. This regimen was repeated twice based on the result of a previous study, in which the median time to response was 43.0 days. 11 Prior to the study treatment, a 5‐HT3 receptor antagonist and dexamethasone were given. Selective neurokinin‐1 receptor antagonistic antiemetics were recommended to reduce the degree of nausea and vomiting. Enrolled patients had to receive neoadjuvant nab‐paclitaxel plus gemcitabine therapy within 12 weeks. Chemotherapy was started when a patients' recovery status fulfilled the following criteria on day 1 in each cycle of treatment: neutrophil count >1500/mm3, platelet count >100 000/mm3, AST/ALT ≤2.5xULN, and no febrile neutropenia, grade 2 or lower mucositis oral, diarrhea or peripheral sensory neuropathy. Dose adjustment was required if there were predefined toxic events in the protocol. During the rest period of 2 to 8 weeks, preoperative restaging scans and staging laparoscopy were performed upon completion of therapy. In the absence of disease progression, patients underwent planned pancreatectomy within 8 weeks (Figure 1). Postoperative adjuvant therapy was unregulated.
FIGURE 1.
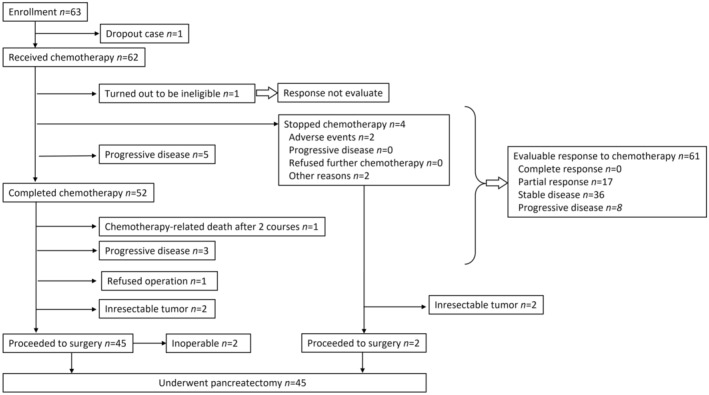
Consort diagram.
2.2. Criteria of dose reduction and discontinuation of the protocol treatment
Toxicities and adverse events were evaluated in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events [CTCAE], version 4.0. If patients had PSN, only nab‐paclitaxel was reduced. The reduced dose was set at 100 or 75 mg/m2 for nab‐paclitaxel, and 800 or 600 mg/m2 for gemcitabine. During the trial, patients were not allowed to receive concomitant radiotherapy or chemotherapy. The number of administrations during two cycles was determined as six times, facilitated by rescheduling to a later date within a 3‐week period.
2.3. Assessment of quality of life
Before and after administration of nab‐paclitaxel plus gemcitabine, enrolled patients completed FACIT‐F (version 4), 20 and questionnaires on any additional concerns, a numerical rating scale test on degree of pain and sensory disorder (cold, burning) and PNQ. FACIT‐F was assessed by the degree of fatigue, and FACT/GOG‐NTX subscale (additional concern) 21 was assessed by degree of PSN, which were converted to numerical values (0: “not at all”; 1: “a little bit”; 2: “somewhat”; 3: “quite a bit”; 4: “very much”). Total values were recorded for each questionnaire before and after therapy. Fatigue and sensory/motor neurotoxicity were assessed with PNQ converted to 0–4.
2.4. Central review for confirmation of eligibility criteria
To ascertain whether or not the subjects met the study criteria by central review, the investigator in charge of case registration submitted all multi‐detector computed tomography images of the chest and abdomen diagnosed as BRPC from each institution to the research office. This was followed by a review by an experienced independent radiologist and surgeon.
2.5. Pathological assessment
The grading of the extent of residual carcinoma in specimens was performed according to the grading scheme reported by Evans et al., which is based on the percentage of residual tumor cells. R0 resectability (R0‐status) was defined as the absence of tumor cell infiltration within 1 mm of the resection margin, and R1 status was defined as the presence of tumor cell infiltration within 1 mm of the resection margin. 22
2.6. Data collection
Data for all patients were collected prospectively, including history, physical examination, laboratory data, pathologic examination, perioperative clinical information, toxicity following treatment, questionnaire for QOL assessment, and complications.
2.7. Study design and statistical analysis
Study design and statistical analysis regarding the primary objective were described previously. 4 , 5 , 15 , 16 , 23 , 24 , 25
3. RESULTS
3.1. Patient characteristics
Between October 4, 2016, and March 31, 2019, 63 patients were enrolled from 15 institutions. A consort flow diagram is shown in Figure 1; one patient dropped out and another turned out to be ineligible after enrollment. The per‐protocol population thus comprised 61 patients (Figure 1). Table 1 shows the baseline characteristics of the intention‐to‐treat population (full analysis set) with PDAC. Subgroups were predominant in the patients with borderline resectable‐artery (BR‐A) at 62% in this study.
TABLE 1.
Patient characteristics (N = 61).
| Baseline | |
| Sex (male/female) | 34 (56%)/27 (44%) |
| Age (years) | 69 [45, 80] |
| PS (ECOG) (0/1/2–4) | 56 (92%)/5 (8%)/0 |
| Body weight (kg) | 54.8 [30.0, 77.7] |
| BMI | 21.5 [14.3, 33.4] |
| Location of pancreatic cancer (Head/Body‐Tail) | 41 (67%)/20 (33%) |
| Tumor maximum size (mm) | 28.1 [15.7, 61.0] |
| Subgroup of borderline category | |
| Vein | 23 (38%) |
| Artery | 38 (62%) |
| Comorbidity, patient no. | |
| No/yes | 32 (52%)/29(48%) |
| Diabetes mellitus | 14 (23%) |
| Hypertension | 17 (28%) |
| Angina pectoris | 2 (3%) |
| Overall response a | |
| CR/PR/SD/PD/NE | 0/17 (28%)/36 (59%)/8 (13%)/0 |
| Resection rate | 45 (74%) |
| Introduction of adjuvant chemotherapy | 38 (62%) |
| S‐1 | 36 (59%) |
| Gemcitabine | 2 (3%) |
| Completion of adjuvant chemotherapy | 24 (39%) |
| Recurrence pattern | |
| Overall recurrence | 38 (62%) |
| Locoregional recurrence | 9 (15%) |
| Systemic recurrence | 6 (10%) |
| Liver | 16 (26%) |
| Peritoneal seeding | 6 (10%) |
| Lung | 5 (8%) |
| Lymph node | 3 (5%) |
| Remnant pancreas | 2 (3%) |
| Para‐aortic lymph node | 1 (2%) |
| Median overall survival time (months) | 25.2 (90% CI [20.4, 34.8], 95% CI [19.2, 38.7]) |
| Median recurrence‐free survival (months) | 12.3 (95% CI [9.4, 16.7]) |
Note: The continuous variable represents the median [minimum, maximum], and the qualitative variable represents the frequency (percentage). BRPC defined as borderline by the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology, pancreatic adenocarcinoma‐Version 2.2016.
Abbreviations: BMI, body mass index; CI, confidence interval; CR, complete response; NE, unevaluable; PD, progressive disease; PR, partial response; PS, performance status; SD, stable disease.
Percentage changes from baseline in size of target lesions according to the RECIST criteria.
3.2. Dose intensity
The median dose intensity of nab‐paclitaxel and gemcitabine was 99.0% [98.1%, 99, 9%] and 98.8% [98.0%, 99.7%], respectively. There was no delay between the last chemotherapy and operative days in accordance with the study protocol.
3.3. Discontinuation of protocol treatment
Reasons for discontinuation of protocol treatment included progression of disease during neoadjuvant chemotherapy (n = 3, 4.9%), not meeting criteria for surgery (n = 10, 16.4%), treatment‐related death (n = 1, 1.6%), withdrawal of consent (n = 1, 1.6%), and exceeding the criteria for the interval period from preoperative treatment to surgery (n = 1, 1.6%).
3.4. Efficacy
Response rate at the last tumor assessment after GnP‐NAT was 27.9% (95% CI 17.1% to 40.8%; 17 partial responses), although no patients had a complete response. The disease control rate was 86.9% (95% CI [75.8%, 94.2%]); 36 patients (67.9%) had stable disease, and eight patients (13.1%) had progressive disease according to RECIST 1.1 criteria. Resection rate was 73.8% (95% CI [60.9%, 84.2%]) (n = 45), and R0 resection rate was 63.9% (95% CI [50.6%, 75.8%]) in overall enrolled patients (intention‐to‐treat population) (n = 39) (Tables 1 and 3).
TABLE 3.
Surgical and pathological outcomes.
| N = 45 | |
|---|---|
| Surgical outcomes | |
| Procedure | |
| Pancreatoduodenectomy | 34 (76%) |
| Distal pancreatectomy | 9 (20%) |
| Total pancreatectomy | 2 (4%) |
| Non‐therapeutic surgery | 0 |
| Combined resection | |
| Portal vein | 30 (67%) |
| Artery (CA/HA/SA/LGA/SMA) | 6 (13%)/7 (16%)/1 (2%)/0/0 |
| Inferior vena cava | 1 (2%) |
| Other organ (gallbladder/left adrenal gland) | 2 (4%)/1 (2%) |
| Operative time (min) | 426 [156, 1056] |
| Blood loss (ml) | 410 [10, 4848] |
| Intraoperative transfusion (yes/no) | 10 (22%)/35 (78%) |
| Dindo's classification | |
| Grade I | |
| Diarrhea | 3 (7%) |
| Others | 0 |
| Grade II | |
| Postoperative pancreatic fistula | 1 (2%) |
| Delayed gastric emptying | 2 (4%) |
| Thromboembolism | 1 (2%) |
| Diarrhea | 2 (4%) |
| Intra‐abdominal abscess | 2 (4%) |
| Atelectasis | 1 (2%) |
| Others | 3 (7%) |
| Grade IIIa | |
| Postoperative pancreatic fistula | 5 (11%) |
| Thromboembolism | 1 (2%) |
| Delayed gastric emptying | 0 |
| Intra‐abdominal bleeding | 0 |
| Intra‐abdominal abscess | 1 (2%) |
| Ascites | 1 (2%) |
| Others | 0 |
| Grade IIIb | |
| Thromboembolism | 1 (2%) |
| Others | 0 |
| Grade IV, V | 0 |
| Re‐admission within 30‐days after discharge | 1 (2%) |
| Pathological outcomes | |
| Cytology (rapid/permanent/not done) | 27(60%)/12(27%)/6(13%) |
| Negative/suspicious positive/positive/NE | 35 (78%)/3 (7%)/2 (4%)/6 (13%) |
| R0 resection rate | 39(64%) |
| T factor (UICC 7th edition) | |
| pT3 | 29 (64%) |
| pT4 | 16(36%) |
| Lymph node metastasis (UICC 7th edition) | |
| pN0 | 35 (78%) |
| pN1 | 10 (22%) |
| UICC‐pStage (UICC 7th edition) | |
| 0/IA/IB | 0/0/0 |
| IIA | 27 (44%) |
| IIB | 9 (15%) |
| III | 25 (41%) |
| IV | 0 |
| Histologic response (Evans grade/CAP score) | |
| I/3 | 10 (16%) |
| IIa/3 | 26 (43%) |
| IIb/2 | 8 (13%) |
| III, IIIM/1 | 1 (2%) |
| IV, IVM/0 (pCR) | 0 |
Note: The continuous variable represents the median [minimum, maximum], and the qualitative variable represents the frequency (percentage). The qualitative variable represents the frequency (percentage). The grading of the extent of residual carcinoma in specimens was performed by integration of two different grading schemes: the grading scheme reported by Evans et al., which is based on the percentage of residual tumor cells, and the scoring protocol recommended by the College of American Pathologists (CAP), which is based on the ratio of residual tumor cells and the stroma.
Abbreviations: CA, celiac artery; HA, hepatic artery; LGA, left gastric artery; NE, unevaluable; pCR, pathological complete response; SA, splenic artery; SMA, superior mesenteric artery.
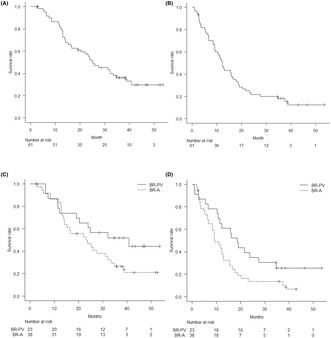
At the last follow‐up in October 12, 2021, median follow‐up time was 25.2 months (IQR 13.0, NA) from the first day of the protocol therapy to death from any cause. Four patients were lost to follow‐up within 5 years after enrolment. Median overall survival, the primary endpoint, was 25.2 months (90% CI [20.4, 34.8]), and the estimated overall survival was 36.2% at 3 years (95% CI [25.6%, 51.1%]) and 29.8% at 5 years (95% CI [19.3%, 46.2%]) (Figure 2A). Median recurrence‐free survival was 12.3 months (95% CI [9.4, 16.7]), and the estimated recurrence‐free survival at 3 years was 18.2% (95% CI [10.6%, 31.2%]) and 12.5% at 5 years (95% CI [5.8%, 26.6%]) (Figure 2B). As for survival time after surgery (n = 45), median overall survival was 28.6 months (95% CI [21.0, NA]), and the median recurrence‐free survival was 12.4 months (95% CI [8.4, 17.0]). Regarding subgroup analysis of borderline resectable category, the median overall survival was 40.8 months (95% CI [23.9, NA]) with borderline resectable‐portal vein (BR‐PV) (n = 23) and 23.2 months (95% CI [15.1, 32.1]) with BR‐A (n = 38) (p = 0.097) (Figure 2C). The median recurrence‐free survival was 18.6 months (95% CI [11.5, NA]) in the BR‐PV group and 9.4 months (95% CI [8.5, 15.5]) in the BR‐A group (p = 0.037) (Figure 2D). The recurrence rate was 84% in patients who underwent pancreatectomy and 62% in patients enrolled in this study. Most initial recurrences were in the liver (n = 12), with the most frequent site of recurrence in the present series being locoregional (n = 9) (Table 1).
FIGURE 2.
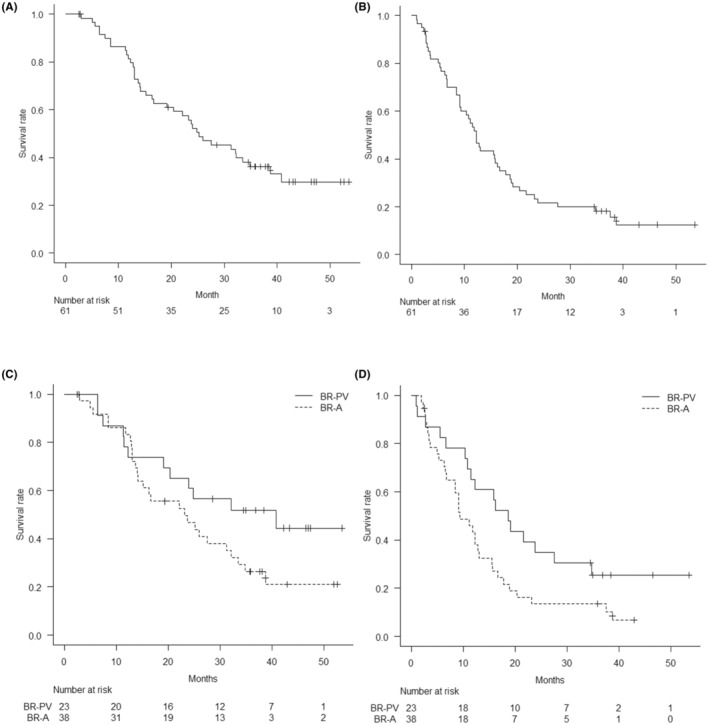
(A) Kaplan–Meier graph shows the median overall survival was 25.2 months (90% CI [20.4, 34.8]), and the estimated overall survival at 3 years was 36.2% (95% CI [25.6%, 51.1%]) and 29.8% at 5 years (95% CI [19.3%, 46.2%]). (B) Kaplan–Meier graph shows the median recurrence‐free survival was 12.3 months (95% CI [9.4%, 16.7%]), and the estimated recurrence‐free survival was 18.2% at 3 years (95% CI [10.6%, 31.2%]) and 12.5% at 5 years (95% CI [5.8%, 26.6%]). C. Kaplan–Meier graph shows the median overall survival was 40.8 months (95% CI [23.9, NA]) with BR‐PV (n = 23) and 23.2 months (95% CI [15.1, 32.1]) with BR‐A (n = 38) (p = 0.097). D. Kaplan–Meier graph shows the median recurrence‐free survival was 18.6 months (95% CI [11.5, NA]) in the BR‐PV group and 9.4 months (95% CI [8.5, 15.5]) in the BR‐A group (p = 0.037).
3.5. Adverse events
Adverse drug reactions deemed to be potentially related to the trial protocol are shown in Table 2. The overall rate of any grade events (CTCAE ver. 4.0 criteria) during the protocol treatment was 96.7%. The overall rate of grade 3 and 4 events was 73.8%. The majority of these adverse events represented expected neutropenia (n = 40). One patient had treatment‐related death in this study; he had a history of irradiation therapy for past esophageal cancer and died from exacerbation via pneumonia. Otherwise, there were no incidences of life‐threatening serious adverse events, such as Grade 4 unexpected non‐hematologic toxicity or Grade 4 hematologic toxicity with fever and hemorrhage. Other adverse events were generally within the expected range (Table 2).
TABLE 2.
Toxicity following treatment with GnP‐NAT therapy (N = 61).
| Treatment toxicity | Any grades | Grade 2 | Grade 3–4 a |
|---|---|---|---|
| Leukopenia | 21 (34%) | 6 (10%) | 14 (23%) |
| Anemia | 16 (26%) | 5 (8%) | 2 (3%) |
| Thrombocytopenia | 16 (26%) | 5 (8%) | 3 (5%) |
| Neutropenia | 50 (82%) | 8 (13%) | 40 (66%) |
| Elevated AST/ALT | 5 (8%) | 1 (2%) | 1 (2%) |
| Elevated Bilirubin | 1 (2%) | 1 (2%) | 0 |
| Appetite loss | 10 (16%) | 2 (3%) | 1 (2%) |
| Fever | 3 (5%) | 1 (2%) | 0 |
| Nausea | 2 (3%) | 1 (2%) | 0 |
| Vomiting | 1 (2%) | 1 (2%) | 0 |
| Urticaria | 5 (8%) | 1 (2%) | 0 |
| Maculopapular rash | 5 (8%) | 3 (5%) | 2 (3%) |
| Papulopustular rash | 1 (2%) | 1 (2%) | 0 |
| Acneiform rash | 2 (3%) | 0 | 0 |
| Abdominal pain | 2 (3%) | 1 (2%) | 0 |
| Diarrhea | 3 (5%) | 0 | 0 |
| Constipation | 3 (5%) | 3 (5%) | 0 |
| Small bowel obstruction | 1 (2%) | 0 | 1 (2%) |
| Fatigue | 14 (23%) | 2 (3%) | 2 (3%) |
| Oral inflammation | 1 (2%) | 1 (2%) | 0 |
| Hair loss | 15 (25%) | 11 (18%) | 0 |
| Febrile neutropenia | 2 (3%) | 0 | 2 (3%) |
| Cholangitis | 1 (2%) | 0 | 1 (2%) |
| Interstitial pneumonia | 1 (2%) | 0 | 1 (2%) |
| Upper respiratory inflammation | 3 (5%) | 2 (3%) | 1 (2%) |
| Peripheral sensory neuropathy | 13 (21%) | 3 (5%) | 0 |
Note: Values are number of events (%). Safety was evaluated in accordance with the Common Terminology Criteria for Adverse Events version 4.0.
Grade 4 toxicity was found in only one patient with leukopenia (2%), and in 13 patients with neutropenia (21%).
3.6. Surgical procedures
Table 3 shows surgical and pathological outcomes of patients who underwent pancreatectomy (n = 45). Thirty‐five (77.8%) patients underwent pancreatectomy combined with vascular resection of the artery or portal vein or both. Two patients underwent pancreatoduodenectomy with en‐bloc resection of common hepatic artery and portal vein, 26 two patients underwent distal pancreatectomy with en‐bloc resection of the celiac axis and portal vein, 27 and one patient underwent pancreatoduodenectomy with en‐bloc resection of the splenic artery. 28
3.7. Postoperative complications
Postoperative complications are shown in Table 3; they were found in 19 patients (42.2%) comprising 24 events, and nine patients (20.0%) had nine events graded ≥ IIIa. No patients had postoperative complications that were assessed to be ≥ grade IIIb morbidity of the Dindo's classification found in patients, with one exception. This patient presented portal venous thromboembolism on postoperative day 4 after distal pancreatectomy combined with portal vein resection, and had intravenous heparin infusion for 5 days. However, this treatment was not effective for the thromboembolism, so the patient underwent laparotomy to place the catheter into the portal vein for thrombolytic therapy with urokinase injection. After the reoperation, the portal vein had re‐perfused, and the patient made a rapid recovery.
3.8. Pathological outcomes
The results of overall R0 resection rates and the intraoperative peritoneal cytology are also shown in Table 3. The R0 resection rates of BR‐A/BR‐PV were 55.3%/78.3% in the per‐protocol population, and 84.0%/90.0% in resected patients. The examination of the histopathological treatment effect based on the Evans grade revealed the following number of patients per grade: grade I = 10, IIa = 26, IIb = 8, III = 1, and ≥IIIM = 0.
3.9. Assessment of quality of life
The QOL assessment before and after GnP‐NAT regarding fatigue and peripheral sensory neuropathy proved an increase from 10.0 [7.0, 12.2] to 14.0 [9.0, 20.0] (p < 0.001) in the FACIT‐F questionnaire, increase from 1.0 [0.0, 2.0] to 3.5 [1.0, 9.0] (p < 0.001) in FACT/GOG‐NTX, and increase from 3.0 [2.0, 3.0] to 4.0 [3.0, 5.0] (p < 0.001) in PNQ, respectively. GnP‐NAT exacerbated fatigue and peripheral sensory neuropathy. However, no patients required postponement of the scheduled surgery due to fatigue or PSN.
3.10. Value change of parameters
In tumor markers of corresponding data of the same cases without missing data before and after GnP‐NAT, median CA19‐9 value (U/mL) (n = 56) decreased from 103.0 [42.3, 395.3] to 20.5 [10.7, 65.4] (p < 0.001) and median CEA value (ng/mL) (n = 55) from 3.0 [2.1, 4.5] to 2.8 [1.8, 4.1] (p = 0.999). The rates of patients with normal range increased from 21.3% [11.9%, 33.7%] to 64.3% [50.4%, 76.6%] in CA19‐9, and decreased from 80.0% [67.7%, 89.2%] to 78.6% [65.6%, 88.4%] in CEA value in all available data. As for metabolic parameter, median SUV‐max value (n = 30) decreased from 6.3 [4.0, 8.3] to 3.2 [2.3, 4.6] (p < 0.001).
3.11. Prognostic factors
The univariate and multivariate logistic regression analyses of prognostic factors among patients who underwent pancreatectomy are shown in Table 4 (n = 45). Among them, elevated CA19‐9 value after GnP‐NAT (HR, 0.271; 95% CI, 0.117–0.631; p = 0.002), distal pancreatectomy (HR, 0.323; 95% CI, 0.142–0.733; p = 0.007), and incompletion of adjuvant chemotherapy (HR, 0.302; 95% CI, 0.138–0.661; p = 0.003) remained independent predictors of prognosis, even after control for the other variables.
TABLE 4.
Univariate and multivariate analysis for prognostic factor in patients who underwent pancreatectomy (N = 45).
| Prognostic factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Hazard ratio | p‐value | Hazard ratio | p‐value | |
| Age ≤69 | 0.590 [0.277, 1.258] | 0.172 | – | – |
| Sex Man | 1.193 [0.573, 2.486] | 0.637 | – | – |
| Normalization of CA 19–9 value after GnP‐NAT | 0.323 [0.147, 0.712] | 0.005 | 0.271 [0.117, 0.631] | 0.002 |
| Pancreatoduodenectomy a | 0.329 [0.151, 0.719] | 0.005 | 0.323 [0.142, 0.733] | 0.007 |
| BR‐artery | 2.643 [1.182, 5.911] | 0.018 | – | – |
| Artery combined resection | 1.653 [0.727, 3.762] | 0.231 | – | – |
| Artery and PV combined resection | 1.016 [0.307, 3.367] | 0.979 | – | – |
| Pathological T3 | 0.398 [0.186, 0.852] | 0.018 | – | – |
| Pathological N0 | 0.834 [0.368, 1.888] | 0.663 | – | – |
| Dindo's classification < grade III | 0.866 [0.352, 2.129] | 0.753 | – | – |
| Completion of adjuvant chemotherapy | 0.247 [0.115, 0.534] | <0.001 | 0.302 [0.138, 0.661] | 0.003 |
| R0 resection | 0.549 [0.207, 1.457] | 0.228 | – | – |
Total pancreatectomy was categorized into the group of distal pancreatectomy.
Abbreviations: CA, carbohydrate antigen; PV, portal vein.
4. DISCUSSION
Results from the NAC‐GA trial showed that GnP‐NAT is an effective regimen in patients with BRPC. 4 This protocol treatment is thus indicated to be a promising treatment regimen. The overall survival was comparable, even when compared with a systematic review and patient‐level meta‐analysis of neoadjuvant FOLFIRINOX in patients with BRPC, which demonstrated the patient‐level median OS was 22.2 months (95% CI [18.8, 25.6]). 8 No deaths were attributed to GnP‐NAT, except for one patient with treatment‐related death that died with exacerbation from pneumonia, probably due to a prior history of irradiation therapy for esophageal cancer. This result cautions against GnP‐NAT therapy in patients with a history of interstitial lung disease and prior pulmonary irradiation. The occurrence of pneumonitis as a result of various neoadjuvant therapies followed by radiation therapy might be of minor importance. Nonetheless, Li et al. reported on patients with lung cancer who had received chemotherapy with gemcitabine. The percentage of lung volume receiving ≥5 Gy was 50% or more, and those with subclinical interstitial lung disease involving ≥25% of lung volume had an increased risk of grade ≥3 radiation pneumonitis in patients with lung cancer with subclinical interstitial lung disease. 29 Elsewhere, Vasiljevic et al. reported that mean total lung doses of >10 Gy escalate the risk of lung tissue complications in adjuvant‐treated patients with breast cancer. 30 Neutropenia and leukopenia were the most commonly reported grade III–IV adverse events, although the incidences of non‐hematological grade III–IV adverse events, such as diarrhea and fatigue, were low. In terms of feasibility and level of toxicity as a neoadjuvant therapy, the setting dose and cycles of the protocol treatment were confirmed to be feasible and safe. In addition, subjective assessment of QOL also revealed tolerable fatigue and PSN under the regimen of this study. Furthermore, unlike FOLFIRINOX therapy, the GnP‐NAT regimen, which is not subject to UGT1A1 restrictions in its indications, may benefit more patients as a neoadjuvant therapy, regardless of race, age, or genetic polymorphism.
The primary endpoint of this study was overall survival time, although the regimen was determined to include only two cycles based on the result of a previous study, which reported the median time to response as 43 days. 11 The histologic response of patients who completed the trial protocol was not as strong as expected. However, this therapeutic effect may be improved by increasing the number of treatment cycles or by adding radiation therapy. Conversely, overall survival would be affected not only by the local treatment effect of neoadjuvant therapy, but also by the systemic effect. The ability to select patients with occult cancer cells may be a benefit of neoadjuvant therapy, but the disappearance of occult tumor cells during neoadjuvant treatment cannot be proven. A recent study reported that the escape mechanism of chemo‐resistant cancer cells could induce early distant metastasis and might not contribute to prolonging survival, even if the local treatment effect was enhanced by stronger chemotherapy or in combination with radiation therapy. 31
The resection rate in the present study, 74%, was comparable with the results of meta‐analysis of neoadjuvant FOLFIRINOX in patients with BRPC, which was 68% (95% CI [60.1% to 74.6%]). However, the R0 resection rate of 64% in the present study was lower than that of FOLFIRINOX, which was 84% (95% CI [76.8% to 89.1%]). 11 The predominant population of the BR‐A subgroup in this study, or conversion diagnosis from R0 to R1 (n = 6, 13%) on dissected peripancreatic tissue margin by the 1‐millimeter rule assessment of this study might be related to the difference between them. 22
Another issue with the borderline resectable category is the poor treatment outcome in the BR‐A group. In this study, the prognosis in the BR‐A group was worse than that of patients in the BR‐PV group due to progression of disease during preoperative treatment and early recurrence after surgery. One explanation for the poor efficacy of GnP‐NAT in patients with BR‐A may be related to its malignant potential as systemic disease. Another would be the difficulty in obtaining adequate surgical margin on the dissection layer in patients with major artery invasion of PDAC. Kato et al. reported the R0 resection rate was significantly lower in patients with BR‐A than in those with BR‐PV, 4 and Bolm et al. recently described that only perivascular stranding around the superior mesenteric artery rather than alterations of the superior mesenteric and portal vein remained a predictive factor of positive resection margins after neoadjuvant treatment. 32 Patients with BR‐A should be assessed with multiple imaging modalities during long‐term treatment rather than undergoing neoadjuvant therapy for a defined period. 33 Algorithmic therapy may be suitable, determining the appropriateness of resection indication according to the therapeutic response. At least, preoperative chemotherapy for GnP‐NAT therapy may be a preferable regimen if limited to patients with BR‐PV. Lastly, we investigated the prognostic factors among patients who underwent pancreatectomy. Elevated CA19‐9 value after GnP‐NAT, distal pancreatectomy, and incompletion of adjuvant chemotherapy remained independent predictors of prognosis. These factors might be helpful in construction of the algorithmic strategy for patients with BRPC.
We note that the exclusion criteria (inappropriate patients including those with high suspicion of distant metastases due to extremely high level of tumor markers) was a major limitation of this study. Nonetheless, there is little evidence regarding adjuvant therapy for patients who underwent neoadjuvant, and non‐regulation of postoperative adjuvant therapy was also a major limitation. GnP‐NAT could still be another important preoperative chemotherapy option for BRPC alongside FOLFIRINOX therapy. However, GnP‐NAT might be avoided, and careful hospitalization might be considered for patients with a history of radiotherapy or pulmonary disease who have a condition similar to interstitial pneumonia. In summary, the survival data from the present study supports further investigations of GnP‐NAT efficacy in patients with BRPC, as well as prospective evaluation of adverse events.
AUTHOR CONTRIBUTIONS
Ken‐ichi Okada and Hiroki Yamaue drafted the manuscript. Manabu Kawai, Seiko Hirono helped to draft the manuscript. Kenjiro Kimura, Yo‐Ichi Yamashita, Kazuto Shibuya, Ippei Matsumoto, Sohei Satoi, Kazuhiro Yoshida, Yasuhiro Kodera, Takahiro Akahori, Hidetoshi Eguchi, Mitsuhiro Asakuma, Masaji Tani, Etsuro Hatano, Hisashi Ikoma, Go Ohira, and Hisashi Ikoma supported acquisition of data. Manabu Kawai and Ke Wan helped to collect data. Hiroki Yamaue, Manabu Kawai helped in the revision of the article. Toshio Shimokawa and Ke Wan supervised statistical analysis.
FUNDING INFORMATION
This work was supported by Taiho Pharmaceutical Co., Ltd., Tokyo, Japan under a research contract. No grant numbers applied.
CONFLICT OF INTEREST STATEMENT
K.Y., Y.K., S.H., and H.Y. are editorial board members of Annals of Gastroenterological Surgery. The authors declare the following relationships: K.O. received lecture fees from Tsumura & Co., Tokyo, Japan. H.Y., K.Y., Y.K., and H.E. received lecture fees and research funding, I.M., K.Y., M.T., E.H. and M.K. received lecture fees from Taiho Pharmaceutical Co. Ltd., Tokyo, Japan. S.S. received research funding from Nihon Servier Co. Ltd., Tokyo, Japan. K.Y. and Y.K. received lecture fees and research funding, and K.Y., M.T. and E.H. received lecture fees from Eli Lilly Japan Co. Ltd., Kobe, Japan. Y.K. received research funding from Pfizer Japan Inc., Tokyo, Japan and Nippon Kayaku Co., Ltd., Tokyo, Japan. Y.K. received lecture fees and research funding from Nippon Kayaku Co., Ltd., Tokyo, Japan. The funding sources had no role in the design, practice, or analysis of this study. All remaining authors have declared no conflicts of interest.
ETHICS STATEMENT
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and local laws/regulations. Patients provided written informed consent before participation. The study protocol and all amendments were reviewed and approved by suitably constituted institutional ethics review board or independent ethics committee in each center.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.
ACKNOWLEDGMENTS
We would like to thank Benjamin Phillis at the Clinical Study Support Center, Wakayama Medical University, for proofreading and editing the manuscript.
Okada K‐i, Kimura K, Yamashita Y‐I, Shibuya K, Matsumoto I, Satoi S, et al. Efficacy and safety of neoadjuvant nab‐paclitaxel plus gemcitabine therapy in patients with borderline resectable pancreatic cancer: A multicenter single‐arm phase II study (NAC‐GA trial). Ann Gastroenterol Surg. 2023;7:997–1008. 10.1002/ags3.12712
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, et al. International study group of pancreatic surgery. Borderline resectable pancreatic cancer: a consensus statement by the international study group of pancreatic surgery (ISGPS). Surgery. 2014. Jun;155(6):977–88. [DOI] [PubMed] [Google Scholar]
- 2. Jang JY, Han Y, Lee H, Kim SW, Kwon W, Lee KH, et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline Resectable pancreatic cancer: a prospective, randomized, open‐label, multicenter phase 2/3 trial. Ann Surg. 2018. Aug;268(2):215–22. [DOI] [PubMed] [Google Scholar]
- 3. Bachellier P, Addeo P, Faitot F, Nappo G, Dufour P. Pancreatectomy with arterial resection for pancreatic adenocarcinoma: how can it Be done safely and with which outcomes?: a single institution's experience with 118 patients. Ann Surg. 2020. May;271(5):932–40. [DOI] [PubMed] [Google Scholar]
- 4. Kato H, Usui M, Isaji S, Nagakawa T, Wada K, Unno M, et al. Clinical features and treatment outcome of borderline resectable pancreatic head/body cancer: a multi‐institutional survey by the Japanese Society of Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2013. Aug;20(6):601–10. [DOI] [PubMed] [Google Scholar]
- 5. Hirono S, Kawai M, Okada KI, Miyazawa M, Shimizu A, Kitahata Y, et al. Treatment strategy for borderline resectable pancreatic cancer with radiographic artery involvement. Pancreas. 2016. Nov;45(10):1438–46. [DOI] [PubMed] [Google Scholar]
- 6. Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011. May 12;364(19):1817–25. [DOI] [PubMed] [Google Scholar]
- 7. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med. 2013. Oct 31;369(18):1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Janssen QP, Buettner S, Suker M, Beumer BR, Addeo P, Bachellier P, et al. Neoadjuvant FOLFIRINOX in patients with borderline Resectable pancreatic cancer: a systematic review and patient‐level meta‐analysis. J Natl Cancer Inst. 2019. Aug 1;111(8):782–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shirasu H, Todaka A, Omae K, Fujii H, Mizuno N, Ozaka M, et al. Impact of UGT1A1 genetic polymorphism on toxicity in unresectable pancreatic cancer patients undergoing FOLFIRINOX. Cancer Sci. 2019. Feb;110(2):707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han FF, Guo CL, Yu D, Zhu J, Gong LL, Li GR, et al. Associations between UGT1A1*6 or UGT1A1*6/*28 polymorphisms and irinotecan‐induced neutropenia in Asian cancer patients. Cancer Chemother Pharmacol. 2014. Apr;73(4):779–88. [DOI] [PubMed] [Google Scholar]
- 11. Ueno H, Ikeda M, Ueno M, Mizuno N, Ioka T, Omuro Y, et al. Phase I/II study of nab‐paclitaxel plus gemcitabine for chemotherapy‐naive Japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2016. Mar;77(3):595–603. [DOI] [PubMed] [Google Scholar]
- 12. Inoue Y, Saiura A, Oba A, Ono Y, Mise Y, Ito H, et al. Neoadjuvant gemcitabine and nab‐paclitaxel for borderline resectable pancreatic cancers: intention‐to‐treat analysis compared with upfront surgery. J Hepatobiliary Pancreat Sci. 2021. Feb;28(2):143–55. [DOI] [PubMed] [Google Scholar]
- 13. Miyasaka Y, Ohtsuka T, Kimura R, Matsuda R, Mori Y, Nakata K, et al. Neoadjuvant chemotherapy with gemcitabine plus nab‐paclitaxel for borderline Resectable pancreatic cancer potentially improves survival and facilitates surgery. Ann Surg Oncol. 2019. May;26(5):1528–34. [DOI] [PubMed] [Google Scholar]
- 14. Yamaguchi J, Yokoyama Y, Fujii T, Yamada S, Takami H, Kawashima H, et al. Results of a phase II study on the use of neoadjuvant chemotherapy (FOLFIRINOX or GEM/nab‐PTX) for borderline‐resectable pancreatic cancer (NUPAT‐01). Ann Surg. 2022. Jun 1;275(6):1043–9. [DOI] [PubMed] [Google Scholar]
- 15. Okada K, Hirono S, Kawai M, et al. Phase I study of nab‐paclitaxel plus gemcitabine as neoadjuvant therapy for borderline resectable pancreatic cancer. Anticancer Res. 2017. Feb;37(2):853–8. [DOI] [PubMed] [Google Scholar]
- 16. Okada KI, Shimokawa T, Hirono S, Kawai M, Sho M, Satoi S, et al. Effect of neoadjuvant nab‐paclitaxel plus gemcitabine therapy on overall survival in patients with borderline Resectable pancreatic cancer: a prospective multicenter phase II trial (NAC‐GA trial). Oncology. 2017;93(5):343–6. [DOI] [PubMed] [Google Scholar]
- 17. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology, pancreatic adenocarcinoma‐Version 2.2016. Available from: http://www.nccn.org/professionals/physician_gls/PDF/pancreatic.pdf. Date last accessed 5 September 2016
- 18. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004. Aug;240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009. Jan;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 20. Salsman JM, Beaumont JL, Wortman K, Yan Y, Friend J, Cella D. Brief versions of the FACIT‐fatigue and FAACT subscales for patients with non‐small cell lung cancer cachexia. Support Care Cancer. 2015. May;23(5):1355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang HQ, Brady MF, Cella D, Fleming G. Validation and reduction of FACT/GOG‐Ntx subscale for platinum/paclitaxel‐induced neurologic symptoms: a gynecologic oncology group study. Int J Gynecol Cancer. 2007. Mar–Apr;17(2):387–93. [DOI] [PubMed] [Google Scholar]
- 22. Menon KV, Gomez D, Smith AM, Anthoney A, Verbeke CS. Impact of margin status on survival following pancreatoduodenectomy for cancer: the Leeds pathology protocol (LEEPP). HPB (Oxford). 2009. Feb;11(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakao A, Kanzaki A, Fujii T, Kodera Y, Yamada S, Sugimoto H, et al. Correlation between radiographic classification and pathological grade of portal vein wall invasion in pancreatic head cancer. Ann Surg. 2012. Jan;255(1):103–8. [DOI] [PubMed] [Google Scholar]
- 24. Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Kondo N, et al. Benefit of portal or superior mesenteric vein resection with adjuvant chemotherapy for patients with pancreatic head carcinoma. J Surg Oncol. 2013. Mar;107(4):414–21. [DOI] [PubMed] [Google Scholar]
- 25. Chen BE, Cook RJ, Lawless JF, Zhan M. Statistical methods for multivariate interval‐censored recurrent events. Stat Med. 2005. Mar 15;24(5):671–91. [DOI] [PubMed] [Google Scholar]
- 26. Amano R, Kimura K, Nakata B, Yamazoe S, Motomura H, Yamamoto A, et al. Pancreatectomy with major arterial resection after neoadjuvant chemoradiotherapy gemcitabine and S‐1 and concurrent radiotherapy for locally advanced unresectable pancreatic cancer. Surgery. 2015. Jul;158(1):191–200. [DOI] [PubMed] [Google Scholar]
- 27. Okada K, Kawai M, Tani M, Hirono S, Miyazawa M, Shimizu A, et al. Surgical strategy for patients with pancreatic body/tail carcinoma: who should undergo distal pancreatectomy with en‐bloc celiac axis resection? Surgery. 2013. Mar;153(3):365–72. [DOI] [PubMed] [Google Scholar]
- 28. Desaki R, Mizuno S, Tanemura A, Kishiwada M, Murata Y, Azumi Y, et al. A new surgical technique of pancreaticoduodenectomy with splenic artery resection for ductal adenocarcinoma of the pancreatic head and/or body invading splenic artery: impact of the balance between surgical radicality and QOL to avoid total pancreatectomy. Biomed Res Int. 2014;2014:219038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li F, Liu H, Wu H, Liang S, Xu Y. Risk factors for radiation pneumonitis in lung cancer patients with subclinical interstitial lung disease after thoracic radiation therapy. Radiat Oncol. 2021. Apr 13;16(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vasiljevic D, Arnold C, Neuman D, Fink K, Popovscaia M, Kvitsaridze I, et al. Occurrence of pneumonitis following radiotherapy of breast cancer—a prospective study. Strahlenther Onkol. 2018. Jun;194(6):520–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun X, He X, Zhang Y, Hosaka K, Andersson P, Wu J, et al. Inflammatory cell‐derived CXCL3 promotes pancreatic cancer metastasis through a novel myofibroblast‐hijacked cancer escape mechanism. Gut. 2022. Jan;71(1):129–47. [DOI] [PubMed] [Google Scholar]
- 32. Bolm L, Pisuchpen N, Qadan M, Kambadakone A, Sondermann S, Mueller K, et al. Prediction of R status in resections for pancreatic cancer using simplified radiological criteria. Ann Surg. 2022. Aug 1;276(2):215–21. [DOI] [PubMed] [Google Scholar]
- 33. Truty MJ, Kendrick ML, Nagorney DM, Smoot RL, Cleary SP, Graham RP, et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann Surg. 2021. Feb 1;273(2):341–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


