Abstract
Background
This study explored perceptions of barriers and facilitators to healthful dietary behaviors among patients with gastrointestinal (GI) cancer and their caregivers, including caregiver preparedness, patient and caregiver self-efficacy for symptom management, and other environmental, social, and familial factors that may serve as barriers and facilitators to healthful eating.
Methods
Using a concurrent mixed methods cross-sectional study design, individuals with GI cancer receiving outpatient chemotherapy and their caregivers completed surveys, dietary assessments, and interviews. Caregiving preparedness, self-efficacy for symptom management, and dietary intake were assessed using validated instruments. Dietary quality was measured using the Healthy Eating Index (HEI)-2020. In-depth interviews explored barriers and facilitators to healthful eating, symptom management, and caregiver preparedness.
Results
Twenty-seven patient-caregiver dyads completed study activities (N = 54). Dietary quality scores ranged from 26 to 81, with a median score of 43 for patients and 42 for caregivers. Thematic analysis identified three barriers to healthful eating: caregiver self-efficacy and preparedness, caregiver needs are neglected, and nutrition as a source of conflict. Overall self-efficacy scores (Mdn, [IQR]) were 69.1 (45.0) for caregivers and 75.6 (34.1) for patients. Caregiver preparedness score was 2.99 ± .87; problem areas were identified, including addressing emotional needs, fluctuating eating habits, advanced disease progression and making care activities pleasant. Despite the challenges, three main facilitators were identified: increased awareness and value of nutrition, influential others, and positive coping.
Conclusion
Our findings suggest the importance of developing interventions that increase nutrition-related preparedness among caregivers and self-efficacy for managing treatment side effects. Future research should continue to explore the relationship between positive coping and dietary behaviors. While engaging patients and caregivers together during dietary interventions is a promising modality, strategies for maintaining personal nutrition-related goals when facing contrasting priorities between patients and caregivers should be addressed.
Keywords: gastrointestinal cancer, cancer caregiver, dietary intake, nutrition, caregiver preparedness, positive coping
Introduction
Prevention and management of gastrointestinal (GI) cancers constitute a major public health challenge. GI cancers represent 26% of the global cancer incidence and 35% of all cancer-related deaths.1,2 In the United States, GI cancers are the most common type of cancer, and include appendix, anal, bile duct, colorectal, esophageal, gall bladder, liver, pancreatic, rectal, small intestine, and stomach cancers. 3 Of these, the five most commonly diagnosed GI cancers include cancers of the colorectum (10.2% incidence of new cases in 2018), stomach (5.7%), liver (4.7%), esophagus (3.2%), and pancreas (2.5%). 4 While there are advances in treatment and early detection of several types of GI cancer, primary prevention remains the most effective strategy for reducing the global burden of this disease. More than half of all GI cancers are caused by modifiable risk factors, including dietary intake and a sedentary lifestyle, obesity, alcohol consumption, and tobacco use.4,5 Despite recent advancements in treatment, prognosis tends to be poor for certain types of GI cancer, including cancers of the pancreas, stomach, and esophagus, in part due to the late stage of most diagnoses.6,7
Management of treatment side effects for individuals receiving GI cancer treatment is critically important for the prevention of malnutrition. 8 Common treatment side effects include fatigue, pain, anorexia, nausea, vomiting and diarrhea; and malnutrition has been reported in up to 50% of patients receiving GI cancer treatment. 9 Malnutrition in patients with GI cancer is associated with adverse clinical outcomes, including increased rates of complications, higher mortality rates, and longer hospital admissions when compared to well-nourished patients; all of which lead to increases in healthcare costs.10,11 The increasing prevalence of GI cancer and advancements in treatment have resulted in more patients being treated in the outpatient setting. Accordingly, family caregivers of these patients play an instrumental role in the cancer treatment and recovery process, including managing treatment side effects. 12
Beyond managing treatment side effects, cancer caregivers provide physical, emotional, and social support to patients. 12 Caregiving for patients with GI cancer can be particularly challenging due to poor prognosis, late detection, and the high level of patient care that is required. 13 As a result, caregivers of people with GI cancer experience high levels of stress, mood disturbances, insomnia, and poor quality of life.13,14 In addition to poor prognosis, caregivers report that patient weight loss, malnutrition, and further deterioration of patients’ nutrition status are major sources of distress, negatively impacting their own emotional and physical wellbeing.8,15,16
Caregivers play an important role in promoting patient survival and are a high-risk group themselves. Yet, few nutrition-related supportive care interventions for caregivers exist despite caregivers’ reported need for this type of support.8,13,16,17 The overarching goal of this research was to inform the development of future behavioral dietary interventions to improve nutrition-related caregiver capacity and the nutrition of both patient and caregiver through changes in dietary intake at the household level. The aim of this study was to seek the perspectives of patients who were undergoing GI cancer treatment and their caregivers to explore barriers and facilitators to healthful dietary behaviors, including caregiver preparedness, patient and caregiver self-efficacy for symptom management, and other environmental, social, and familial factors.
Methods
Study Design
In this study, a cross-sectional concurrent explanatory mixed methods study design was used, where quantitative data (structured surveys and dietary assessment) and qualitative data (in-depth interviews) were collected roughly around the same time. 18 In concurrent explanatory mixed methods research, the results of both sets of data are compared and contrasted to determine how qualitative findings can inform the interpretation of the quantitative results. A core assumption in mixed methods research is that the collective strength of combining statistical trends with stories and personal experiences provides a better understanding of the research problem than either form of data alone. 18 The Institutional Review Boards of Drexel University and Thomas Jefferson University approved this study (Ethics Approval # 18-069, January 28th 2019). Written informed consent was obtained from the participants for their anonymized information to be published in this article. Data collection occurred between January and September of 2019. Our research team consists of eight women and two men representing diverse age ranges, socioeconomic and geographic backgrounds, and highest level of training ranging from Bachelors’, Masters’, Nurse Practitioner, and PhD degrees. Members of the team have combined expertise in clinical nutrition, community nutrition, oncology care, cancer survivorship, qualitative field methods, and mixed methods approaches. The reporting of this study conforms to COREQ guidelines. 19
Participants
Patient and caregiver participants were recruited at a single oncology care setting, Asplundh Cancer Pavilion. While study eligibility criteria were broad, in that patients with any type of GI cancer diagnosis and stage were eligible to participate, we purposively sampled for greater representation of high-burden cancers, such as pancreatic and esophageal cancers. Colorectal cancers are more prevalent and, in many cases, do not have the same outcome burden as cancers that are typically diagnosed later in stage, such as pancreatic and esophageal cancers. Hence, our team was particularly interested in understanding patient and caregiver nutrition-related experiences among those who were faced with these less common yet more severe cancers because these scenarios are often more clinically challenging. Participants were required to be at least 18 years of age, able to read and speak English, able to provide consent, and not cognitively impaired. Additional patient inclusion criteria included diagnosis of GI cancer, undergoing active chemotherapy treatment for cancer, and having a caregiver also willing to participate in this study. Patients who were on enteral or parenteral (tube) feeding were excluded. Caregivers were recruited if they were providing care for an individual who was diagnosed with GI cancer; thus, caregiver eligibility depended on patient eligibility. In this study, cancer caregivers were defined as individuals who provide unpaid, informal care to a relative or friend diagnosed with cancer. 20 In addition to cancer diagnoses, participants were purposively sampled to the extent possible across demographic and personal characteristics. The prevailing standard for qualitative sample size is to interview to saturation. 21 While it can be difficult to determine a sample size in advance, our recruitment goal was to recruit between 25 and 30 cancer caregiver and patient dyads.
Procedure
Interested patients and caregivers were referred to the research study team, who were available on-site to describe the research purpose and activities in detail and answer questions. Written informed consent was obtained from all participants. Participants were asked to complete four contacts with the study team for data collection during which they completed a structured survey, three dietary recalls, and an in-depth interview. During the initial visit, the survey, in-depth interview, and one dietary recall were completed in person at Asplundh Cancer Center. Two additional 24-hour dietary recalls took place as scheduled telephone calls within a three-week time window. All data were collected within a three-week time period. In-person data collection was conducted in patient chemotherapy infusion rooms with patients and in a private conference room with caregivers. For patients, the survey asked about demographic and cancer diagnosis characteristics, occurrence and severity treatment side effects, and self-efficacy for managing their side effects. For caregivers, the survey asked about demographic and caregiving characteristics, self-efficacy for managing their loved ones’ treatment side effects, and caregiving preparedness. Twenty-four-hour dietary recalls provided a snapshot of dietary intake during the time of study participation. Participants each were compensated $50 for completing all study activities.
Quantitative Data Collection
Participant demographic and descriptive characteristics included age, gender, race, marital status, educational attainment, and employment status. Caregiver experience information included relationship to care recipient, number of months as a caregiver, and hours per day providing care; and patient information included diagnosis type, stage, and treatment characteristics. Supplementary Table 1 describes the quantitative data collection in detail.
The prevalence and severity of patient treatment side effects was assessed in two ways: (1) prevalence of 14 common treatment side effects and the extent to which they disturbed patient eating and drinking behaviors; and (2) the Chemotherapy-induced Taste Alteration Scale (CiTAS). 22 The CiTAS has demonstrated good validity (Cronbach α = .82) and modest test-retest reliability (r = .41, P < .003). 22 Patient and caregiver self-efficacy for managing cancer treatment side effects was measured using the Self-efficacy Scale for Managing Cancer Symptoms.23,24 Prior studies using this instrument in lung cancer patients,23,24 prostate cancer patients and their caregivers, 25 caregivers of cancer patients at the end of life, 26 and the effects of a caregiver training program have demonstrated internal consistency and construct validity. 27 The Preparedness for Caregiving Scale was used to assess caregivers’ readiness to provide care for the patient. 28 Cronbach alphas of .88 – .93 have been reported,28-31 and construct validity has been demonstrated by negative correlations between preparedness and caregiver worry and lack of resources. 30 The research team added an open-ended question to the end of the scale asking participants to share if there was anything specific they would like to be better prepared for. Dietary quality was measured using the Healthy Eating Index-2020 (HEI-2020). 32 Three 24-hour dietary recalls were collected and analyzed using the Automated Self-Administered 24-hour Recall (ASA24) Dietary Assessment Tool, version 2018 (https://epi.grants.cancer.gov/asa24/). Each participant’s HEI-2020 score was calculated from the mean intake values of three 24-hour dietary recalls; scores range from 0 to 100. A radar graph was created to provide an illustration of how patients and caregivers obtained their overall HEI-2020 scores (https://epi.grants.cancer.gov/hei/interpret-visualize-hei-scores.html). 33 Each axis on the radar graph represents a unique component score. Component scores were graphed as percentages of the maximum possible score, for example, a Seafood and Plant Proteins score of 3/5 was graphed as 60%. A graded approach was also used to aid interpretation of the HEI scores, as recommended by experts in this field (https://epi.grants.cancer.gov/hei/interpret-visualize-hei-scores.html). 34 The grading system is as follows: Overall scores of 90 to 100 or component scores that are 90% to 100% of maximum score were given an “A.” Overall scores of 80 to 89 or component scores that are 80% to 89% of maximum score were given a “B.” Overall scores of 70 to 79 or component scores that are 70% to 79% of maximum score were given a “C.” Overall scores of 60 to 69 or component scores that are 60% to 69% of maximum score were given a “D.” Lastly, overall scores of 0 to 59 or component scores that are 50% to 59% of maximum score were given an “F.”
Qualitative Data Collection
Participants who completed the survey and dietary recalls were eligible to participate in in-depth interviews, which explored barriers and facilitators to healthful eating, symptom management and caregiver preparedness. Using a qualitative descriptive approach, two members (DD and BJM) of the research team (one male, one female), with experience in conducting qualitative research, including in-depth interviews, conducted all interviews. In-depth interviews with patients and caregivers occurred separately and lasted between 60 and 90 minutes. All interviews were semi-structured, and probes and transitions followed questions when necessary. Throughout the study, the interview guide was reviewed and revised by the research team to ensure the collection of rich data (see supplementary files). All interviews were audio recorded and transcribed verbatim. Interviewers took field notes during and after interviews. Repeat interviews were not conducted.
Quantitative and Qualitative Data Analyses
Descriptive statistics were used to summarize demographic and personal characteristics, prevalence and severity of side effects, self-efficacy for managing side effects, caregiving preparedness, and dietary quality (HEI-2020). All quantitative data were analyzed using IBM SPSS Statistics, Version 28.0 (Armonk, NY: IBM Corp) and were reported as Mean ± Standard Deviation, Mean (range), or Median (Interquartile Range, IQR); categorical data were displayed as Frequency (Percentage). Participants with missing data were excluded from analyses.
Qualitative data were thematically analyzed using a hybrid inductive and deductive coding approach. Data were collected until saturation was reached. Transcripts were read several times and a preliminary codebook was developed. Data were analyzed using inductive and deductive thematic analysis. Two researchers independently performed open coding on the same sample of interviews. Coding was compared and discussed to identify descriptive codes. Two researchers then coded each transcript. Themes and sub-themes were developed and reviewed by other research team members to ensure they captured the expression of participants’ experiences. Any interpretation or coding disagreement between the coders was discussed with the project team until consensus was reached. NVivo11 software (QSR International Pty Ltd. Version 11, 2015) was used for coding and organization into themes and sub-themes. The integration of quantitative and qualitative data occurred during the analysis and interpretation phases. 35 In the results section, we use data triangulation and interpretation to simultaneously present the quantitative and qualitative findings. 18
The confirmability, credibility, dependability, and transferability criteria were used to ensure trustworthiness. 36 Confirmability was established by recording all research-related activities. Credibility was ensured through peer-checking and modified member-checking. In peer-checking, the research team assessed the codes and themes and verified the accuracy of data analysis. In the modified member-checking, the research team presented the major qualitative findings to key clinical stakeholders. Transcripts were not returned to participants for comment. Dependability was maintained through data analysis by two team members. Transferability was ensured through purposive sampling of research participants.
Results
Demographic, Personal, and Cancer Diagnosis-Related Characteristics
Twenty-seven patient-caregiver dyads (54 total participants) were included in the analysis. Six individuals withdrew from the study due to severe illness and were not included in the analysis. Table 1 displays participant demographic and personal characteristics for patients and caregivers. Forty-one percent of patients and 59% of caregivers were female. The majority of individuals in both groups were older than 55 years and were married. For patients, 85% were Caucasian and 15% were African American. For caregivers, 81% were Caucasian, 15% were African American, and 4% were Asian. Although annual income was not assessed, 100% of patients and 93% of caregivers reported their financial resources in the past 30 days as “adequate.” Forty-eight percent of patients and 67% of caregivers reported their highest educational attainment as college graduate or graduate school. The majority of participants were retired (59% of patients and 52% of caregivers) and married (93% of dyads were spouses and 7% were parent/child). Hypertension was the most frequently reported additional medical condition (63% of patients and 48% of caregivers), and 19% of caregivers were cancer survivors themselves. Caregivers were typically engaged in caregiving activities seven days a week and spent over seven hours each day undertaking caregiving activities.
Table 1.
Demographic and Personal Characteristics of Patients With GI Cancer and Their Caregivers.
| Characteristics | Patients (N = 27) | Caregivers (N = 27) |
|---|---|---|
| N (%) | N (%) | |
| Gender | ||
| Female | 11 (41) | 16 (59) |
| Male | 16 (59) | 11 (41) |
| Age | ||
| 35–54 years | 1 (4) | 4 (15) |
| 55 or older | 26 (96) | 23 (85) |
| Race | ||
| White | 23 (85) | 22 (81) |
| Black | 4 (15) | 4 (15) |
| Asian | 0 (0) | 1 (4) |
| Ethnicity Latinx Descent (Yes) | 0 (0) | 0 (0) |
| Marital status | ||
| Never married | 0 (0) | 1 (4) |
| Married | 25 (93) | 26 (96) |
| Widowed | 2 (7) | 0 (0) |
| Education | ||
| High school graduate or GED | 7 (26) | 5 (18) |
| Some college | 7 (26) | 4 (15) |
| College graduate | 9 (33) | 11 (41) |
| Graduate school | 4 (15) | 7 (26) |
| Employment | ||
| Full-time paid work | 5 (19) | 5 (19) |
| Part-time paid work | 3 (11) | 2 (7) |
| Homemaker | 0 (0) | 2 (7) |
| Unemployed | 1 (4) | 2 (7) |
| Receiving disability | 2 (7) | 1 (4) |
| Retired | 16 (59) | 14 (52) |
| Other | 0 (0) | 1 (4) |
| Insurance status | ||
| Private insurance | 10 (37) | 9 (33) |
| HMO | 4 (15) | 3 (11) |
| Medicaid | 2 (7) | 0 (0) |
| Medicare | 11 (41) | 15 (56) |
| Additional medical condition | ||
| Hypertension | 17 (63) | 13 (48) |
| Diabetes | 6 (22) | 3 (11) |
| Heart disease | 0 (0) | 5 (19) |
| Stroke | 3 (11) | 1 (4) |
| Arthritis | 11 (41) | 11 (41) |
| Cancer | 27 (100) | 5 (19) |
| Adequate financial resources in past 30 days (Yes) | 27 (100) | 25 (93) |
| Dyad relationship | ||
| Spousal | 25 (93) | 25 (93) |
| Familial (parent/child) | 2 (7) | 2 (7) |
| Days per week providing care (mean ± SD) | – | 6.7 ± .95 |
| Hours per day providing care (mean ± SD) | – | 7.4 ± 7.87 |
| Cancer diagnosis | ||
| Colon or rectal | 4 (15) | — |
| Pancreatic | 15 (56) | |
| Esophageal | 4 (15) | |
| Gallbladder | 2 (7) | |
| Other (gastrointestinal or intestinal malignancies) | 2 (7) | |
| Stage | ||
| I | 2 (7) | — |
| II (incudes A & B) | 8 (30) | |
| III | 8 (30) | |
| IV | 9 (33) | |
| Side effects experienced (“quite often” or “very often”) | ||
| Fatigue | 14 (45) | — |
| Reduced appetite | 11 (36) | |
| Nausea | 9 (29) | |
| Constipation | 9 (29) | |
| Dry mouth | 8 (26) | |
| Diarrhea | 8 (26) | |
| Trouble swallowing | 4 (13) | |
| Chemotherapy-induced taste alteration scale (CiTAS) score a | 8.5 (IQR 5.9) | — |
aThe overall CiTAS score ranges from 4 (no taste alteration) to 20 (maximum severity of taste alteration); score is displayed as median (Interquartile Range).
Table 1 also displays patient cancer diagnosis and treatment side effects information. Over half of the patients were diagnosed with pancreatic cancer (56%). The remaining patients were diagnosed with cancers of the colon or rectum (15%), esophagus (15%), gallbladder (7%), and other GI malignancies, such as bile duct cancer (7%). Cancer stage at diagnosis varied: 33% had stage IV, 30% stage III, 30% stage II, and 7% stage I. The most commonly reported treatment-related side effect was fatigue (45%), followed by poor appetite (36%), nausea (29%), constipation (29%), dry mouth (26%), diarrhea (26%), and trouble swallowing (13%). The median CiTAS score was 8.5 (IQR 5.9) from a range of 4 (no taste alteration) to 20 (maximum severity of taste alteration).
Dietary Quality
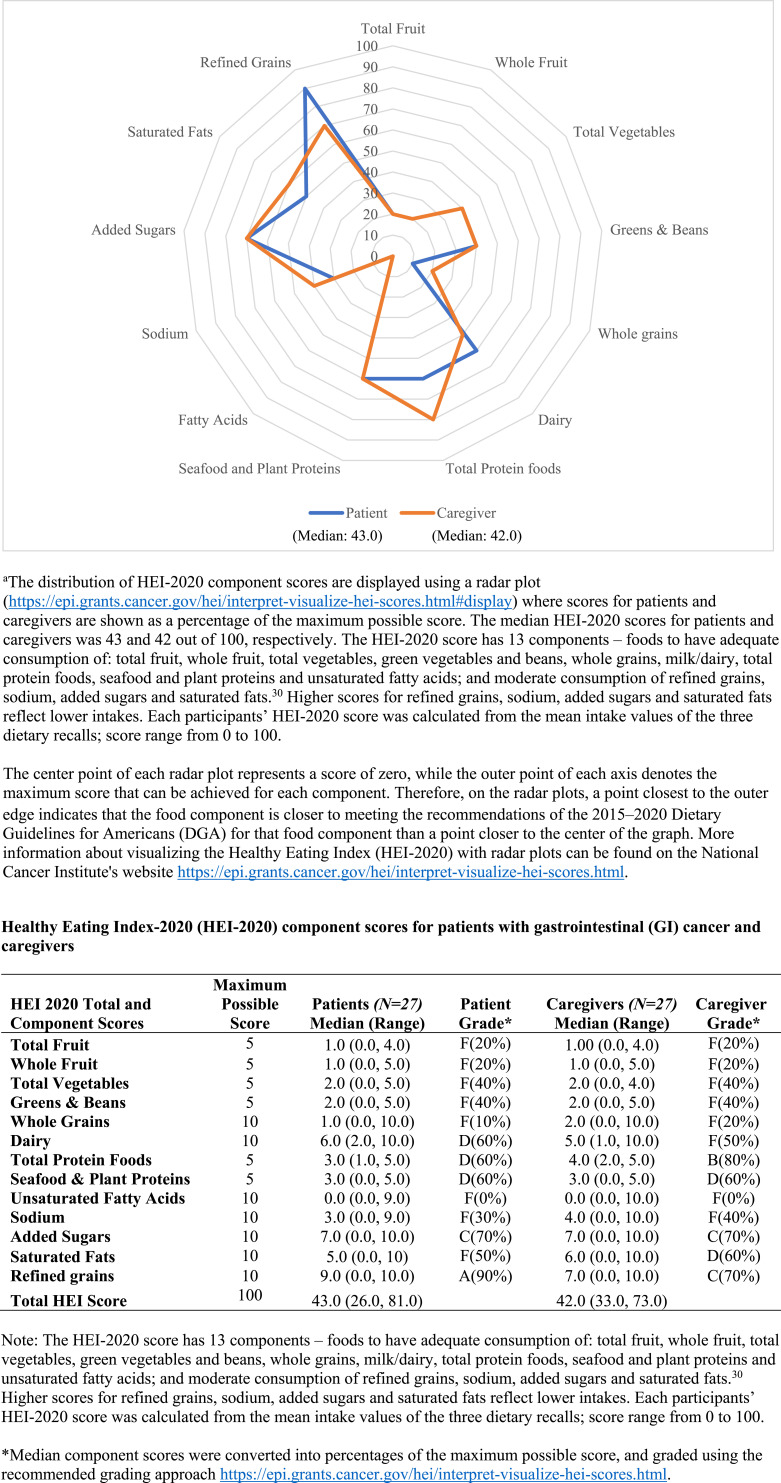
HEI-2020 scores ranged from a low of 26 (indicating many areas of poor nutrition) to a high of 81, with a median score of 43 for patients and 42 for caregivers. Table 2 displays the distribution of HEI-2020 scores by quartile range and Figure 1 displays the distribution of HEI-2020 component scores as a percentage of the maximum possible score. Figure 1 also includes a table that displays the HEI-2020 component, total scores (median and range), and HEI grading systems for patients and caregivers to further support interpretation. For context, the average HEI score for the US population as assessed through the National Health and Nutrition Examination Survey (NHANES; 2017–2018), was 58 for adults between the ages of 31 and 70 years; 32 and the average score for cancer survivors between the ages of 42 and 64 years was 54. 37
Table 2.
Healthy Eating Index-2020 (HEI-2020) Scores by Quartile Range Among Patients and Caregivers. a
| Healthy Eating Index (HEI) Score | All Participants (n = 54) | Patients (n = 27) | Caregivers (n = 27) |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| 26–37 | 15 (28) | 9 (33) | 6 (22) |
| 38–43 | 14 (26) | 5 (19) | 9 (34) |
| 44–54 | 12 (22) | 6 (22) | 6 (22) |
| 55–81 | 13 (24) | 7 (26) | 6 (22) |
aHEI-2015 score included 13 components – foods to have adequate consumption of: total fruit, whole fruit, total vegetables, green vegetables and beans, whole grains, milk/dairy, total protein foods, seafood and plant proteins and fatty acids; and moderate to low consumption of refined grains, sodium, added sugars and saturated fats. 30
Each participants’ HEI-2015 score was calculated from the mean intake values of the three dietary recalls; scores can range from 0 to 100.
Figure 1.
Distribution of Healthy Eating Index-2020 (HEI-2020) component scores as a percentage of the maximum possible score for patients with gastrointestinal (GI) cancer and caregivers.a
Both patients and caregivers had a median component score of 20% (grade F) for total fruit and whole fruit, 40% (grade F) for total vegetables and greens and beans, and patients 10% (grade F) and caregivers 20% (grade F) for whole grain consumption, all indicative of low intakes (Figure 1). For dairy consumption, patients had a median component score of 60% (grade D) and caregivers scored 50% (grade F) indicating low intakes. For total protein food consumption, patients scored 60% (grade D), whereas caregivers scored 80% (grade B). Both patients and caregivers had a median component score of 60% (grade D) for seafood and plant proteins consumption and 0% (grade F) for fatty acids consumption, respectively, indicating low intakes.
For sodium consumption, both patients and caregivers had a median component score of 30% and 40%, respectively (grade F), indicating high sodium intake. For added sugars, both patients and caregivers scored 70% (grade C) indicating moderate intake. Patients had a median score of 50% (grade F), whereas caregivers scored 60% (grade D) for saturated fats consumption, indicating high intake. For refined grain consumption, patients scored 90% (grade A), whereas caregivers scored 70% (grade C), indicating moderate intake.
Barriers to Healthful Dietary Behaviors
Three main barriers to healthful dietary behaviors emerged from this study: caregiver self-efficacy and preparedness, caregiver needs are neglected, and nutrition as a source of conflict.
Caregiver Self-Efficacy and Preparedness
For the first theme related to barriers to healthy eating, quantitative analysis focused on self-efficacy for managing treatment side effects among caregivers and patients, and caregivers’ readiness to provide care for the patient (caregiving preparedness). Self-efficacy scores for caregivers and patients include overall self-efficacy (caregivers Mdn = 69.1, IQR = 45.0; patients Mdn = 75.6, IQR = 34.1) as well as the individual domains: function-related (caregivers Mdn = 75.0, IQR = 34.2; patients Mdn = 80.0, IQR = 31.7); pain management (caregivers Mdn = 64.6, IQR = 54.4; patients Mdn = 69.3, IQR = 30.0); and management of “other” side effects, such as fatigue, lack of appetite, nausea, shortness of breath, and feeling blue and frustrated (caregivers Mdn = 67.5, IQR = 40.5; patients Mdn = 78.3, IQR = 38.3). Table 3 displays caregiver and patient self-efficacy scores.
Table 3.
Patient and Caregiver Self-Efficacy for Managing Treatment-Related Side Effects.
| Self-Efficacy a | Patients (N = 27) | Caregivers (N = 27) |
|---|---|---|
| Overall self-efficacy | 75.6 (34.1) | 69.1 (45.0) |
| Function-related self-efficacy | 80.0 (31.7) | 75.0 (34.2) |
| Pain management self-efficacy | 69.3 (30.0) | 64.6 (54.4) |
| Management of “other” side effects b | 78.3 (38.3) | 67.5 (40.5) |
aSelf-efficacy scores range from 10 to 100; data are displayed as median (Interquartile Range [IQR]).
b“Other” side effects included fatigue, lack of appetite, nausea, shortness of breath, feeling blue and frustration.
The mean caregiver preparedness scores were 2.99 ± .87. Caregivers reported low preparation in three domains: the stress of caregiving, making care activities pleasant, and taking care of emotional needs. Common themes from answers to the open-ended question at the end of the scale, which invited caregivers to list specific areas they would like to be better prepared for, included (1) preparation for fluctuating eating habits, (2) advanced disease progression, and (3) addressing emotional needs (again). Table 4 displays caregiver preparedness by the frequency of reported preparedness (“minimally prepared” and “prepared”) and mean scores. Table 5 displays qualitative themes and exemplar quotes.
Table 4.
Caregiver Preparedness by Frequency (Percent) of Reported Preparation and Mean Score (N = 27).
| Aspect of Caregiver Preparedness a | Minimally Prepared b | Well Prepared c | Mean + SD |
|---|---|---|---|
| N (%) | N (%) | ||
| To handle emergencies | 3 (11) | 24 (89) | 3.33 ± 1.00 |
| To take care of physical needs | 5 (19) | 22 (81) | 3.15 ± 1.10 |
| To find and set-up services | 5 (19) | 22 (81) | 3.19 ± 1.11 |
| General preparedness for caregiving | 5 (19) | 22 (81) | 3.26 ± .98 |
| To get help from health care system | 7 (26) | 20 (74) | 3.26 ± 1.06 |
| For the stress of caregiving | 12 (44) | 15 (56) | 2.63 ± 1.28 |
| To make care activities pleasant | 13 (48) | 14 (52) | 2.52 ± 1.16 |
| To take care of emotional needs | 14 (52) | 13 (48) | 2.56 ± 1.16 |
| Overall preparedness d | 2.99 ± .87 |
aThe Preparedness for Caregiving Scale uses a 5-point Likert scale, ranging from 0 (Not at all prepared) to 4 (Very well prepared).
b“Minimally prepared” includes participants who reported “not at all,” “not too well,” or “somewhat” prepared.
c“Well Prepared” includes participants who reported “pretty well” and “very well” prepared.
dOverall preparedness is calculated by taking a mean of all items answered; score range of 0 to 4, with higher scores indicating better preparedness.
Table 5.
Qualitative Themes and Exemplar Quotes.
| Themes | Codes | Illustrative Quotations |
|---|---|---|
| Barriers | ||
| Caregiver self-efficacy and preparedness | Distress related to eating behaviors and weight loss | “But he’s eating. He’s getting calories in. So if I find out today that he’s back to 147, I think I’ll have a stroke because then I don’t know what we’re doing wrong. I worry about that. What am I doing wrong? Why isn’t he gaining? Why aren’t I getting more weight on him, and weight’s going on me, but it’s not going on him.” (Katie, pancreatic cancer caregiver, HEI score 42) |
| “But if he would just get the calories in… even milkshakes for a while, he was drinking. And I feel like sugar is something that just really does feed into cancer. And so I feel like when I’m giving it to him, I’m giving him stuff that just wants to feed that tumor, which makes me feel guilty. But he doesn’t have that belief. So, and he just wants nutrition and he wants to eat, and he’s really stubborn just to eat with me.” (Katie) | ||
| Caregiver needs are neglected | Caregiver stress management | When asked how she manages the stress of caregiving… “I don’t. I don’t, I just do a lot of smiling and I just don’t. I have a dog.” (Leah, esophageal cancer caregiver, HEI score 38) |
| “I used to work out and stuff. Now I don’t. I don’t mean that I’m frustrated…I just don’t feel like doing more than I’m already doing for everybody else. You know, it’s funny because I feel like I do feel stressed, but I don’t think anyone else thinks that I should be feeling stressed.” (Helen, colorectal cancer caregiver, HEI score 38) | ||
| Nutrition as a source of conflict | Conflict | On accommodating loved one’s food preferences… “I don’t know how to explain it. I’m still trying after 31 years to get over the five things that he likes…I feel like – I hate cooking now. I’ll put it that way.” (Helen) |
| “I find that I eat what he likes. I don’t eat much of what I like. I entered this marriage thrilled that I was going to be cooking all kinds of things, and I found myself limited because his diet was limited. And I wasn’t getting what I used to always have.” (Leah, esophageal cancer caregiver, HEI score 38) | ||
| Facilitators | ||
| Increased awareness and value of nutrition | Changes in shopping and meal preparation | “I watch the seasoning, what I’m putting in it, how much, you know. And the texture, I make sure it’s okay for her to take.” (Daniel, pancreatic cancer caregiver, HEI score 62) |
| “If I’m making a chicken cutlet for him, instead of frying it up all the time, what I try and do is bread and bake it. It might not taste as great but it’s still, it’s healthier. I don’t fry many foods anymore. So that’s probably changed.” (Helen, colorectal cancer caregiver, HEI score 38) | ||
| Misinformation about nutrition | “Well, so now I’m actually obviously more conscious of what I cook, how I cook it. We’ve added more vegetables…I don’t really cook red meat anymore, because he won’t eat it.” (Ruth, colorectal cancer caregiver, HEI score 40) | |
| “But you just get so much information. Just like, you know, uncles just [sent] me, “Oh. Try these pills. They're from a plant over in,” you know… “And they say this is going to stop cancer.” And it's like, “Thanks, uncle.” Like, take them and dump them in the trash.” (Matthew, esophageal cancer patient, HEI score 43) | ||
| “So - but, you know, you hear rumors that cancer patients shouldn’t eat anything with sugar in it. It’s bad for them, I’m like jeeze, then we won’t be eating, you know” (Josh, gallbladder cancer patient, HEI score 48) | ||
| Influential others | Influence of caregiver | “But more so my wife, she tries to put the stuff out there that I should have. And shame on me for not eating.” (Chris, colorectal cancer patient, HEI score 57) |
| “I mean I know it’s not like I’m getting big, gigantic steaks put in front of me every day. And I’m getting the right stuff to eat. I've just got to eat it. I mean so no, I would say for the most part, it’s my fault.” (Chris) | ||
| “She (wife) does a good job of buying the stuff that I should be eating…. You look at our refrigerator – there’s a gigantic bag of walnuts, so instead of eating that Kandy Kake or Jelly Crimpet, I eat walnuts. And I can tell you – they don’t taste anything like jelly crimpets, but I eat them.” (Chris, colorectal cancer patient, HEI score 57) | ||
| Family interactions and meal sharing | “Both of my kids and their spouses love to cook. And they love to eat healthy…So when we eat with them, I feel like – ‘Oh my gosh, we had a great, really healthy thing.’" (Katie) | |
| “I really like to go out, you know, with everybody. You know, and sit there and…as a family, we all go out a bunch of times a year. And it’s a big thing. You know? So I always like to eat – everybody in my family knows what I’m doing and everything. And they seem to get a kick, because I eat everything.” (Mike, pancreatic cancer patient) | ||
| Positive coping | Positive attitude | “I just take it day-by-day and just have fun.” (Mike, pancreatic cancer patient) |
| “I think your attitude’s probably a big part of it. You know? Keep a good attitude, strong- you know, fight the fight” (Matthew, esophageal cancer patient, HEI score 43) | ||
| “Well, I truly believe your attitude has a lot to do with it. And if you don’t try to help yourself, it’s not going to work. You just can’t give up.” (Gertrude, pancreatic cancer patient) | ||
| “The only thing…if I was just going to give a blanket of advice, I would just – keep your chin up, and keep eating.”(Mike) | ||
| Humor | (When asked about changes in appetite) “Probably my stubbornness to conform affects it -- because we don’t have tasty cakes. You got any on you in your bag?” (Chris, colorectal cancer patient, HEI score 57) | |
| (When asked about changes in appetite) “I used to eat – I could eat a whole hoagie. A whole 12-inch hoagie. Now, I’ll eat half of it, and put it in the refrigerator and hope my daughter doesn’t take it for lunch. And the next day, you go and get it out of the refrigerator and it’s gone and you’re depressed. So, there’s the depression. But no, it hasn’t affected me.” (Chris) | ||
| Focusing on the things you can control | “There's things you have no control over. There's things that you depend on your doctor for. There's support from your family and the love – but there are things that you have to be able to do yourself. So, I felt like nutrition and exercise were the two main things that I felt I, myself, could control. And that's where it led me to. Even before I started my chemotherapy the first time, I got in touch with a dietitian here.” (Rob, colorectal cancer patient, HEI score 60) | |
| “But the eating good is about the number one thing I can do to help myself to be healthy, is eating good food. It’s about the only thing I can do.” (Barry, pancreatic cancer patient, HEI score 26) | ||
| “But one of the comments doctors said – patting me on the back, he said, ‘Whatever you're doing, just keep doing it’ – that was probably one of the biggest encouragements that I felt… [Voice breaks]. That I can control – control...so it's sort of a psychological thing too. Like, help yourself to be able to – I can help things a little bit. So, I feel like every day when I wake up and I'm conscious of that when I'm eating, I'm saying to myself, ‘Well this is only going to help me.’” (Rob) | ||
| Adopting strategies to improve caregiving | “Keep chin up. Just patience, lot’s of it. Don’t give up. And you got to be the glass half-full person, because they’re definitely going to be the half-empty person.” (Leah, esophageal cancer caregiver, HEI score 38) | |
| “Patience. Be patient…Have patience. Just sit back and say ‘all right, it’s not that big of a deal what he’s asking me to do.” (Ruth, colorectal cancer caregiver, HEI score 40) | ||
| “Well, just being attentive to the patients… Making sure they get what they need, that’s the main thing. Have meetings with other doctors and be in communication… Make sure that the patient is getting what they need.” (Daniel, pancreatic cancer caregiver, HEI score 62) | ||
In interviews, caregivers’ felt powerless when faced with patients’ fluctuating eating behaviors and weight loss, and responsible for patients’ dietary choices and inability to maintain or gain weight. For example, Katie, a pancreatic cancer caregiver with a lower HEI-2020 score (HEI = 42) shared: “But he’s eating. He’s getting calories in. So, if I find out today that he’s back to 147, I think I’ll have a stroke because then I don’t know what we’re doing wrong. I worry about that. What am I doing wrong? Why isn’t he gaining? Why aren’t I getting more weight on him…?” She later shared: “I feel like sugar is something that just really does feed into cancer. I feel like when I’m giving it to him, I’m giving him stuff that just wants to feed that tumor, which makes me feel guilty.”
Caregiver Needs Are Neglected
In this study, patient preferences and needs took precedence, while caregivers’ emotional and physical self-care behaviors received less attention. Both patients and caregivers recognized the importance of receiving support. Yet, while the patients we spoke with cherished the support they received from their caregivers, family, and friends, caregivers expressed mixed reactions towards the support they received, some finding it difficult to prioritize themselves enough to ask for support. And despite recommendations, caregivers reported that their motivation wasn’t to take care of themselves first. Helen, who was a colorectal cancer caregiver with a lower HEI score (HEI = 38), shared: “I used to work out and stuff. Now I don’t. I don’t mean that I’m frustrated… I just don’t feel like doing more than I’m already doing for everybody else. You know, it’s funny because I feel like I do feel stressed, but I don’t think anyone else thinks that I should be feeling stressed.” When asked how she manages the stress of caregiving, Leah, an esophageal cancer caregiver with a lower HEI score (HEI = 38) shared: “I don’t. I don’t, I just do a lot of smiling and I just don’t. I have a dog.”
Nutrition as a Source of Conflict
For some, nutrition appeared to be a source of conflict, particularly when one group compromised their meal preferences for the other, as Leah and Helen illustrate below. “I find that I eat what he likes. I don’t eat much of what I like. I entered this marriage thrilled that I was going to be cooking all kinds of things, and I found myself limited because his diet was limited. And I wasn’t getting what I used to always have” (Leah); and “I don’t know how to explain it. I’m still trying after 31 years to get over the five things that he likes… I feel like – I hate cooking now. I’ll put it that way.” (Helen). While dietary mismatches may have preceded the cancer experience, the diagnosis seemed to exacerbate the issues.
Facilitators to Healthful Dietary Behaviors
Despite the challenges faced by patients with GI cancer and their caregivers in their attempts to consume a healthy diet, several factors were identified that encouraged and helped support healthy eating and dietary choices. These facilitators were organized into three themes: increased awareness and value of nutrition, influential others, and positive coping.
Increased Awareness and Value of Nutrition
Most of the caregivers we spoke with relied on the Oncology Dietitian at the Cancer Center to provide them with guidance and support. As a result, caregivers, who were typically responsible for household grocery shopping and food preparation, described changes they made in their culinary practices. These changes reflected a balance between adhering to the dietary guidance they’ve received and catering to the changes in taste, tolerance and food preferences experienced by the family member they provided care for. Caregivers reported “being more conscious” of what they cook and how they cook it. For example, many reported including more vegetables and less red meat, and baking instead of frying. Others reported focusing on the types of seasoning they use and the texture of foods due to treatment-related changes in their loved ones’ sense of taste, tolerance, and food preferences.
It was apparent that patients and caregivers were also learning how to discern the difference between evidence-based information and misinformation. Josh, who had gallbladder cancer and an HEI score above the median for the patient population (HEI = 48), shared: “So - you hear rumors that cancer patients shouldn’t eat anything with sugar in it. It’s bad for them. I’m like jeeze, then we won’t be eating, you know.” Similarly, Matthew, who had esophageal cancer and an HEI score of 43, shared: “But you just get so much information. Just like, you know, uncle just [sent] me, “Oh. Try these pills. They're from a plant over in,” you know, “And they say this is going to stop cancer.” And it's like, “Thanks, uncle.” Like, take them and dump them in the trash.”
Influential Others
While caregivers relied on the guidance of healthcare providers, including the Oncology Dietitian and Nurse Practitioner, patients relied on their caregivers for nutrition support. Additionally, the dietary behaviors and food practices of caregivers served as an important influence for patients’ dietary intake. For example, Chris, who had colorectal cancer and a higher HEI score (HEI = 57) shared: “She (wife) does a good job of buying the stuff that I should be eating…. You look at our refrigerator – there’s a gigantic bag of walnuts, so instead of eating that Kandy Kake or Jelly Krimpet, I eat walnuts. And I can tell you – they don’t taste anything like Jelly Krimpets, but I eat them.”
Lastly, caregivers, specifically, perceived maximizing family interactions during meal sharing as important facilitators of healthful dietary behaviors. Many participants recalled fond interactions with family and friends during mealtimes and how these interactions positively influenced their diet, as Katie shared: “Both of my kids and their spouses love to cook. And they love to eat healthy… So when we eat with them, I feel like – ‘Oh my gosh, we had a great, really healthy thing.’" Mike, who was a pancreatic cancer patient, shared: “I really like to go out, you know, with everybody. You know, and sit there and… as a family, we all go out a bunch of times a year. And it’s a big thing. You know? So I always like to eat – everybody in my family knows what I’m doing and everything. And they seem to get a kick, because I eat everything.”
Positive Coping
Nearly all the patients we interviewed mentioned the importance of having a positive mental attitude, as it related to their cancer experience broadly and in their experiences with nutrition-related side effects and food. Often framing their insights and experiences as advice to others with a new cancer diagnosis, our patient participants recommended to take it “day-by-day,” to “fight the fight,” and as Mike shared: “The only thing…if I was just going to give a blanket of advice, I would just – keep your chin up, and keep eating.” Similarly, many of our patients described their experiences with humor and sarcasm. For example, when asked about the ways that changes in his appetite have affected him, Chris shared: “I used to eat – I could eat a whole hoagie. A whole 12-inch hoagie. Now, I’ll eat half of it, and put it in the refrigerator and hope my daughter doesn’t take it for lunch. And the next day, you go and get it out of the refrigerator and it’s gone… and you’re depressed. So, there’s the depression. But no, it hasn’t affected me.”
Patients also emphasized that maintaining control over their health behaviors, including dietary intake and physical activity, helped them cope with the uncertainty of their diagnosis. Rob, who had colorectal cancer and a high HEI score (HEI = 60), shared: “There's things you have no control over. There's things that you depend on your doctor for. There's support from your family and the love – but there are things that you have to be able to do yourself. So, I felt like nutrition and exercise were the two main things that I felt I, myself, could control. And that's where it led me to. Even before I started my chemotherapy the first time, I got in touch with a dietitian here.” Similarly, Barry, a pancreatic cancer patient, shared: “But the eating good is about the number one thing I can do to help myself to be healthy, eating good food. It’s about the only thing I can do.”
Adopting strategies to improve their caregiving was an important coping mechanism among caregivers. Slowing down, being attentive, and having patience were noted as important caregiver qualities, as Peter, a colorectal cancer caregiver with a higher HEI score (HEI = 73), shared: “And if people are talking to you, advising you to slow down, do this, take a break, they do have your best intentions in their heart, and it's okay to listen once in a while. To slow down is very important. But there's a desire to help, but you have to help well. It's a very difficult balance.”.
Discussion
The barriers and facilitators that emerged through this research underscore the value of developing dietary interventions that harness the influential role that each member of the dyad holds, while simultaneously being responsive to their unique needs. These findings may be particularly relevant as the patients and caregivers in this study generally reported low dietary quality (median HEI score of 43 for patients and 42 for caregivers with a maximum possible score of 100). The average HEI score for the US population as assessed through the NHANES (2017–2018) was 58 for adults between the ages of 31 and 70 years; 32 and the average score for cancer survivors between the ages of 42 and 64 years was 54. 37 Lee and colleagues evaluated the dietary quality of self-reported cancer survivors using NHANES (2005–2016) data where the term “cancer survivor” referred to people who had been diagnosed with any cancer in their lifetime, including those who had a current diagnosis and those who were cancer-free. 37 The average HEI-2020 scores for respondents who reported a diagnosis of bladder, colorectal, or “other” cancer site, which included other GI cancers, was 53.5, 56.7, and 54.0, respectively. The findings of a recent mortality assessment of a large, multiethnic cohort of adult men and women help provide context for these HEI scores. Panizza and colleagues measured the association between the HEI-2015 (which is the exact same scoring system as the HEI-2020) and mortality from all-cause, cardiovascular disease (CVD), and cancer in the Multiethnic Cohort (MEC). 38 Comparing those with the lowest quality diets (men = 17.9 to 56.1; women = 23.5 to 59.8) to those with the highest quality diets (men = 74.1 to 98.7; women = 78.1 to 99.8), the reduction in risk of mortality from all-cause, CVD, and cancer was 21%, 24%, and 20%, respectively, for men and 21%, 25%, and 16%, respectively, for women. The subsequent discussion will compare study findings to previous research and synthesize perceived barriers and facilitators into implications for future interventions to improve dietary quality among caregivers and patients.
In our study, we identified three main barriers to healthful eating: low preparedness for caregiving and self-efficacy for managing treatment side effects among caregivers, caregiver emotional and physical self-care behavioral neglect, and nutrition as a source of conflict among caregivers and patients. Caregivers in our study reported somewhat low self-efficacy in managing treatment side effects, especially in managing fatigue, loss of appetite, and nausea, which were also the most common treatment side effects reported by patients. As in earlier studies,39-41 we found that caregivers felt less prepared for the stress of caregiving, making care activities pleasant, and taking care of emotional needs. However, they also desired additional guidance in addressing fluctuating eating habits, the occurrence of which led to feelings of fear and guilt, as expressed through interviews. This is similar to previous research that has shown that changes in appetite and weight loss are significant sources of anxiety and distress among caregivers;13,42,43 and caregivers experience guilt and frustration when they perceive they are not meeting certain caregiving expectations, such as the provision of adequate nutrition for patients with a reduced ability to eat.44,45
From the perspectives of caregivers in our study, caregivers’ emotional and physical self-care behaviors were neglected during their caregiving experience. Many caregivers in our study found it difficult (or impossible) to prioritize their personal health and wellness needs, including engaging in physical activity, preparing foods they enjoy, and stress reduction. Similar to our findings, Ross et al. found that 60% of caregivers in their study reported a decline in physical activity since becoming a caregiver, and nearly 50% reported their diet was worse. 46 Caregivers with higher caregiving burden and those who provided care for additional people were more likely to report worsened health behaviors. 46 A similar pattern was reported by Lee et al. in a study that examined levels of cardiometabolic risk biomarkers and their correlates in caregivers of patients with colorectal cancer. 47 In their study, caregivers experiencing higher caregiver burden and higher psychological distress demonstrated an elevated risk for cardiometabolic disease.
Despite challenges, several factors were identified as facilitators that encouraged and helped support healthy eating and dietary choices. These themes included increased awareness and value of nutrition, influential others, and positive coping. Both patients and caregivers in our study were aware of the importance of a healthy diet during cancer treatment and survivorship. Caregivers’ knowledge about the role of nutrition in cancer survivorship influenced their food shopping and preparation behaviors, whereas patients more often described relying on their caregivers for dietary guidance and support. Several studies have reported caregivers as a primary influence of patient dietary change.16,48-50 For example, in a study among patients with colorectal cancer and their caregivers, Lee et al. found that patients were more likely to adopt healthful diets if their caregivers prioritized improving their own diets. 50 Similarly, in two qualitative investigations of barriers and facilitators of dietary change after prostate cancer diagnosis, Kassianos et al. and Avery et al. found that participants’ wives and partners played central roles in food choice and preparation.48,49 Additional research has shown that mutuality, or the strength of the relationship between the patient and caregiver, was an influential factor in caregivers’ participation in health promotion behaviors. 46 In that study, when patients and their caregivers reported strong and healthy relationships, caregivers were more likely to participate in health promoting behaviors.
Despite being faced with a life-threatening illness, an uncertain future, and frequent, debilitating treatment side effects, participants emphasized important positive coping strategies they found helpful in making the diagnosis of cancer, its treatment, and the side effects they experienced more manageable. For patients, coping with their diagnosis and treatment by keeping a positive mental attitude was important, particularly as it pertained to their dietary intake, because they felt that was one aspect of their life they could control. Similarly, Gouzmen et al. (2015) reported that positive coping abilities among GI cancer patients may influence engagement in healthy behaviors, such as improvements in dietary intake. 51 In their study, patient resilience was associated with positive changes in dietary practices, and this association was mediated by post-traumatic growth. Other research has also focused on resilience as one of the most common coping strategies for individuals living with cancer. 52 In a recent systematic review and inductive content analysis of literature pertaining to resilience in colorectal cancer patients, resilience was associated with a variety of factors, including social support, hope, mental and physical burden, quality of life, and post-traumatic growth. 53 Other research has shown that cancer patients with higher resiliency report lower levels of emotional distress 54 and better quality of life. 55 Recently, Dionne-Odom et al. found higher caregiver-reported resilience was associated with higher caregiver preparedness. 56 This is similar to patterns we observed among caregivers in our study, where they described how adopting strategies to improve their caregiving was viewed as an important coping strategy. Slowing down and being attentive were commonly endorsed, as was the cultivation of patience, which is a well-documented effective coping strategy in the literature. 39
In some ways, our findings align with previous research that investigated the associations between health behaviors and coping strategies among caregivers of lung and colorectal cancer patients. 57 In that study, “positive reframing” and “acceptance” were frequently endorsed emotion-focused coping strategies reported by caregivers. Further, caregivers who engaged in physical activity reported greater use of emotion-focused coping when compared to caregivers who were physically inactive; and those with a moderate amount of daily activity reported less use of dysfunctional coping strategies when compared to caregivers who were current smokers, binge drinkers, and less-rested. 50 Other research has suggested that both family and patient resilience may alleviate caregiver burden, and family resilience also appears to promote patients’ resilience. 58 Taken together, these findings support the pursuit of future research to elucidate the mechanisms connecting caregiver resilience, caregiving preparedness, and dietary behaviors.
Strengths and Limitations
This study presents several significant insights into the barriers and facilitators that individuals with GI cancer and their caregivers experience in their attempts to consume a healthy diet. Additional strengths include the use of a mixed methods study design, inclusion of both patient and caregiver perspectives, purposive sampling that resulted in greater representation from less common yet more severe GI cancer types (such as pancreatic and esophageal), objective measurement of dietary intake, and assessment of dietary quality using the HEI-2020. While mixed methods and qualitative inquiry provide rich detail into the perspectives and experiences of the participants, the findings of this study may not be generalizable to all patients with GI cancer or caregivers who provide care for these patients. Due to the extensive nature of our data collection with both patients and their caregivers, we focused recruitment and data collection at a single oncology care setting. While this facilitated participant recruitment and engagement, and resulted in high quality structured and qualitative data, the stage and distribution of GI cancer types among our participants does not mirror national patterns, where early-stage cancers and colorectal cancers are more prevalent. The generalizability of our findings may also be limited by the demographic characteristics of the group who were primarily older, well-educated, non-Hispanic white patients and caregivers. These data were also collected prior to the COVID-19 pandemic. During the pandemic, it may have been more challenging for patients and caregivers to receive supportive care, and the nature of how post-pandemic care will evolve remains unclear in many cases. Yet, while cancer treatment is always evolving with the goal of becoming more effective and less arduous, healthful nutrition throughout the treatment trajectory will remain a vital determinant of patient outcomes. Lastly, nearly 20% of our caregivers previously experienced a cancer diagnosis. Considering the average age of the cancer caregiving population, it isn’t unexpected that some caregivers will have had a history of cancer. It is important to note, however, that none of the caregivers in our study were experiencing a current cancer diagnosis or undergoing cancer treatment. Future research should include patients and caregivers from a wider range of socio-demographic backgrounds and could include oncology healthcare providers to understand their perspectives and experiences.
Conclusion
Understanding the lived experience of both patients and caregivers regarding factors that influence their dietary behaviors can yield multiple benefits. First, healthcare professionals, including clinicians, researchers, and educators, can become better versed in helping caregivers prepare for and practice nutrition-related strategies for alleviating cancer treatment side effects (thereby reducing caregiver burden and distress). This type of augmented support holds great potential for improving the nutrition and health of caregivers, and ultimately that of the patient and household. Second, this study suggests the importance of patient and caregiver resilience as a coping strategy and future research that explores and elucidates the mechanisms connecting caregiver resilience, caregiving preparedness, and dietary behaviors is needed. While engaging patients and caregivers together during dietary interventions continues to be a promising avenue for future research, strategies for maintaining personal nutrition-related goals when facing contrasting priorities between patients and caregivers should be addressed. In doing so, interventions could dramatically improve the physical, psychosocial, and emotional health of patients with GI cancer and their caregivers.
Supplemental Material
Supplemental Material for “Keep Your Chin Up, and Keep Eating”: Perceptions of Barriers and Facilitators to Healthful Dietary Behaviors Among Individuals With Gastrointestinal Cancer and Caregivers by Brandy-Joe Milliron, Cynthia Klobodu, Jonathan Deutsch, Karon Martyn, Dan Dychtwald, Emily Riahi, Shawn Carro, Taylor Hisek, Natalie Darcy, and Ann C Klassen in Cancer Control
Supplemental Material for “Keep your Chin up, and Keep Eating”: Perceptions of Barriers and Facilitators to Healthful Dietary Behaviors Among Individuals With Gastrointestinal Cancer and Caregivers by Brandy-Joe Milliron, Cynthia Klobodu, Jonathan Deutsch, Karon Martyn, Dan Dychtwald, Emily Riahi, Shawn Carro, Taylor Hisek, Natalie Darcy, and Ann C Klassen in Cancer Control
Author Contributions: Conceptualization: BJM, ACK, KM; Project administration and supervision: BJM, DD, KM; Data collection: BJM, DD, ER, SC, TH; Data analysis: BJM, CNK, JD, KM, ND, ACK; Writing- original draft preparation: BJM, CNK, JD, ACK; Funding acquisition: BJM, ACK; Writing-review and editing: BJM, CNK, JD, DD, SC, TH, ER, KM, ND, ACK. All authors have read and agreed to the published version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the Transdisciplinary Integrated Population Science Pilot Grants Program, Thomas Jefferson University’s Sidney Kimmel Cancer Center.
Statement of Human and Animal Rights: All of the research activities were conducted in accordance with the Institutional Review Boards of Drexel University and Thomas Jefferson University.
Informed Consent: Written informed consent was obtained from the participants for their anonymized information to be published in this article.
Supplemental Material: Supplemental material for this article is available online.
Ethical Statement
Ethical Approval
This study was approved by the Institutional Review Boards of Drexel University and Thomas Jefferson University (Approval Number: 18-069, January 28, 2019).
ORCID iD
Brandy-Joe Milliron https://orcid.org/0000-0003-1113-9043
References
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2018;144(8):1941-1953. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Abnet C, Neale R, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159(1):335-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GI Cancers Alliance . Gastrointestinal cancer by the numbers. 2022. Available from: gicancersalliance.org.
- 4.Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31-54. [DOI] [PubMed] [Google Scholar]
- 5.World Cancer Research Fund/American Institute for Cancer Research . Diet, Nutrition, Physical Activity, and Cancer: A Global Perspective. A Summary of the Third Expert Report; 2018. Available from: https://www.wcrf.org/wp-content/uploads/2021/02/Summary-of-Third-Expert-Report-2018.pdf [Google Scholar]
- 6.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold M, Rutherford MJ, Bardot A, et al. Progress in cancer survival, mortality, and incidence in seven high- income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20(11):1493-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milliron BJ, Klobodu C, Dychtwald D, et al. When eating becomes torturous: Understanding nutrition-related cancer treatment side effects among individuals with cancer and their caregivers. Nutrients 2022;14(2):356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hebuterne X, Lemarie E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J Parenter Enteral Nutr. 2014;38(2):196-204. [DOI] [PubMed] [Google Scholar]
- 10.Nitenberg G, Raynard B. Nutritional support of the cancer patient: issues and dilemmas. Crit Rev Oncol Hematol. 2000;34:137-168. [DOI] [PubMed] [Google Scholar]
- 11.Fettes SB, Davidson HIM, Richardson RA, Pennington CR. Nutritional status of elective gastrointestinal surgery patients pre- and post-operatively. Clin Nutr. 2002;21:249-254. [DOI] [PubMed] [Google Scholar]
- 12.American Cancer Society . Caregivers and Family: what a cancer caregiver does. 2016. Available from. https://www.cancer.org/treatment/caregivers/what-a-caregiver-does.html
- 13.Shaw J, Harrison J, Young J, et al. Coping with newly diagnosed upper gastrointestinal cancer: a longitudinal qualitative study of family caregivers’ role perception and supportive care needs. Support Care Cancer. 2013;21:749-756. [DOI] [PubMed] [Google Scholar]
- 14.Shaw J, Young J, Butow P, et al. Improving psychosocial outcomes for caregivers of people with poor prognosis gastrointestinal cancers: a randomized controlled trial (Family Connect). Support Care Cancer. 2016;24(2):585-595. [DOI] [PubMed] [Google Scholar]
- 15.Findlay M, Rankin N, Bauer J, Collett G, Shaw T, White K. “Completely and utterly flummoxed and out of my depth”: patient and caregiver experiences during and after treatment for head and neck cancer – a qualitative evaluation of barriers and facilitators to best-practice nutrition care. Support Care Cancer. 2020;28:5771-5780. [DOI] [PubMed] [Google Scholar]
- 16.Packel L, Dychtwald DK, Pontiggia L, et al. Physical activity and nutrition-related beliefs, behaviors, and challenges in individuals living with cancer and their caregivers. Rehabilitation Oncology 2022. [Google Scholar]
- 17.Taleghani F, Ehsani M, Farzi S, et al. Nutritional challenges of gastric cancer patients from the perspectives of patients, family caregivers, and health professionals: a qualitative study. Support Care Cancer. 2021;29(7):3943-3950. [DOI] [PubMed] [Google Scholar]
- 18.Creswell JW. A Concise Introduction to Mixed Methods Research. 1st ed. Sage Publications; 2015. [Google Scholar]
- 19.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349-357. [DOI] [PubMed] [Google Scholar]
- 20.Hunt GG, Longacre ML, Kent EE, et al. Cancer caregiving in the U.S. An intense, episodic, and challenging care experience. National alliance for caregiving in partnership with the national cancer institute and the cancer support community. 2016.
- 21.Hennick M, Kaiser BN. Sample sizes for saturation in qualitative research: a systematic review of empirical tests. Soc Sci Med. 2021;292(6):114523. [DOI] [PubMed] [Google Scholar]
- 22.Campagna S, Gonella S, Sperlinga R, et al. Prevalence, severity, and self-reported characteristics of taste alterations in patients receiving chemotherapy. Oncol Nurs Forum. 2018;45(3):342-353. [DOI] [PubMed] [Google Scholar]
- 23.Porter LS, Keefe FJ, Garst J, McBride CM, Baucom D. Self-efficacy for managing pain, symptoms, and function in patients with lung cancer and their informal caregivers: associations with symptoms and distress. Pain. 2008;137(2):306-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porter LS, Keefe FJ, McBride CM, Pollack K, Fish L, Garst J. Perceptions of patients’ self-efficacy for managing pain and lung cancer symptoms: correspondence between patients and family caregivers. Pain. 2002;117:340-348. [DOI] [PubMed] [Google Scholar]
- 25.Campbell LC, Keefe FJ, McKee DC, et al. Prostate cancer in African Americans: relationship of patient and partner self-efficacy to quality of life. J Pain Symptom Manag. 2004;28:433-444. [DOI] [PubMed] [Google Scholar]
- 26.Keefe FJ, Ahles TA, Porter LS, et al. The self-efficacy of family caregivers for helping cancer patients manage pain at end-of-life. Pain. 2003;1(2):157-162. [DOI] [PubMed] [Google Scholar]
- 27.Hendrix CC, Bailey DE, Steinhauser KE, et al. Effects of enhanced caregiver training program on cancer caregiver’s self-efficacy, preparedness, and psychological well-being. Support Care Cancer. 2016;24(1):327-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Archbold PG, Stewart BJ, Greenlick MR, Harvath T. Mutuality and preparedness as predictors of caregiver role strain. Res Nurs Health. 1990;13(6):375-384. [DOI] [PubMed] [Google Scholar]
- 29.Carter JH, Stewart BJ, Archbold PG, et al. Living with a person who has Parkinson's disease: the spouse's perspective by stage of disease. Parkinson’s Study Group. Mov Disord. 1998;13(1):20-28. [DOI] [PubMed] [Google Scholar]
- 30.Silver HJ, Wellman NS, Galindo-Ciocon D, Johnson P. Family caregivers of older adults on home enteral nutrition have multiple unmet task-related training needs and low overall preparedness for caregiving. J Am Diet Assoc. 2004;104(1):43-50. [DOI] [PubMed] [Google Scholar]
- 31.Hudson PL, Hayman-White K. Measuring the psychosocial characteristics of family caregivers of palliative care patients: psychometric properties of nine self-report instruments. J Pain Symptom Manag. 2006;31(3):215-228. [DOI] [PubMed] [Google Scholar]
- 32.U.S. Department of Agriculture, Food and Nutrition Service, Center for Nutrition Policy and Promotion . Average healthy eating index-2015 scores for adults by age groups. What We Eat in America NHANES 2017-2018; 2021. Available from: https://fns-prod.azureedge.us/sites/default/files/media/file/HEI-2015_Adults_NHANES2017-2018.pdf
- 33.Vinyard M, Zimmer M, Herrick KA, Story M, Juan W, Reedy J. Healthy Eating Index-2015 scores vary by types of food outlets in the United States. Nutrients. 2021;13:2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the healthy eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fetters MD, Curry LA, Creswell JW. Achieving integration in mixed methods designs – principles and practices. Health Serv Res. 2013;48(6):2134-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guba EG. Criteria for assessing the trustworthiness of naturalistic inquiries. Educ Technol Res Dev. 1981;29:75-91. [Google Scholar]
- 37.Lee E, Zhu J, Velazquez J, et al. Evaluation of diet quality among American adult cancer survivors: results from 2005-2016 national health and nutrition examination survey. J Acad Nutr Diet. 2021;121(2):217-232. [DOI] [PubMed] [Google Scholar]
- 38.Panizza CE, Shvetsov YB, Harmon BE, et al. Testing the predictive validity of the Healthy Eating Index-2015 in the Multiethnic Cohort: is the score associated with a reduced risk of all-cause and cause-specific mortality? Nutrients. 2018;10:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mastel-Smith B, Stanley-Hermanns M. “It’s like we’re grasping at anything” caregivers’ education needs and preferred learning methods. Qual Health Res. 2012;22(7):1007-1015. [DOI] [PubMed] [Google Scholar]
- 40.Deshields T, Rihanek A, Potter P, et al. Psychosocial aspects of caregiving: perceptions of cancer patients and family caregivers. Support Care Cancer. 2012;20(2):349-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sklenarova H, Krumpelmann A, Haun MW, et al. When do we need to care about the caregiver? Supportive care needs, anxiety, and depression among informal caregivers of patients with cancer and cancer survivors. Cancer. 2015;121(9):1513-1519. [DOI] [PubMed] [Google Scholar]
- 42.Wang T, Molassiotis A, Chung B, Tan JY. Unmet care needs of advanced cancer patients and their informal caregivers: a systematic review. BMC Palliat Care. 2018;17:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amano K, Morita T, Koshimoto S, Uno T, Katayama H, Tatara R. Eating-related distress in advanced cancer patients with cachexia and family members: a survey in palliative and supportive care settings. Support Care Cancer. 2019;27:2869-2876. [DOI] [PubMed] [Google Scholar]
- 44.Penner JL, McClement S, Lobchuk M, Daeninck P. Family members’ experiences caring for patients with advanced head and neck cancer receiving tube feeding: a descriptive phenomenological study. J Pain Symptom Manag. 2012;44(4):563-571. [DOI] [PubMed] [Google Scholar]
- 45.Fisher CL, Mullis MD, Kastrinos A, et al. “Home wasn’t really home anymore”: understanding caregivers’ perspectives of the impact of blood cancer caregiving on the family system. Support Care Cancer. 2021;29(6):3069-3076. [DOI] [PubMed] [Google Scholar]
- 46.Ross A, Lee L, Wehren L, et al. Factors that influence health-promoting behaviors in cancer caregivers. Oncol Nurs Forum. 2020;47(6):692-702. [DOI] [PubMed] [Google Scholar]
- 47.Lee L, Kim Y, Shamburek R, Ross A, Yang L, Bevans MF. Caregiving stress and burden associated with cardiometabolic risk in family caregivers of individuals with cancer. Stress. 2022;25(1):258-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avery KN, Donovan JL, Horwood J, et al. The importance of dietary change for men diagnosed with and at risk of prostate cancer: a multi-centre interview study with men, their partners and health professionals. BMC Fam Pract. 2014;15(1):81. doi: 10.1186/1471-2296-15-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kassianos AP, Coyle A, Raats MM. Perceived influences on post-diagnostic dietary change among a group of men with prostate cancer. Eur J Cancer Care. 2015;24(6):818-826. doi: 10.1111/ecc.12357 [DOI] [PubMed] [Google Scholar]
- 50.Lee MK, Park SY, Choi GS. Facilitators and barriers to adoption of a healthy diet in survivors of colorectal cancer. J Nurs Scholarsh. 2019;51(5):509-517. [DOI] [PubMed] [Google Scholar]
- 51.Gouzman J, Ben-Zur H, Shacham-Shmeuli E, Aderka D, Siegelmann-Danieli N, Beny A. Resilience and psychosocial adjustment in digestive system cancer. J Clin Psychol Med Settings. 2015;22:1-13. [DOI] [PubMed] [Google Scholar]
- 52.Seiler A, Jenewein J. Resilience in cancer patients. Front Psychiatr. 2019;10:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sihvola S, Kuosmanen L, Kvist T. Resilience and related factors in colorectal cancer patients: a systematic review. Eur J Oncol Nurs. 2022;56:102079. [DOI] [PubMed] [Google Scholar]
- 54.Min JA, Yoon S, Lee CU, et al. Psychological resilience contributes to low emotional distress in cancer patients. Support Care Cancer. 2013;21(9):2469-2476. [DOI] [PubMed] [Google Scholar]
- 55.Zhang H, Zhao Q, Cao P, Ren G. Resilience and quality of life: exploring the mediator role of social support in patients with breast cancer. Med Sci Monit. 2017;23:5969-5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dionne-Odom JN, Azuero A, Taylor RA, et al. Resilience, preparedness, and distress among family caregivers of patients with advanced cancer. Support Care Cancer. 2021;29:6913-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Litzelman K, Kent E, Rowland JH. Interrelationships between health behaviors and coping strategies among informal caregivers of cancer survivors. Health Educ Behav. 2018;45(1):90-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y, Wang K, Yin Y, Li Y, Li S. Relationships between family resilience, breast cancer survivors’ individual resilience, and caregiver burden: a cross-sectional study. Int J Nurs Stud. 2018;88:79-84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for “Keep Your Chin Up, and Keep Eating”: Perceptions of Barriers and Facilitators to Healthful Dietary Behaviors Among Individuals With Gastrointestinal Cancer and Caregivers by Brandy-Joe Milliron, Cynthia Klobodu, Jonathan Deutsch, Karon Martyn, Dan Dychtwald, Emily Riahi, Shawn Carro, Taylor Hisek, Natalie Darcy, and Ann C Klassen in Cancer Control
Supplemental Material for “Keep your Chin up, and Keep Eating”: Perceptions of Barriers and Facilitators to Healthful Dietary Behaviors Among Individuals With Gastrointestinal Cancer and Caregivers by Brandy-Joe Milliron, Cynthia Klobodu, Jonathan Deutsch, Karon Martyn, Dan Dychtwald, Emily Riahi, Shawn Carro, Taylor Hisek, Natalie Darcy, and Ann C Klassen in Cancer Control