Abstract
Although intermittent hypoxia training (IHT) has proven effective against various clinical disorders, its impact on mild cognitive impairment (MCI) is unknown. This pilot study examined IHT’s safety and therapeutic efficacy in elderly patients with amnestic MCI (aMCI). Seven patients with aMCI (age 69 ± 3 years) alternately breathed 10% O2 and room-air, each 5 minutes, for 8 cycles/session, 3 sessions/wk for 8 weeks. The patients’ resting arterial pressures fell by 5 to 7 mm Hg (P < .05) and cerebral tissue oxygenation increased (P < .05) following IHT. Intermittent hypoxia training enhanced hypoxemia-induced cerebral vasodilation (P < .05) and improved mini-mental state examination and digit span scores from 25.7 ± 0.4 to 27.7 ± 0.6 (P = .038) and from 24.7 ± 1.2 to 26.1 ± 1.3 (P = .047), respectively. California verbal learning test score tended to increase (P = .102), but trail making test-B and controlled oral word association test scores were unchanged. Adaptation to moderate IHT may enhance cerebral oxygenation and hypoxia-induced cerebrovasodilation while improving short-term memory and attention in elderly patients with aMCI.
Keywords: cerebral tissue O2 saturation, cerebrovascular response, cognitive function, elderly, hypoxemia
Introduction
Intermittent hypoxia (IH) can inflict harm or confer benefit, depending on the frequency, duration, and intensity of the hypoxia exposures. 1,2 Brief bouts of high-intensity IH applied up to 8 to12 h/day 3 -5 to model obstructive sleep apnea (OSA) in rats, increase arterial pressures, 3 -5 intensify inflammation, 6 impair neurobehavioral function, 7,8 and worsen ischemic injury of heart 9 and brain. 10 In contrast, mild to moderate intensity IH training (IHT) programs (eg, 9%-12% O2, 30-90 min/day for 3-5 weeks) afford robust protection of myocardium 11 -13 and brain 14 -18 in animals. The IH intensity and cumulative hypoxia duration per session appear to be critical determinants of beneficial versus detrimental responses to IH. 2
Increasingly, training with low-dose hypoxic exposures is being applied as nonpharmacological therapy for various pathological disorders in humans. Burtscher et al 19 reported that normobaric IHT (alternating 3-5 minutes of exposure to 10%-14% O2 and 3-minute normoxic recovery, 3-5 cycles per daily session, 15 sessions over 3 weeks) increased peak aerobic capacity of older men with or without coronary artery disease. Haider et al 20 documented that a similar IHT regimen improved baroreflex sensitivity and autonomic cardiovascular function in patients with chronic obstructive pulmonary disease. Intermittent hypoxia training also has been applied to lower arterial pressures in patients with hypertension, 21 improve quality of life and physical performance in patients with heart failure 22 and spinal cord lesions, 23 and decrease serum glucose concentrations and enhance glucose tolerance in prediabetic patients. 24 Recently, Bayer et al 25 reported that a hypoxia–hyperoxia regimen alternating 4- to 7-minute exposures to 10% to 14% O2 and 2- to 4-minute exposures to 30% to 40% O2 for 4 to 8 cycles/session, 3 sessions/week for 5 to 7 weeks, combined with physiotherapy, occupational therapy, and cycling, augmented cognitive performance on the Dementia-Detection and Sunderland Clock-Drawing tests as well as physical performance on the 6-minute walk test in geriatric patients. However, IHT’s impact on cognitive performance in patients with amnestic mild cognitive impairment (aMCI) has not been reported.
Intermittent hypoxia exposures inevitably lower arterial oxygen saturation (SaO2) 26 and, in turn, cerebral tissue O2 saturation (ScO2) while enhancing cerebral perfusion or cerebrovasodilation. 27 Nonetheless, most previous IH studies did not document the participants’ ScO2 and SaO2. Establishing the relationships between IH, SaO2, and ScO2 may facilitate clinical application of IH and foster understanding of the mechanisms of therapeutic adaptation. It remained to be determined whether elderly adults with cognitive impairments would benefit from IHT programs readily tolerated by healthy young adults. 26,27 This pilot clinical study addressed 2 objectives: first, to demonstrate that elderly patients with aMCI could tolerate cyclic, brief, moderate hypoxemia, sufficient to lower SaO2 to approximately 70% within 5 minutes; and second, to examine whether IHT could improve cognitive function and physiological responses to hypoxia in elderly patients with aMCI. Cognitive performance, along with cardioventilatory and cerebrovascular function, arterial pressures, SaO2, and ScO2 were assessed in elderly adults with aMCI and compared before and after an 8-week IHT program.
Methods
Participants
All participants provided a written consent which was approved by the University of North Texas Health Science Center’s Institutional Review Board for Protection of Human Subjects. Fourteen nonsmokers with aMCI were recruited. The assessment and/or diagnosis of MCI was based on Petersen et al’s criteria 28 including the patient’s and/or his/her family members’ complaints of memory problem, cognitive decline exceeding that considered to represent normal aging, but intact independent functioning, and no diagnosis of Alzheimer’s disease (AD) and/or dementia, and, in addition, a mini-mental status examination (MMSE) score 1 standard deviation below the group mean of the participant’s age and education cohort, and/or an absolute MMSE score between 18 and 26. The patients were evaluated by licensed practitioners in the Memory Disorders Clinic of Geriatric Assessment and Planning Program at the University of North Texas Health Science Center. The inclusion criteria included that the presence of aMCI and the patient’s ability and willingness to visit the laboratory and tolerate intermittent-hypoxia ventilation via an air-cushioned, disposable face-mask. Patients were included who had controlled stabilized chronic conditions ≥1 year duration, including hypertension, coronary artery disease, ischemic stroke, diabetes or metabolic disease, chronic bronchitis, degenerative arthritis, and/or other aging-related chronic conditions. Exclusion criteria were MMSE score <18; diagnosis of dementia, moderate to severe cognitive impairment or AD; current enrollment in another clinical trial or interventional study; current smoker; uncontrolled chronic conditions including hypertension (systolic/diastolic pressures >140/90 mm Hg with medications), diabetes, chronic renal failure, nephritis, lung fibrosis, emphysema, infectious disease, cancer, or congenital heart disease; recurrent acute conditions such as chest pain, stroke, migraine, headaches, seizures or epileptic episodes, or exacerbation of asthma or allergic rhinitis, myocardial ischemia or infarct, a finding of second or third degree atrioventricular block on electrocardiogram; had or was expecting a major surgery or transplant; or neurological disorders including Parkinson’s disease, amyotrophic lateral sclerosis, vertigo, Tourette’s syndrome, Huntington’s disease, schizophrenia, or bipolar disorder.
Prior to enrollment, all participants visited the laboratory for orientation and assessment of their cardiovascular, respiratory, and tissue oxygen responses to hypoxia. During the orientation, participants were familiarized with the laboratory, study procedures, equipment, and measurements; and their IHT tolerance was determined. Five patients were excluded from the study because of ScO2 below 50% while breathing room air (one patient), phobic reaction to the facemask (2 patients), and multiple premature ventricular contractions or arrhythmia during hypoxia breathing (2 patients). Another 2 participants began but did not complete the IHT program because of unanticipated schedule conflicts and a nonstudy related surgery, respectively. Seven participants diagnosed with aMCI completed the 8-week IHT program. Table 1 summarizes the characteristics of these 7 patients, 6 of whom had histories of hypertension managed with medications.
Table 1.
Characteristics of Study Participants.
| Patients (n = 7) With MCI | 1 Man, 6 Women |
| Age | 69.3 ± 2.7 years (range 58-78 years) |
| Body mass index | 28 ± 4 kg/m2 (5 overweight or obese) |
| Patients with treated hypertension (n = 6) | On one or more medications: Ca2+ channel blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, β-adrenoceptor blockers, diuretics |
| Other conditions |
|
| Education | 14.5 ± 1.0 years (range 12-20 years) |
Abbreviation: MCI, mild cognitive impairment.
Procedures
After completing the orientation, all eligible patients underwent baseline evaluation of cognitive function, followed on a separate day by assessment of baseline physiological function. The patients then completed an IHT program consisting of 8 cycles per IH sessions of alternately breathing 10% O2 and room air, each for 5 minutes, 3 sessions per week for 8 weeks. Physiological function was re-evaluated 1 to 2 days after the last IH session, and post-training cognitive function was assessed 1 to 2 days after the physiological reassessment. All participants were instructed to maintain their regular routine and medications throughout the study.
Measurements
Cognitive assessment
Cognitive function was assessed by a licensed geriatric neuropsychologist using MMSE, California verbal learning test-second edition (CVLT-II short version), digit span (forward and backward), trail making test-B (TMT-B), and controlled oral word association test (COWAT) before and after the 8-week IHT program. All tests were conducted between 10:00 and 11:30 Am, with the patient in the seated position.
Physiological assessment
Cardiovascular, respiratory, and tissue O2 variables at rest and during 5-minute hypoxia exposures were measured for physiological assessment 1 to 2 days before starting and 1 to 2 days after completing the IHT program. During the test, the patient’s heart rate (HR) was monitored beat-to-beat by a standard electrocardiography limb lead. Continuous systolic arterial pressures (SAP) and diastolic arterial pressures (DAP) were monitored noninvasively by double finger cuffs placed on the proximal phalanges of the left index and middle fingers (CNAP 500, Graz, Austria). Mean arterial pressure (MAP) was estimated as (SAP + 2·DAP)/3. Systemic arterial O2 saturation (SaO2) was continuously monitored from the right earlobe by a radiometer sensor (TOSCA 500, Radiometer America Inc, Westlake, Ohio). The probe was maintained at 42°C to dilate and thereby arterialize the subcutaneous capillary blood in the earlobe. Regional ScO2 of the prefrontal cortex was monitored by near infrared spectroscopy (Somanetics, 5100 INVOS Cerebral Oximeter, Troy, Michigan) with a sensor placed on the right side of the forehead. Preliminary studies confirmed that ScO2 on right and left sides shows the same pattern and magnitude in response to hypoxic exposures in elderly patients with MCI. Middle cerebral artery flow velocity (VMCA) was monitored by transcranial Doppler sonography using a 2 MHz probe (EZ-Dop DWL System, Singen, Germany) placed on the left temporal window. Cerebral vascular conductance (CVC) was estimated as mean VMCA/MAP. These measurements and the techniques of transcranial Doppler sonography combined with near infrared spectroscopy have been applied in our previous studies. 27,29 -32
Continuous inspired and expired fractions of O2 and CO2 were measured using a mass spectrometer (Perkin-Elmer, 1100 Medical Gas Analyzer, St Louis, Missouri). Gas was sampled via a tubing embedded in the inlet of a VacuMed Universal Ventilation Meter (VacuMed, Ventura, CA, USA), which monitored ventilation frequency (breathing frequency f Br) and tidal volume (VT). Minute ventilation was calculated as VT·f Br. Partial pressures of end-tidal O2 (PETO2) and CO2 (PETCO2) were computed as ambient barometric pressure times the expired fractions of O2 and CO2, respectively. Analog data were continuously digitized online at 250 Hz by a computer interfaced with a data acquisition system (MP150 BIOPAC, Santa Barbara, CA). All measurements were made with the patient wearing a face mask (VacuMed) while resting in the supine position. All tests were performed approximately 2 hours after a light meal, between 9:00 and 11:00 am or 2:00 and 4:00 pm.
Intermittent Hypoxia Training
All participants breathed hypoxic air (10% ± 0.1% O2) for 5 min/cycle, alternated with 5-minute room air breathing, repeated for 8 cycles during each IH training (IHT) session (Figure 1), 3 sessions/week for 8 weeks. Two participants’ first 6 training sessions were 10 cycles of 4-minute breathing 10% O2 and 4-minute breathing room air, after 2 weeks, these patients were transitioned to the standard 5-minute 10% O2 5-minute room air cycles. This selection was based on the rate of decreases in SaO2 and/or ScO2 in the orientation IH session. Cumulative hypoxic time was 40 minutes for a single session, and total hypoxia exposure was 960 minutes over the IHT program. Electrocardiogram, SaO2, and ScO2 were continuously monitored during all IHT sessions. No adverse or unexpected events were detected in any of the patients during the IHT sessions.
Figure 1.
Cardiorespiratory responses to a typical intermittent hypoxia (IH) session. Each IH session consisted of 8 cyclic bouts of breathing 10% O2 for 5 minutes and room air for another 5 minutes. From top to bottom, the panels show the fractions of inspired and expired O2, arterial O2 saturation, cerebral tissue O2 saturation, heart rate, tidal volume, and the fraction of expired CO2. Arterial O2 saturation fell gradually during 5-minute hypoxic exposures and then recovered completely during room-air breathing. Cerebral tissue O2 saturation fell in lockstep with arterial O2 saturation. Heart rate and tidal volume increased during hypoxia, while end-tidal CO2 fell due to increased ventilation.
Data Analysis and Statistics
Each 5-minute hypoxic exposure was divided into five 1-minute intervals, within which SaO2, ScO2, HR, SAP, DAP, f Br, VT, minute ventilation, PETCO2, and PETO2 values were averaged and compared with the respective prehypoxia baseline values. The reported data represent the average values collected during the last 30 seconds of each 1-minute interval, as described previously. 26 The VMCA data were collected at rest and during the fifth minute of each hypoxic exposure, respectively, from 5 patients, because 2 patients did not have a clear transcranial Doppler signal. Group data or differences before and after IHT were compared by paired t tests after the data set passed the Shapiro-Wilk normality test. Raw cognitive function test scores were reported. Two-factor analyses of variance were applied to assess the effects of the 5-minute hypoxia and IHT. Least-squares linear regression was applied to define the relationship between ScO2 and SaO2, and a general linear model was applied to evaluate the interactions of IHT with SaO2 and ScO2 during hypoxia exposures. Statistical analyses were performed with Statistical Analysis System software (SAS Version 9.4, Cary, North Carolina). All data are reported as group mean values ± standard error of the mean. Statistical significance was accepted at P ≤ 0.05.
Results
Resting Physiological Variables
Eight-week IHT significantly decreased resting MAP (P = .008), SAP (P = .047), and DAP (P = .031) in older adults with MCI (Table 2). Resting SaO2 was slightly (approximately 1%), but significantly (P = .037) lower after IHT, possibly due to decreased resting ventilation (P = .097). Cerebral tissue oxygenation (ScO2) at rest increased (P = .035) from 67.9% ± 1.2% before to 70.7% ± 1.6% after IHT (Table 2). However, IHT did not affect (P = .207) VMCA at rest (baseline 46.8 ± 3.0 cm/s; post-IHT 44.2 ± 1.9 cm/s). Similarly, CVC at rest was not different (P = .632) before (0.479 ± 0.020 cm/mm Hg·s) and after (0.468 ± 0.021 cm/[mm Hg·s]) IHT. Neither VT (P = .402), breathing frequency (f Br, P = .117), PETCO2 (P = .421), nor PETO2 (P = .554) at rest differed before and after 8-week IHT (Table 2).
Table 2.
Cardiovascular and Respiratory Function During 5-Minute Acute Hypoxia Before and After IHT.
| 0 minute | 1 minute | 2 minutes | 3 minutes | 4 minutes | 5 minutes | P Value | ||
|---|---|---|---|---|---|---|---|---|
| HR, bpm | Before | 70 ± 5 | 72 ± 5 | 75 ± 5 | 78 ± 5 | 80 ± 6 | 82 ± 5 | Hypoxia: .037 IHT: .382 |
| After | 67 ± 5 | 70 ± 4 | 73 ± 5 | 76 ± 4 | 78 ± 4 | 78 ± 4 | ||
| MAP, mm Hg | Before | 101 ± 3 | 100 ± 3 | 99 ± 3 | 98 ± 3 | 97 ± 3 | 98 ± 3 | Hypoxia: .413 IHT: .021 |
| After | 95 ± 3a | 97 ± 2 | 94 ± 2 | 94 ± 2 | 92 ± 2 | 92 ± 2 | ||
| SAP, mm H) | Before | 139 ± 2 | 139 ± 2 | 137 ± 3 | 136 ± 2 | 135 ± 3 | 135 ± 3 | Hypoxia: .338 IHT: .006 |
| After | 132 ± 3a | 135 ± 3 | 131 ± 4 | 130 ± 4 | 128 ± 4 | 128 ± 4 | ||
| DAP, mm Hg | Before | 82 ± 3 | 81 ± 3 | 80 ± 3 | 79 ± 3 | 78 ± 4 | 79 ± 3 | Hypoxia: .319 IHT: .002 |
| After | 77 ± 3a | 78 ± 2 | 76 ± 3 | 76 ± 2 | 74 ± 2 | 74 ± 2 | ||
| SaO2, % | Before | 97.3 ± 0.3 | 91.6 ± 0.5 | 83.3 ± 1.4 | 78.8 ± 1.8 | 74.8 ± 2.2 | 70.3 ± 2.9 | Hypoxia: .001 IHT: .657 |
| After | 96.3 ± 0.5a | 90.4 ± 0.7 | 83.8 ± 0.8 | 79.7 ± 1.1 | 76.2 ± 1.5 | 73.8 ± 1.4 | ||
| ScO2, % | Before | 67.9 ± 1.2 | 62.2 ± 1.4 | 57.7 ± 1.8 | 55.1 ± 1.9 | 52.8 ± 2.1 | 50.9 ± 2.4 | Hypoxia: .001 IHT: .004 |
| After | 70.7 ± 1.6a | 65.9 ± 1.9 | 61.8 ± 1.7 | 59.2 ± 1.7 | 56.7 ± 1.7 | 55.3 ± 2.0 | ||
| f Br, br/min | Before | 14 ± 1 | 13 ± 1 | 13 ± 1 | 13 ± 1 | 13 ± 1 | 13 ± 1 | Hypoxia: .872 IHT: .818 |
| After | 12 ± 1 | 12 ± 2 | 13 ± 2 | 13 ± 2 | 14 ± 2 | 13 ± 2 | ||
| VT, L | Before | 0.70 ± 0.06 | 0.85 ± 0.11 | 0.95 ± 0.09 | 0.93 ± 0.09 | 0.96 ± 0.10 | 1.08 ± 0.16 | Hypoxia: .002 IHT: .597 |
| After | 0.76 ± 0.10 | 0.93 ± 0.08 | 0.95 ± 0.09 | 0.99 ± 0.07 | 1.00 ± 0.06 | 1.01 ± 0.11 | ||
| Vminute, L/min | Before | 9.40 ± 0.96 | 10.46 ± 1.07 | 11.82 ± 1.31 | 12.42 ± 1.98 | 12.24 ± 1.68 | 13.71 ± 2.21 | Hypoxia: .008 IHT: .898 |
| After | 8.66 ± 1.21 | 11.19 ± 1.47 | 11.56 ± 1.55 | 12.58 ± 1.39 | 13.52 ± 1.70 | 13.22 ± 2.08 | ||
| PETCO2, mm Hg | Before | 42.4 ± 0.5 | 41.0 ± 0.8 | 40.0 ± 0.6 | 39.6 ± 1.0 | 39.8 ± 0.9 | 39.6 ± 0.9 | Hypoxia: .001 IHT: .580 |
| After | 43.1 ± 0.5 | 41.0 ± 0.3 | 40.0 ± 0.2 | 39.3 ± 0.5 | 38.8 ± 0.7 | 39.0 ± 0.6 | ||
| PETO2, mm Hg | Before | 105 ± 2 | 60 ± 2 | 49 ± 1 | 46 ± 1 | 44 ± 1 | 42 ± 1 | Hypoxia: .001 IHT: .448 |
| After | 103 ± 1 | 60 ± 1 | 51 ± 1 | 48 ± 1 | 46 ± 1 | 43 ± 1 | ||
Abbreviations: DAP, diastolic blood pressure; f Br, breathing frequency; HR, heart rate; IHT, intermittent hypoxia training; MAP, mean arterial pressure; PETCO2, partial pressure of end-tidal CO2; PETO2, partial pressure of end-tidal O2; SAP, systolic blood pressure; SaO2, arterial oxygen saturation; ScO2, cerebral tissue oxygen saturation; SEM, standard error of the mean; VT, tidal volume; Vent, ventilation.
a A statistically significant difference in the baseline valuables (0 minute) before and after IHT (paired t test). P values indicate the outcome of 2-factor analysis of variance: hypoxia factor (room air vs hypoxic air) and training factor (before vs after IHT). Mean values ± SEM from 7 patients.
Physiological Responses to Hypoxia Exposures
Heart rate, blood pressure, and ventilatory responses to hypoxemia
Five-minute exposures to 10% O2 produced moderate hypoxemia (P < .001), indicated by progressive decreases in SaO2 to approximately 70% and 74% during the fifth minute of hypoxia before and after the 8-week IHT program, respectively. Intermittent hypoxia training did not modulate the SaO2 response to acute hypoxia (IHT factor P = .657; Table 2). Heart rate progressively increased during 5-minute hypoxia exposures (hypoxia factor P = .037), but IHT did not alter these HR responses (IHT factor P = .382). Although systemic arterial pressure was not affected by acute hypoxia exposures, MAP, SAP, and DAP were consistently lower after IHT (Table 2). As expected, minute ventilation increased during hypoxia (IHT factor P = .008); IHT did not alter this physiological response (IHT factor P = .898). The augmented minute ventilation was due entirely to increases in VT (hypoxia factor P = .002) at constant f Br (hypoxia factor P = .872); again, IHT altered neither VT nor f Br during hypoxia (Table 2).
Cerebral tissue hypoxia and cerebrovascular response during hypoxemia
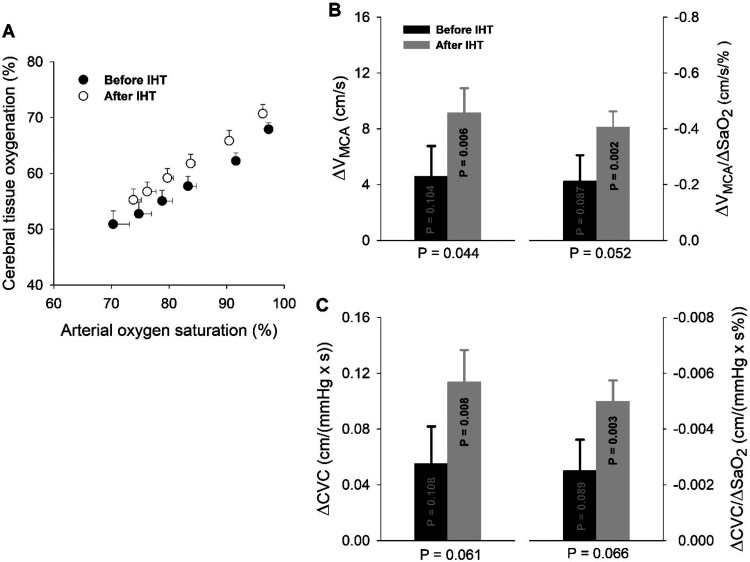
During 5-minute hypoxia, ScO2 progressively fell (hypoxia factor P < .001), concordant with the hypoxia-induced acute hypoxemia (Figure 2A). Although the changes in ScO2 per unit change in SaO2 were similar before and after IHT, ScO2 was consistently greater after IHT versus pre-IHT baseline (P = .004). Five-minute hypoxia increased VMCA by 4.5 ± 2.2 cm/s (10% increase) before and 9.2 ± 1.8 cm/s (+21% increase) after IHT, respectively (Figure 2B); thus, IHT doubled the cerebrovascular hyperemic response to acute hypoxia (P = .044). This IHT enhancement of cerebrovasodilation remained after normalizing the ΔVMCA values to the decreases in SaO2; thus, ΔVMCA/ΔSaO2 (cm/[s·%]) increased from −0.21 ± 0.09 before to −0.41 ± 0.06 after IHT (P = .052). Figure 2C presents the hypoxia-induced changes in estimated CVC (ΔCVC), that is, ΔVMCA/MAP, without normalization (left graph) and normalized to the hypoxia-induced decrease in SaO2 (ΔCVC/ΔSaO2; right graph). Cerebral vasodilation indicated by ΔCVC or ΔCVC/ΔSaO2 during the fifth minute of hypoxic breathing was doubled after the IHT program.
Figure 2.
Cerebral tissue oxygenation and cerebrovascular responses during hypoxemia. Panel A, Cerebral tissue hypoxia is highly correlated with hypoxemia (P < .001) during 5-minute acute hypoxic exposure, and cerebral tissue O2 saturation is consistently greater (P = .004) after intermittent hypoxic training (n = 7), but the changes in ScO2 per unit change in SaO2 are similar (P = .232) before and after IHT. Panel B, The changes in blood flow velocity of the middle cerebral artery (ΔVMCA) and ΔVMCA values normalized by hypoxemia (ΔVMCA/ΔSaO2) during the fifth minute of acute hypoxia (n = 5). Although only nonstatistically significant trends in these cerebrovascular responses were noted before IHT (ΔVMCA: P = .104; ΔVMCA/ΔSaO2: P = .087), IHT’s effects on both were statistically significant (ΔVMCA: P = .006; ΔVMCA/ΔSaO2: P = .002), and the magnitudes of these ΔVMCA (P = .044) and ΔVMCA/ΔSaO2 (P = .052) responses nearly doubled after IHT versus pre-IHT. Panel C, Cerebral vasodilation in terms of changes in estimated cerebrovascular conductance (ΔCVC) or ΔCVC normalized by hypoxemia (ΔCVC/ΔSaO2); both measures of vasodilation were appreciably enhanced after 8-week IHT. The P values within the bars apply to the changes in these variables during acute hypoxia versus the respective prehypoxia baselines. The P values below the abscissa apply to the comparison of IH-induced increases in these variables post- versus pre-IHT (ie, grey bar vs black bar). IHT indicates intermittent hypoxia training; SaO2, arterial oxygen saturation; ScO2, cerebral tissue O2 saturation.
Cognitive Function
Table 3 presents the raw scores of cognitive testing in the participants with aMCI before and after 8-week IHT, except scaled scores are reported for TMT-B because its raw scores failed the normality test. The participants’ performance on MMSE and digit span tests significantly improved after IHT: MMSE score increased from pre-IHT 25.7 ± 0.4 to 27.7 ± 0.6 after IHT (P = 0.038), and digit span score increased from 24.7 ± 1.2 before to 26.1 ± 1.3 after IHT (P = .047). Although not statistically significant, a trend toward improvement in the CVLT-II test after versus before IHT (P = .102) was noted. However, neither TMT-B nor COWAT was not altered by IHT (Table 3).
Table 3.
Cognitive Performance Measures Before and After 8-Week Intermittent Hypoxic Training.a
| MMSE (Point) | CVLT-II (Total Score) | Digit Span (Total Score) | TMT-B (Scaled Score)b | COWAT (Words) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Before | After | Before | After | Before | After | Before | After | Before | After |
| 1 | 24 | 29 | 45 | 40 | 24 | 24 | 5 | 8 | 24 | 26 |
| 2 | 26 | 30 | 58 | 72 | 23 | 24 | 11 | 14 | 24 | 36 |
| 3 | 26 | 27 | 66 | 74 | 21 | 21 | 10 | 8 | 28 | 26 |
| 4 | 25 | 25 | 60 | 60 | 22 | 26 | 8 | 7 | 32 | 19 |
| 5 | 26 | 27 | 62 | 67 | 29 | 30 | 9 | 9 | 57 | 71 |
| 6 | 26 | 29 | 42 | 63 | 28 | 31 | 8 | 8 | 33 | 49 |
| 7 | 27 | 27 | 49 | 51 | 26 | 27 | 1 | 6 | 45 | 48 |
| Mean ± SEM | 25.7 ± 0.4 | 27.7 ± 0.6 | 54.6 ± 3.5 | 61.0 ± 4.6 | 24.7 ± 1.2 | 26.1 ± 1.3 | 7.4 ± 1.3 | 8.6 ± 1.0 | 34.7 ± 4.6 | 39.3 ± 6.8 |
| P value | .038 | .102 | .047 | .280 | .285 | |||||
Abbreviations: COWAT, controlled oral word association test; CVLT-II, California verbal learning test-second edition (short version); MMSE, mini-mental state examination; SEM, standard error of the mean; TMT-B, trail making test-version B.
a P value indicates the outcome from the paired t test.
b Scaled score reported for TMT-B because the unit score (in seconds) failed the normality test for the paired t test. All other data represent the raw scores.
Discussion
Brain has the highest metabolic rate and O2 demand of the organs and tissues, and the notion that reduced O2 supply could benefit the brain may seem counterintuitive. Indeed, interruptions of O2 delivery due to ischemic stroke, cardiac arrest, and asphyxiation inflict devastating damage to the human brain. On the other hand, moderate hypoxia, which causes hypoxemia, but not ischemia, evokes adaptations that increase the brain’s resistance to hypoxia or ischemia. 33 Moreover, IH-induced hypoxemia activates cerebrovasodilation and may improve cognitive function in the early stages of neurodegenerative disease. This study tested, for the first time, the impact of IHT on cognitive performance and cerebrovascular function in elderly adults with aMCI. Two patients were excluded from the IHT program due to cardiac rhythm disturbances during the orientation screening with acute hypoxia exposure, another 2 could not tolerate the facemask, and another patient did not undergo acute hypoxia due to low SaO2 on room air, but of the 9 patients who passed the initial screening, 7 completed the IHT program with no untoward effects, and the other 2 left the study for reasons unrelated to the IHT. Thus, the 8-week program of IH exposures eliciting brief, cyclic, moderate hypoxemia was tolerated by the older adults with aMCI who passed the initial screening without unexpected or advent incidents or cardiac arrhythmias. Furthermore, some measures of cognitive performance, particularly short-term memory and concentration ability, were improved by IHT in these patients with aMCI
The IH exposures lowered resting arterial pressure and enhanced cerebral tissue oxygenation and cerebrovascular dilation. During 5-minute breathing of hypoxia air (10% O2), SaO2 in older adults with aMCI fell to approximately 70% of baseline before IHT and to approximately 74% of baseline after IHT. This moderate hypoxemia did not cause the elderly participants any distress or discomfort. The cerebral tissue hypoxia induced by moderate hypoxemia was associated with compensatory cerebrovasodilation (Figure 2). Moreover, following 8-week IHT, the patients’ mean, SAP, and DAP were lowered by 5 to 7 mm Hg versus the respective pre-IHT values, bringing them nearer the current targets. 34 This favorable IHT effect was concordant with those reported by Lyamina et al, 21 who demonstrated that a 20-day IHT program of 4 to 10 daily cycles of 3-minute exposures to 10% O2 alternated with 3-minute room air breathing reduced 24-hour SAP from 151 ± 1 to 129 ± 1 mm Hg and DAP from 95 ± 1 to 79 ± 1 mm Hg in young adult patients (ca 32 years) with stage 1 hypertension. Since 6 of the 7 patients with aMCI had medication-controlled hypertension (Table 1), the present study not only demonstrated the safety of IHT, but extended its antihypertensive effect to older adults with aMCI.
The hemodynamic and ventilatory responses to the hypoxia exposures distinguished the IH exposures from OSA, where interruptions in ventilation produce hypercapnia and provoke hypertension. Intermittent hypoxia exposures augmented the patients’ minute ventilation, an effect entirely due to increased VT (Table 2). Unlike the hypercapnia seen in OSA, the increased ventilation during IH produced acute hypocapnia and, consequently, transient respiratory alkalosis. Moreover, the increased VT would have lowered intrathoracic pressure 35 and, thus, augmented the cephalothoracic pressure gradient, thereby enhancing cerebral perfusion by facilitating venous return from the brain to the heart. 36 The augmented cerebral drainage would help maintain cerebral volume homeostasis in the face of compensatory increases in cerebral perfusion during acute hypoxemia. The coordinated augmentation of cerebral perfusion afforded by cerebral vasodilation and VT-enhanced cerebrovascular drainage could promote clearance of metabolic wastes and excessive interstitial fluid and solutes from the cerebral parenchyma during cyclic IH exposures.
Although IHT did not modulate resting VMCA, the VMCA response to acute hypoxia exposure was appreciably augmented after 8-week IHT. Thus, acute IH exposures provoked cerebral vasodilation and increased cerebral perfusion. It therefore can be concluded that the cerebral tissue hypoxia during IH exposures resulted from hypoxemia, not cerebral ischemia. This repeated cerebrovascular activation over the course of the IHT program may activate beneficial adaptations potentiating hypoxemia-induced cerebral vasodilation in older adults with aMCI.
The IHT program increased resting ScO2 in older patients with aMCI. Since IHT did not alter resting VMCA, these data suggested that an increased ScO2 at rest was more likely associated with an enrichment of microvasculature in the cortical region following IHT. Intermittent hypoxia induction of angiogenesis is likely mediated by increases in vascular endothelial growth factor, erythropoietin, and brain-derived neurotrophic factor after the activated expression of hypoxia-inducible factor 1 (HIF-1) and its target genes. 37 Erythropoietin, a powerful neuroprotectant 38,39 which is expressed by astrocytes in a HIF-1 activated manner, 40 promotes brain angiogenesis after focal ischemia in mice. 41 In humans, 2-minute moderate IH exposures (reduced PETO2 to 45 mm Hg) interspersed with 2-minute recovery increased erythropoietin concentration in blood, 42 enabling sufficient circulating erythropoietin to cross the blood–brain barrier to afford neuroprotection. 43 Furthermore, hypoxia adaptation is reported to augment cerebral expression of neuroglobin, an O2-binding heme protein, in rat, 44 particularly in the ischemia-vulnerable hippocampus. 45 Augmented neuroglobin combined with angiogenesis may provide the basis for the IHT-related increase in ScO2 observed in the present study.
In parallel with enhanced cerebral tissue oxygenation, this study also demonstrated that IHT improved the participants’ MMSE and digit span scores, and tended to improve their performance on the CVLT-II test (Table 3). Although TMT-B and COWAT performance improved in 4 and 5 of the 7 participants, respectively, neither of these improvements attained statistical significance following the 8-week IHT program. This discrepancy was probably associated with an age-related increase in intraindividual variability in executive function and/or reaction time 46 -48 as indicated by TMT-B and COWAT scores, in addition to a possible type II or β error. Nonetheless, the improved short-term memory and concentration ability following IHT suggested that this intervention could be potentially applied to help improve overall cognitive function in patients with aMCI. The persistence of the IHT-induced cognitive improvements and the possibility that more protracted IHT programs may produce even greater benefits, merit investigation.
Study Limitations and Perspectives
The main study limitation was that we were unable to distinguish whether improvement in some cognitive performance tests resulted from the IHT intervention or a practice effect due to the study’s repeated-measures design, because there was no sham-IHT or control group in this pilot study. In addition, the patients might experience a placebo effect since the intervention was not blind. Future clinical trials utilizing a randomized, double-blind design to compare pre- and postintervention cognitive performance in IHT versus sham-IHT groups are needed. Nonetheless, this pilot study suggests that IHT with brief cyclic moderate hypoxemia is tolerated in elderly with aMCI and potentially effective to prevent or retard neurodegenerative progression to AD-dementia before cognitive decline becomes irreversible. To assure patient safety, before IHT commences, the patient should undergo acute hypoxia exposure while electrocardiogram, SaO2, and ScO2 are monitored, as was done in the orientation visit of this pilot study.
In summary, this exploratory pilot study confirms that brief, cyclic moderate hypoxemia is tolerable by elderly adults. Intermittent hypoxia training may be adapted as a novel intervention for treating patients with MCI or early AD. An 8-week IHT program lowers resting arterial pressure and enhances cerebrocortical tissue O2 saturation and cerebral vasodilation during hypoxia challenge and potentially improves cognitive performance on tests for attention and short-term memory in older adults with MCI.
Acknowledgments
We thank all participants for their cheerful cooperation during this clinical study. Drs Sid O’Bryant and Meharvan Singh, Departments of Pharmacology and Neuroscience, University of North Texas Health Science Center; Benjamin Williams, Departments of Neurology and Neurotherapeutics, University of Texas Southwestern Medical Center; and Roderick McColl, Department of Radiology, University of Texas Southwestern Medical Center, provided incisive advice during the development of this study. Study data are available from the corresponding author on request.
Footnotes
Author Contributions: HW, PC, XS, and RTM contributed to hypothesis development; HS, HW, and XS contributed to subject recruitment and screening; XS, HS, JRH, SER, and GPK contributed to data collection; all of the authors contributed to data analysis and interpretation; HW and PC contributed to drafting of manuscript; XS and RTM contributed to preparation, editing, and revision of manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a Texas Alzheimer’s Research & Care Consortium—Investigator Grant Program award to XR from the Texas Council on Alzheimer’s Disease and Related Disorders, and an intramural research award to RTM from UNTHSC’s Division of Research Development and Commercialization.
ORCID iD: Xiangrong Shi  https://orcid.org/0000-0002-5422-489X
https://orcid.org/0000-0002-5422-489X
References
- 1. Serebrovskaya TV, Manukhina EB, Smith ML, Downey HF, Mallet RT. Intermittent hypoxia: cause of or therapy for systemic hypertension? Exp Biol Med (Maywood). 2008;233(6):627–650. [DOI] [PubMed] [Google Scholar]
- 2. Navarrete-Opazo A, Mitchell GS. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol. 2014;307(10):R1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tahawi Z, Orolinova N, Joshua IG, Bader M, Fletcher EC. Altered vascular reactivity in arterioles of chronic intermittent hypoxic rats. J Appl Physiol. 2001;90(5):2007–2013; discussion 2000. [DOI] [PubMed] [Google Scholar]
- 4. Phillips SA, Olson EB, Morgan BJ, Lombard JH. Chronic intermittent hypoxia impairs endothelium-dependent dilation in rat cerebral and skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol. 2004;286(1):H388–393. [DOI] [PubMed] [Google Scholar]
- 5. Wu Q, Cunningham JT, Mifflin S. Transcription factor ΔFosB acts within the nucleus of the solitary tract to increase mean arterial pressure during exposures to intermittent hypoxia. Am J Physiol Heart Circ Physiol. 2018;314(2):H270–H277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu XM, Yao D, Cai XD, et al. Effect of chronic continual- and intermittent hypoxia-induced systemic inflammation on the cardiovascular system in rats. Sleep Breath. 2015;19(2):677–684. [DOI] [PubMed] [Google Scholar]
- 7. Li RC, Row BW, Kheirandish L, et al. Nitric oxide synthase and intermittent hypoxia-induced spatial learning deficits in the rat. Neurobiol Dis. 2004;17(1):44–53. [DOI] [PubMed] [Google Scholar]
- 8. Gozal D, Nair D, Goldbart AD. Physical activity attenuates intermittent hypoxia-induced spatial learning deficits and oxidative stress. Am J Respir Crit Care Med. 2010;182(1):104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramond A, Ribuot C, Levy P, Joyeux-Faure M. Deleterious myocardial consequences induced by intermittent hypoxia are reversed by erythropoietin. Respir Physiol Neurobiol. 2007;156(3):362–369. [DOI] [PubMed] [Google Scholar]
- 10. Wang Y, Guo SZ, Bonen A, et al. Monocarboxylate transporter 2 and stroke severity in a rodent model of sleep apnea. J Neurosci. 2011;31(28):10241–10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mallet RT, Ryou MG, Williams AG, Jr, Howard L, Downey HF. β1-Adrenergic receptor antagonism abrogates cardioprotective effects of intermittent hypoxia. Basic Res Cardiol. 2006;101(5):436–446. [DOI] [PubMed] [Google Scholar]
- 12. Manukhina EB, Belkina LM, Terekhina OL, et al. Normobaric, intermittent hypoxia conditioning is cardio- and vasoprotective in rats. Exp Biol Med (Maywood). 2013;238(12):1413–1420. [DOI] [PubMed] [Google Scholar]
- 13. Zong P, Setty S, Sun W, et al. Intermittent hypoxic training protects canine myocardium from infarction. Exp Biol Med (Maywood). 2004;229(8):806–812. [DOI] [PubMed] [Google Scholar]
- 14. Zhu XH, Yan HC, Zhang J, et al. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci. 2010;30(38):12653–12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dale EA, Ben Mabrouk F, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology (Bethesda). 2014;29(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goryacheva AV, Kruglov SV, Pshennikova MG, et al. Adaptation to intermittent hypoxia restricts nitric oxide overproduction and prevents beta-amyloid toxicity in rat brain. Nitric Oxide. 2010;23(4):289–299. [DOI] [PubMed] [Google Scholar]
- 17. Jung ME, Simpkins JW, Wilson AM, Downey HF, Mallet RT. Intermittent hypoxia conditioning prevents behavioral deficit and brain oxidative stress in ethanol-withdrawn rats. J Appl Physiol. 2008;105(2):510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ryou MG, Mallet RT, Metzger DB, Jung ME. Intermittent hypoxia training blunts cerebrocortical presenilin 1 overexpression and amyloid-beta accumulation in ethanol-withdrawn rats. Am J Physiol Regul Integr Comp Physiol. 2017;313(1): R10–R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burtscher M, Pachinger O, Ehrenbourg I, et al. Intermittent hypoxia increases exercise tolerance in elderly men with and without coronary artery disease. Int J Cardiol. 2004;96(2):247–254. [DOI] [PubMed] [Google Scholar]
- 20. Haider T, Casucci G, Linser T, et al. Interval hypoxic training improves autonomic cardiovascular and respiratory control in patients with mild chronic obstructive pulmonary disease. J Hypertens. 2009;27(8):1648–1654. [DOI] [PubMed] [Google Scholar]
- 21. Lyamina NP, Lyamina SV, Senchiknin VN, Mallet RT, Downey HF, Manukhina EB. Normobaric hypoxia conditioning reduces blood pressure and normalizes nitric oxide synthesis in patients with arterial hypertension. J Hypertens. 2011;29(11):2265–2272. [DOI] [PubMed] [Google Scholar]
- 22. Saeed O, Bhatia V, Formica P, et al. Improved exercise performance and skeletal muscle strength after simulated altitude exposure: a novel approach for patients with chronic heart failure. J Card Fail. 2012;18(5):387–391. [DOI] [PubMed] [Google Scholar]
- 23. Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology. 2014;82(2):104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Serebrovska TV, Portnychenko AG, Drevytska TI, et al. Intermittent hypoxia training in prediabetes patients: Beneficial effects on glucose homeostasis, hypoxia tolerance and gene expression. Exp Biol Med (Maywood). 2017;242(15):1542–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bayer U, Likar R, Pinter G, et al. Intermittent hypoxic-hyperoxic training on cognitive performance in geriatric patients. Alzheimers Dement (NY). 2017;3(1):114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang P, Downey HF, Shi X. Acute intermittent hypoxia exposures enhance arterial oxygen delivery. Exp Biol Med (Maywood). 2010;235(9):1134–1141. [DOI] [PubMed] [Google Scholar]
- 27. Liu X, Xu D, Hall JR, et al. Enhanced cerebral perfusion during brief exposures to cyclic intermittent hypoxemia. J Appl Physiol. 2017;123(6):1689–1697. [DOI] [PubMed] [Google Scholar]
- 28. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, Tangelos EG. Aging, memory, and mild cognitive impairment. Internat Psychoger. 1997;9(suppl 1):65–69. [DOI] [PubMed] [Google Scholar]
- 29. Zhang P, Huang G, Shi X. Cerebral vasoreactivity during hypercapnia is reset by augmented sympathetic influence. J Appl Physiol. 2011;110(2):352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Formes K, Zhang P, Tierney N, Schaller F, Shi X. Chronic physical activity mitigates cerebral hypoperfusion during central hypovolemia in elderly humans. Am J Physiol Heart Circ Physiol. 2010;298(3): H1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo H, Tierney N, Schaller F, Raven PB, Smith SA, Shi X. Cerebral autoregulation is preserved during orthostatic stress superimposed with systemic hypotension. J Appl Physiol. 2006;100(6):1785–1792. [DOI] [PubMed] [Google Scholar]
- 32. Zhang P, Shi X, Downey HF. Two-week normobaric intermittent-hypoxic exposures stabilize cerebral vasoreactivity during hypocapnia and hypercapnia. Exp Biol Med (Maywood). 2015;240(7):961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baillieul S, Chacaroun S, Doutreleau S, Detante O, Pepin JL, Verges S. Hypoxic conditioning and the central nervous system: a new therapeutic opportunity for brain and spinal cord injuries? Exp Biol Med (Maywood). 2017;242(11):1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Whelton, PK Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2018;138(17): e426–e483. [DOI] [PubMed] [Google Scholar]
- 35. Moreno AH, Burchell AR, Van der Woude R, Burke JH. Respiratory regulation of splanchnic and systemic venous return. Am J Physiol. 1967;213(2):455–465. [DOI] [PubMed] [Google Scholar]
- 36. Convertino VA, Ryan KL, Rickards CA, et al. Optimizing the respiratory pump: harnessing inspiratory resistance to treat systemic hypotension. Respir Care. 2011;56(6):846–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bernaudin M, Nedelec AS, Divoux D, MacKenzie ET, Petit E, Schumann-Bard P. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22(4):393–403. [DOI] [PubMed] [Google Scholar]
- 38. Mallet RT, Ryou M. Erythropoietin: endogenous protection of ischemic brain. Vitam Horm: 2017;105:197–232. [DOI] [PubMed] [Google Scholar]
- 39. Rabie T, Marti HH. Brain protection by erythropoietin: a manifold task. Physiology (Bethesda). 2008;23(5):263–274. [DOI] [PubMed] [Google Scholar]
- 40. Ruscher K, Freyer D, Karsch M, et al. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22(23):10291–10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li Y, Lu Z, Keogh CL, Yu SP, Wei L. Erythropoietin-induced neurovascular protection, angiogenesis, and cerebral blood flow restoration after focal ischemia in mice. J Cereb Blood Flow Metab. 2007;27(5):1043–1054. [DOI] [PubMed] [Google Scholar]
- 42. Brugniaux JV, Pialoux V, Foster GE, et al. Effects of intermittent hypoxia on erythropoietin, soluble erythropoietin receptor and ventilation in humans. Eur Resp J. 2011;37(4):880–887. [DOI] [PubMed] [Google Scholar]
- 43. Brines ML, Ghezzi P, Keenan S, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97(19):10526–10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Avivi A, Gerlach F, Joel A, et al. Neuroglobin, cytoglobin, and myoglobin contribute to hypoxia adaptation of the subterranean mole rat Spalax. Proc Natl Acad Sci USA. 2010;107(50):21570–21575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu Q, Yu KX, Ma QS, Liu YN. Effects of intermittent hypobaric hypoxia preconditioning on the expression of neuroglobin and Bcl-2 in the rat hippocampal CA1 area following ischemia-reperfusion. Genet Mol Res. 2015;14(3):10799–10807. [DOI] [PubMed] [Google Scholar]
- 46. Hultsch DF, MacDonald SW, Dixon RA. Variability in reaction time performance of younger and older adults. J Gerontol B Psychol Sci Soc Sci. 2002;57(2): P101–115. [DOI] [PubMed] [Google Scholar]
- 47. West R, Murphy KJ, Armilio ML, Craik FI, Stuss DT. Lapses of intention and performance variability reveal age-related increases in fluctuations of executive control. Brain Cogn. 2002;49(3):402–419. [DOI] [PubMed] [Google Scholar]
- 48. Dykiert D, Der G, Starr JM, Deary IJ. Age differences in intra-individual variability in simple and choice reaction time: systematic review and meta-analysis. PLoS One. 2012;7(10):e45759. [DOI] [PMC free article] [PubMed] [Google Scholar]