Abstract
Objective:
To investigate the association of a 6-month Zumba intervention with cognition and quality of life among older cognitively unimpaired apolipoprotein ∊4 (APOE4) carrier and noncarrier women.
Methods:
Fifty-three women were randomly assigned to either twice-weekly Zumba group classes or maintenance of habitual exercise (control group) for 6 months. At baseline, 3, and 6 months, all participants underwent neuropsychological, physical activity, and quality-of-life assessments.
Results:
Overall, neuropsychological test scores and level of physical activity did not differ between intervention and control groups at any time. However, compared to the control group, quality of life was higher at 3 months, and visuospatial working memory and response inhibition improved more in the intervention group by 6 months. Apolipoprotein ∊4 status did not affect the results.
Discussion:
Zumba may strengthen performance on visuospatial working memory among cognitively unimpaired older women but this needs to be tested in a larger clinical trial.
Keywords: Zumba, cognition, quality of life, apolipoprotein ∊4
Introduction
A large body of research in cognitively unimpaired elderly persons suggests that physical exercise has a positive effect on cognitive function. For example, although one meta-analysis failed to show evidence for a beneficial effect of aerobic exercise on cognitive function, 1 several meta-analyses including randomized controlled trials provide support for a beneficial effect of physical activity on cognitive function with effect sizes ranging from small to moderate. 2 –6 Furthermore, various prospective cohort studies have shown that physical activity is associated with a decreased risk of incident cognitive impairment. 7 –14 Exercise engagement has also been associated with fewer alterations of neuroimaging biomarkers that may be indicative of Alzheimer’s disease (AD) pathology in older cognitively unimpaired adults. 15,16 There are conflicting data regarding whether individuals who carry apolipoprotein ∊4 (APOE4) alleles, a common genetic risk factor for late-onset AD, are likely to benefit from exercise more, less, or the same as those without this genetic susceptibility. 12,14,17 –23
It has also been discussed in the literature that cognitive engagement during physical exercise (often referred to as gross-motor cognitive training) might be a potential factor influencing the association between exercise activity and cognitive function. 24 For example, French researchers reported greater improvement of cognitive function after combined aerobic and mental training as compared to either aerobic or mental training alone, 25 and investigators from Germany observed a significant effect on both physical and cognitive outcomes after combined physical and cognitive training in older adults. 26 A recent meta-analysis found that combining physical and cognitive activity in older unimpaired adults was associated with greater cognitive improvement than control conditions and the effects tended to be more pronounced for studies using simultaneous versus sequential designs. 27 Another systematic review and meta-analysis of mind–body exercise demonstrated that tai chi and dance, in particular, were associated with improved global cognition, cognitive flexibility, working memory, verbal fluency, and learning in cognitively intact or impaired older adults compared to controls. 28 One study examined the effects of a “specially designed aerobic dance routine” on cognition in patients with mild cognitive impairment (MCI) and found greater improvements in memory and processing speed at 6 months in those who did the 3-month intervention compared to the usual care control group. 29 Finally, research has shown that a 16-week dance activity program is also associated with increased engaging social activity in older adults and can be regarded as a promising alternative to traditional exercise programs to support successful aging. 30 Therefore, it is important to further investigate the cognitive effects of physical activity that merges both physical and mental stimulations and to focus on not only cognitive but also clinically significant outcomes such as quality of life. 24 Also, the previous studies did not quantify the level of physical activity in the intervention and control conditions in order to separate the effects of exercise from the mentally stimulating activity.
In this study, we investigated whether a type of aerobic exercise (Zumba) that repetitively engages visuospatial working memory and response inhibition to master the ever-changing dance steps might improve performance on tests that measure these domains of executive function. Zumba, which involves Latin-inspired dance in a group/social setting, has gained popularity in the past 10 years. 31 Reported positive effects of Zumba on physical health include muscular strength, 32,33 trunk strength endurance and balance, 34 aerobic/cardiovascular fitness, 33 –37 body composition, 35 –37 inflammatory biomarkers, 35 and pain. 36,38 In addition, a few studies reported beneficial effects on mental health, such as quality of life, 34,35 purpose in life, 33 and intrinsic motivation to exercise. 37 Uncontrolled, brief feasibility studies have also been conducted among patients with diabetes and/or metabolic syndrome 37,39 and patients with Parkinson disease, 40 suggesting that Zumba is safe, enjoyable, and associated with learning the dance steps and improved activity levels or physical fitness in these clinical populations.
Although Zumba might provide a unique opportunity to study aerobic exercise that also engages visuospatial memory, we are unaware of any published data examining the impact of Zumba on cognition. Therefore, in this pilot study, we compared cognitive test performance over time and, in particular, change in measures of visuospatial working memory and response inhibition between cognitively unimpaired women exposed to 6 months of twice-weekly, 60-minute Zumba group classes (intervention group) and those instructed to maintain their habitual physical activity (control group). The rationale for a 6-month, twice-weekly, 60-minute class was derived from the meta-analysis by Colcombe and Kramer who reported that interventions with longer (≥6 months) versus shorter durations, and sessions with longer (≥45 minutes) versus shorter durations had greater effect sizes with regard to cognitive function. 3 We expected Zumba practice to be associated with improvements in measures of cognitive function, particularly visuospatial working memory, as a main characteristic of Zumba is the combination of aerobic exercise with memory challenges, which might make this type of exercise uniquely positioned to impact cognitive function. In addition, we predicted Zumba to be associated with improved measures of quality of life. Lastly, we explored the association of Zumba with changes in other measures of executive function and memory and whether outcomes were influenced by APOE4 carrier status. We monitored potential changes in self-reported physical exercise and body composition among baseline, 3-month, and 6-month assessments.
Methods
Participants
Participants were cognitively unimpaired women aged 55 to 80 years. They were recruited from the community via a community registry, flyers, and a pool of individuals who had previously expressed interest in participating in our ongoing prospective observational study of cognitively unimpaired APOE4 homozygotes, heterozygotes, and noncarriers, 41 but who had not yet participated in longitudinal neuropsychological testing. To determine eligibility for study participation, individuals were screened using a complete medical history, the Folstein Mini-Mental State Examination (MMSE), 42 and the Hamilton Depression Rating Scale (HAM-D). 43 Individuals with MMSE scores <28; HAM-D scores ≥10; and with significant medical, psychiatric, neurological illnesses, substance abuse, and cognitive complaints or impairment were excluded from the study. Persons were also excluded if they were taking psychoactive medication that might clinically impair cognition (eg, narcotics) or improve cognition (eg, memantine or cholinesterase inhibitors or stimulants) or had previously engaged in regular Zumba. All participants underwent APOE genotyping using standard methods and with the understanding that they would not receive information about their APOE genotype status. All participants gave written informed consent, and the study was approved by the Mayo Clinic institutional review board and registered with clinicaltrials.gov (ID # NCT01012791).
Study Design
We conducted a 2-group randomized controlled pilot study; enrolled individuals were randomized in a 1:1 ratio to either the Zumba intervention group or control group (maintain habitual physical activity), and randomization was stratified by APOE4 status. All participants received written educational material about memory loss, stress management, healthy diet and physical activity, alcohol consumption, and smoking. Participants in the control group were encouraged to maintain their regular physical activity program consisting of any exercise that did not include dance or Zumba, such as walking, swimming, or biking. They were further asked to communicate regularly with the study coordinator and report on their engagement in physical activity using weekly exercise logs, with the intention to (1) motivate participants to maintain their habitual physical activity program and (2) to monitor the type of exercise being done.
Zumba Intervention
Participants in the intervention group were enrolled in a twice-weekly Zumba group class for 6 months, with the goal of completing at least 40 sessions with a duration of 60 minutes per session. Zumba classes were offered at several locations in metropolitan Phoenix, Arizona. Study participants joined classes offered to the general public with typically 15 to 20 individuals per class, although often only 1 study participant in any given class. However, all classes were taught by certified Zumba instructors who followed guidelines specifically developed for this study to ensure that the classes were equivalent in number and type of dance moves. We required that the classes not include variations such as more rigorous adding of weights or the slower paced “Zumba Gold” variety of Zumba. Each class typically involved following the instructor performing dance and aerobic movements, which variously incorporated simple elements of samba, salsa, merengue, mambo, hip-hop, squats, or lunges, performed to music. The first Zumba routine, approximately 5 minutes, was a slower, warm-up, and the last 5 minutes involved cooldown and stretching. Similar to the control group, participants in the intervention group kept a weekly log of hours and type of exercise they were doing, which was sent to the study coordinator each week.
Neuropsychological Assessment
At baseline, 3, and 6 months, participants from both groups underwent an extensive battery of neuropsychological tests (Table 1). The primary outcome measure was the Groton Maze Learning Test (GMLT) 44 total errors, which assesses visuospatial memory and executive function using a maze learning paradigm. In this task, the participant is shown a 10 × 10 grid of boxes on a computer screen. A 28-step pathway is hidden among these 100 possible locations. Each box represents move locations, and the grid refers to the box array (ie, 10 × 10). Participants are required to find the hidden pathway guided by 4 search rules. These rules are do not move diagonally, do not move more than 1 box (ie, do not jump), do not move back on the pathway, and return to the last correct location after an error. At each step, only the most recently selected box is shown. Feedback is given with visual and auditory cues to indicate whether the selected box is correct or incorrect. The primary outcome is total number of errors made in attempting to learn the same hidden pathway over 5 consecutive trials.
Table 1.
Overview of Neuropsychological Tests.
| Test | Assessed Domains | Outcome Measure |
|---|---|---|
| Groton Maze Learning Test (GMLT) | Visuospatial working memory, error monitoring, information processing speed, short-term delayed recall for a complex hidden maze | Total errors (higher score indicates poorer performance) |
| One-card learning (OCL) | Visual recognition memory and learning | Accuracy (higher score indicates better performance) |
| Two-back test | Working memory, attention | Accuracy (higher score indicates better performance) |
| Set-shifting (SETS) | Impulsivity, inhibition | Accuracy (higher score indicates better performance) |
| Continuous paired associate learning (CPAL) | Visual learning and memory | Accuracy (higher score indicates better performance) |
| Delis-Kaplan Executive Functioning System (DKEFS) Color-Word Interference Test (includes subscores of color naming time, word reading time, inhibition time, inhibit/switch time) | Executive functioning | Time to completion (higher score indicates poorer performance) |
| Delis-Kaplan Executive Functioning System (DKEFS) Sorting Test | Executive functioning | Correct sorts (higher score indicates better performance) |
| Trail-making Test (TMT) parts A and B | Visual attention, task switching | Time to completion (higher score indicates poorer performance) |
| Rey’s Auditory Verbal Learning Test (AVLT) long-term memory score | Verbal memory | Number of words recalled at 30 minutes delay (higher score indicates better performance) |
Other computerized cognitive tests (http://cogstate.com) included one-card learning task (OCL), 2-back task, set-shifting task (SETS), and continuous paired associate learning task (CPAL). We chose these tests, in part, due to their demonstrated minimal practice effect with repeated testing and because they were previously validated as reliable measures of cognitive change in older healthy individuals. 45 Noncomputerized tests included Delis-Kaplan Executive Functioning System (DKEFS) Color-Word Interference Test, Sorting Test, and Verbal fluency test 46 ; Trail-making Test parts A and B 47 ; and Rey’s Auditory Verbal Learning Test, 48 using alternate forms at 3 and 6 months to minimize practice effect. 49 Test administration was randomized for either the noncomputerized tests first or the computerized tests first. Examiners were blind to the participant’s group and APOE status. Immediately prior to the testing session, the study coordinator reminded each participant that they should not share their group assignment with the psychometrist.
Additional Measurements
At baseline, 3, and 6 months, participants in both groups completed the Short-Form Health Survey (SF-36) 50 and the Community Healthy Activities Model Program for Seniors (CHAMPS) physical activity questionnaire for older adults. 51 The SF-36 is a quality-of-life measure of general physical and mental health. The CHAMPS surveys information about frequency and time spent doing all types of physical activities over the previous 4-week period and measures both total time and intensity of physical exercise. We calculated kcal/wk for total exercise and kcal/wk for exercise with at least moderate intensity using CHAMPS. Furthermore, all participants had their body weight and height measured at baseline, 3, and 6 months and underwent an objective measure of aerobic fitness (6-minute walk test) 52,53 at baseline. Finally, participants in the intervention group were asked to rate Zumba class in terms of “enjoyment of Zumba class,” “ability to follow dance moves,” and “feeling it was a good work-out” at the end of each week (ordinal scale of 1-5, higher score indicating more positive subjective feeling).
Statistical Methods
Groton Maze Learning Test total errors postintervention (at 6 months) was chosen as the primary outcome measure due to our hypothesis that the unique combination of both physical and cognitive engagement in Zumba exercise and the need for participants to learn and memorize the changing dance steps might especially benefit spatial working memory and error monitoring. Mean baseline levels were compared using the 2-sample t test. Mean scores were compared between groups at 6 months using analysis of covariance to adjust for the corresponding baseline scores. For participants who did not complete the 6-month assessment (N = 2 in the intervention group; N = 3 in the control group), the 3-month assessment was used in the primary analysis. Each outcome was also analyzed using a responder definition approach in which a Pearson χ2 or Fisher exact test was used to compare, between arms, the proportion of women whose score improved by a half standard deviation (SD) of the baseline scores at the 6-month time point (or 3-month time point if no data were provided at the 6-month time point). A half SD of the baseline scores is commonly used as a clinically meaningful difference between groups, which is a medium effect size based on the work of Cohen. 54 Norman and colleagues 55 showed that this effect is applicable to patient quality-of-life survey data in a wide variety of studies, so much so that they described it as remarkably universal. The effect of Zumba in APOE4 carriers was compared to the effect of Zumba in APOE4 noncarriers by using a multiple logistic regression model with terms for treatment, APOE4 type, and the treatment-by-type interaction. Practice effects were assessed by calculating the change from baseline in the control group with statistical significance based on a paired t test. The analyses included all participants who provided follow-up data, regardless of compliance with the treatment regimen or the number of follow-up visits.
The study was designed as a pilot study to capture feasibility data and provide a preliminary estimate of the impact of Zumba on a variety of outcomes. As such, the study was not powered to detect small-to-moderate effects. Specifically, for a 2-sided α = .05, 2-sample t test with 30 participants in the intervention and 23 participants in the control group, this study had 80% power to detect a 0.74 SD effect-size difference between groups in the primary end point (a moderate-to-large effect). Given the pilot nature of this study, P values <.05 were considered statistically significant throughout without adjustment for multiple testing, with the understanding that significant effects would be tested in a subsequent randomized trial.
Results
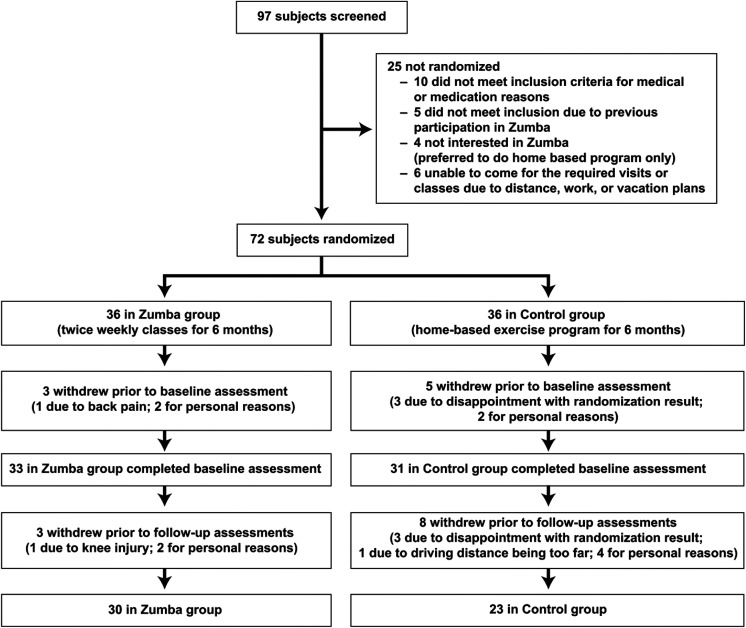
A total of 97 participants were screened and 72 were randomized to either Zumba intervention group or control group (Figure 1). Six women in the intervention group and 13 in the control group withdrew prematurely and without providing primary follow-up data, so the final analysis included 30 women in the intervention group and 23 women in the control group.
Figure 1.
Disposition of participant enrollment.
Demographics at Baseline
All baseline characteristics were well balanced between groups when comparing participants with baseline data, regardless of dropouts. No significant differences between control and intervention groups were found for age, ethnicity, race, diabetes, hypertension, family history of AD, depression score, MMSE, smoking, marital status, or education. After dropouts, there were more APOE4 carriers in the control group (P = .05), but groups otherwise remained well balanced for all other characteristics, including neuropsychological test scores and physical exercise. None of the 10 SF-36 measures differed between groups at baseline, with the exception of bodily pain (Zumba group reported higher quality of life for bodily pain, P = .04; data not shown). Most dropouts occurred before the 3-month follow-up, and the number of participants at 3 and 6 months was similar. Baseline characteristics for participants with follow-up data are displayed in Table 2.
Table 2.
Comparison of Baseline Characteristics for Participants With Follow-Up Data.
| Zumba | Control | P | |
|---|---|---|---|
| N | 30 | 23 | |
| Age (years), mean (SD) | 63.1 (6.0) | 63.6 (6.6) | .80a |
| APOE4 carrier | 9 (30%), 1 ∊4 homozygote | 13 (57%), 1 ∊4 homozygote | .05b |
| Weight (kg), mean (SD) | 72 (11) | 71 (13) | .79a |
| BMI (kg/m2), mean (SD) | 28.0 (5.0) | 27.8 (4.2) | .89a |
| Total exercise (1000 kcal/wk), mean (SD) | 11.6 (6.8) | 11.6 (10.2) | >.99a |
| Moderate exercise (1000 kcal/wk), mean (SD) | 6.7 (6.4) | 7.1 (6.9) | .81a |
| GMLT total errors, mean (SD) | 72 (35) | 69 (22) | .73a |
| DKEFS inhibition, mean (SD) | 57 (15) | 58.4 (8.4) | .74a |
| DKEFS sorting, mean (SD) | 10.0 (2.9) | 9.8 (2.6) | .79a |
| TMT-B, mean (SD) | 71 (28) | 71 (25) | .93a |
| OCL accuracy, mean (SD) | 0.751 (0.073) | 0.744 (0.081) | .75a |
| SETS accuracy, mean (SD) | 0.78 (0.11) [N = 19] | 0.78 (0.10), N = 14 | .82a |
| CPAL accuracy, mean (SD) | 0.56 (0.20) | 0.57 (0.18) | .85a |
| Two-back accuracy, mean (SD) | 0.87 (0.12) | 0.85 (0.13) | .51a |
| AVLT long-term memory, mean (SD) | 9.7 (3.6) | 11.0 (2.4) | .14a |
| SF-36 PCS, mean (SD) | 53.8 (3.3) | 52.8 (4.1), N = 22 | .33a |
| SF-36 MCS, mean (SD) | 55.9 (4.6) | 53.4 (7.9), N = 22 | .16a |
Abbreviations: APOE, apolipoprotein E; AVLT, Rey’s Auditory Visual Learning Test; CPAL, Continuous Paired Associate Learning; DKEFS, Delis-Kaplan Executive Functioning System; GMLT, Groton Maze Learning Test; MCS, mental composite score; OCL, one-card learning (visual continuous learning); PCS, physical composite score; SD, standard deviation; SETS, set-shifting; SF-36, Short-Form Health Survey; TMT, trail-making test B.
a P value based on 2-tailed 2-group t test.
b P value based on 2-tailed Pearson χ2 test.
Zumba and Physical Exercise Adherence and Body Composition
Attendance and rating of Zumba class
The median number of attended Zumba classes per week in the intervention group was 2. Class rating in the Zumba group (scale of 1 [not at all] to 5 [very much]) over the 26 weeks was generally high for enjoyment of Zumba class (mean 4.18, SD 0.74), ability to follow dance moves (mean 3.83, SD 0.56), and feeling it was a good workout (mean 4.42, SD 0.58). No participant of the control group reported having attended a Zumba class during the study.
Physical exercise and body composition
There were no statistically significant differences at any time point between groups in physical exercise as measured by CHAMPS or number of hours per week of exercise reported on the weekly logs (Table 3). Neither group increased mean exercise from the baseline mean at last follow-up. Also, there were no significant differences in weight or body mass index (BMI) at any time point between groups. However, change in weight was different between groups at 3 months (mean change −1 kg for the Zumba group vs 0.6 kg for the control group, P = .01) but did not persist at 6 months.
Table 3.
Comparison of Mean Scores at 6 Months or Last Follow-Up Between Intervention and Control Groups.
| Zumba | Control | Δ | 95% CI | P | |
|---|---|---|---|---|---|
| N | 30 | 23 | |||
| Total exercise (1000 kcal/wk) | 11.7 | 9.9 | 1.8 | −1.5 to 5.2 | .28a |
| Moderate exercise (1000 kcal/wk) | 7.0 | 5.4 | 1.7 | −0.9 to 4.2 | .19a |
| Exercise (h/wk) from weekly logd | 4.77 (2.77) | 3.97 (2.16), N = 22 | 0.81 | −0.62 to 2.24 | .26b |
| Zumba (h/wk)d | 1.76 (0.84) | 0.00 (0.00), N = 22 | 1.76 | NA | NA |
| GMLT total errors | 61 | 59 | 2.0 (0.07)c | −7.1 to 12 | .59a |
| DKEFS inhibition | 51.8 | 55.0 | −3.3 (0.26)c | −8.6 to 2.1 | 0.23a |
| DKEFS sorting | 12.7 | 10.7 | 2.0 (0.72)c | −0.7 to 4.7 | .22a |
| TMT-B | 62 | 64 | −2.0 (0.07)c | −11 to 7.1 | .67a |
| OCL accuracy | 0.75 | 0.74 | 0.01 (0.13)c | −0.03 to 0.05 | .61a |
| SETS accuracy | 0.81 | 0.76 | 0.05 (0.47)c | −0.01 to 0.11 | .08a |
| CPAL accuracy | 0.57 | 0.60 | −0.03 (0.16)c | −0.14 to 0.08 | .61a |
| Two-back accuracy | 0.88 | 0.90 | −0.02 (0.16)c | −0.07 to 0.04 | .61a |
| AVLT long-term memory | 11.7 | 10.9 | 0.9 (0.29)c | −0.3 to 2.1 | .15a |
| SF-36 PCS | 53.3 | 51.1, N = 22 | 2.1 (0.57)c | −0.8 to 5.0 | .15a |
| SF-36 MCS | 54.1 | 53.1, N = 22 | 1.0 (0.16)c | −1.9 to 3.9 | .50a |
Abbreviations: APOE, apolipoprotein E; AVLT, Rey’s Auditory Visual Learning Test; CI, confidence interval; CPAL, Continuous Paired Associate Learning; DKEFS, Delis-Kaplan Executive Functioning System; GMLT, Groton Maze Learning Test; MCS, mental composite score; NA, not applicable; OCL, one-card learning (visual continuous learning); PCS, physical composite score; SD, standard deviation; SETS, set-shifting; SF-36, Short-Form Health Survey; TMT, trail-making test B.
a P value based on 2-tailed analysis of covariance comparison adjusting for the corresponding baseline score.
b P value based on 2-tailed 2-group t test.
c Effect size based on pooled baseline SD.
d No baseline data. Values represent the mean (SD) at 6 months or last follow-up if participant did not provide data at 6 months.
Major Findings: Intervention Group Versus Control Group
Neuropsychological tests
There was no significant difference in neuropsychological test scores between intervention and control groups at any time (refer to Table 3 for a comparison of mean scores at 6 months or last follow-up). However, the percentage of women with more than a 0.5 SD (15 points) improvement in GMLT total errors score at 6 months or last follow-up was substantially higher in the intervention group than in the control group (P = .04; Table 4) and remained statistically significant even when considering all randomized participants without data to be nonresponders (15/36 GMLT responders in the Zumba group vs 5/36 GMLT responders in the control group; P = .03). Likewise, the percentage of women with more than a 0.5 SD (6.3 seconds) improvement in the DKEFS color-word interference inhibition score was significantly higher for the intervention group than for the control group (P = .04). We did not see a difference in the rate of improvement between groups for any other test (Table 4). We did not observe a significant difference between groups in worsening of score for any neuropsychological test. Neither the GMLT total error scores nor the DKEFS inhibition scores showed less of a practice effect in the control group than the other measures of executive cognitive function.
Table 4.
Comparison of Improvement (>0.5 SD) in Outcome Measures Between Baseline and 6-Month or Last Follow-Up.
| Zumba | Control | Δ | 95% CI | P | |
|---|---|---|---|---|---|
| N | 30 | 23 | |||
| Total exercise change >4200 kcal/wk | 6 (20%) | 3 (13%) | .07 | −0.12 to 0.27 | .72a |
| Moderate exercise change >3000 kcal/wk | 8 (27%) | 2 (9%) | .18 | −0.03 to 0.38 | .16a |
| GMLT total errors change <−15 | 15 (50%) | 5 (22%) | .28 | 0.04 to 0.53 | .04b |
| DKEFS inhibition change <−6.3 | 13 (43%) | 4 (17%) | .26 | 0.02 to 0.49 | .04b |
| DKEFS sorting change >1.4 | 16 (53%) | 7 (30%) | .23 | −0.03 to 0.49 | .10b |
| TMT-B change <−13 | 8 (27%) | 7 (30%) | −.04 | −0.28 to 0.21 | .76b |
| OCL accuracy change >0.038 | 13 (43%) | 7 (30%) | .13 | −0.13 to 0.39 | .34b |
| SETS accuracy change >0.053 | 6/19 (32%) | 3/14 (21%) | .10 | −0.20 to 0.40 | .70a |
| CPAL accuracy change >0.094 | 10 (33%) | 8 (35%) | −.01 | −0.27 to 0.24 | .91b |
| Two-back accuracy change >0.062 | 9 (30%) | 7 (30%) | −.004 | −0.25 to 0.25 | .97b |
| AVLT long-term memory change >1.6 | 14 (47%) | 7 (30%) | .16 | −0.10 to 0.42 | .23b |
| SF-36 PCS change >1.8 | 8 (27%) | 6/22 (27%) | −.01 | −0.25 to 0.24 | .96b |
| SF-36 MCS change >3.1 | 5 (17%) | 2/22 (9%) | .08 | −0.10 to 0.26 | .68a |
Abbreviations: APOE, apolipoprotein E; AVLT, Rey’s Auditory Visual Learning Test; CI, confidence interval; CPAL, Continuous Paired Associate Learning; DKEFS, Delis-Kaplan Executive Functioning System; GMLT, Groton Maze Learning Test; MCS, mental composite score; OCL, one-card learning (visual continuous learning); PCS, physical composite score; SD, standard deviation; SETS, set-shifting; SF-36, Short-Form Health Survey; TMT, trail-making test B.
a P value based on 2-tailed Fisher exact test.
b P value based on 2-tailed Pearson χ2 test.
Interaction with APOE4
There was no evidence of interaction between intervention group and APOE genotype. Among APOE4 carriers, 4 (44%) of the 9 women in the Zumba group improved more than 15 points in the GMLT total error score compared to 2 (15%) of the 13 in the control group. Among APOE4 noncarriers, such improvement in GMLT score occurred in 11 (52%) of the 21 Zumba group participants compared to 3 (30%) of the 10 in the control group (interaction P = .68, APOE4 effect P = .41). Similarly, the percentage of women scoring more than 6.3 seconds faster at 6 months or last follow-up in DKFES inhibition was 29 percentage points higher in the Zumba group than the control group for APOE4 carriers versus 23 percentage points higher in the Zumba group than the control group for APOE4 noncarriers (interaction P = .78, APOE4 effect P = .92).
Quality of life
Several of the quality-of-life measures (SF-36) differed significantly between groups at 3 months: physical functioning (pooled SD = 15.5, P = .02), role-physical (pooled SD = 17.3, P = .004), bodily pain (pooled SD = 17.8, P = .02), vitality (pooled SD = 15.6, P = .01), social functioning (pooled SD = 18.5, P = .002), role emotional (pooled SD = 17.8, P = .005), physical composite (pooled SD = 6.0, P = .02), and mental composite (pooled SD = 6.9, P = .02), with the Zumba group reporting higher quality of life than the control group on all of these items (data not shown). However, statistical significance did not persist at 6 months or last follow-up for any of these scales.
Sensitivity analyses
Two sensitivity analyses were conducted to assess whether missing data impacted results. The first employed Markov Chain Monte Carlo multiple imputation in which treatment group, age, APOE4 status, baseline BMI, and baseline GMLT total errors were used to predict 6-month GMLT total errors for participants with missing data prior to comparison between groups using analysis of covariance. The second considered all participants without primary end point data to be nonresponders in the responder definition approach. All sensitivity analyses produced results (multiple imputation results not shown) consistent with the analysis based on observed scores only.
Discussion
In this randomized controlled pilot study of cognition and quality of life in healthy older women, we investigated the effect of a 6-month Zumba intervention on cognitive function and quality of life and also assessed the potential impact of APOE4 genotype status. The Zumba and control groups engaged in the same level of exercise at baseline, 3, and 6 months. Thus, Zumba participants partly substituted Zumba for their previous exercise, which made it possible to dissociate the impact of Zumba from that of other forms of physical exercise.
Although the Zumba intervention group and the control group did not differ in neuropsychological test scores, 6 months of regular Zumba intervention were associated with a higher proportion of participants experiencing improved visuospatial working memory and response inhibition relative to a control group that was instructed to maintain baseline physical activity level. We hypothesize that the improvements noted in the Zumba group with regard to visuospatial working memory and response inhibition reflect the greater reliance upon spatial working memory associated with remembering the patterns of dance moves as well as the need to inhibit dance moves as the sequence changes. There was no evidence that practice effect accounted for the observed cognitive findings on the main outcomes of GMLT and DKEFS.
The lack of significant differences in mean scores of cognitive tests between groups at any time point was likely both a function of these being healthy, cognitively unimpaired individuals who at baseline were already physically active and our small sample size. It is possible that the novelty of the Zumba may have been a factor; however, the improvement in GMLT and DKEFS scores was not apparent until 6 months of practice, and if it were related to novelty, one might expect the benefit to have occurred also at 3 months. As expected, given our instruction to maintain habitual physical activity level, there was no increase in either physical exercise between baseline and 6 months for either group. Thus, it is unlikely that the improvements in GMLT and DKEFS scores were simply related to either frequency or intensity of exercise. It is also unlikely that cognitive changes in GMLT and DKEFS color-word interference inhibition scores were due to individual amount of exercise evolution during the 6 months because there was no correlation between the change from baseline calories of total or moderate exercise and improvement in either GMLT or DKEFS. The strongest correlation was for OCL accuracy—a test of visual recognition and learning. A 10 kcal/wk change in moderate exercise was associated with a 10% improvement in OCL accuracy (r = 0.44). All other measures had weaker or negative correlations with exercise.
Our study is partly in line with a randomized controlled trial from Australia that compared the impact of an 8-month ballroom dancing intervention versus walking program on cognition. Similar to our observation, the dancing intervention was only associated with improved performance in spatial memory, but not any other cognitive domains that the investigators had assessed. 56 In contrast, Korean investigators reported that a 6-month dance exercise intervention was associated with better performance in verbal fluency, word list delayed recall and recognition, and total score on the Consortium to Establish a Registry for Alzheimer’s disease Korean version. 57 However, their study was conducted among cognitively unimpaired older males and females with metabolic syndrome, whereas we included cognitively unimpaired females regardless of metabolic and cardiovascular health status. Finally, a recent meta-analysis concluded that dance interventions in older adults are, overall, associated with improved cognitive function including global cognition and memory but not executive function. 58
Our study does not provide evidence for an interaction between intervention group and APOE genotype on the outcome of cognitive function. Just as many APOE4 carriers improved with the Zumba intervention relative to the controls as did noncarriers. Although some researchers have suggested a potentially larger benefit of physical activity on cognitive function in APOE4 carriers, 12,18,19 other studies also failed to provide support for a potential impact APOE4 genotype status on the association between physical activity and cognitive performance. 17,23
Zumba appears to be an attractive form of exercise for middle-aged and older women in that dropouts were lower in the Zumba group than in the control group, and the majority experienced the class in favorable terms. The initial novelty of Zumba may have influenced quality-of-life measures to be better at 3 months in the intervention group relative to the control group; but by the last follow-up, quality-of-life measures did not differ between groups. In part, our finding is in line with previous research that has shown quality-of-life improvement after a brief, 8-week Zumba intervention. 34,35
The strengths of this study include its rigorous randomized controlled design, the use of an extensive neuropsychological test battery to assess different cognitive domains, and the relatively long intervention of 6 months with classes being taught by certified Zumba instructors. The fact that there was no overall increase in exercise over 6 months for either group helped to dissociate the mentally stimulating effect of Zumba from that of physical exercise.
This study was mainly limited by its small sample size, exclusion of men, lack of adjustment for multiple testing, and greater number of dropouts than expected. Despite the participants’ agreement at the time of consent to be randomized to either group, 6 controls dropped out of study due to disappointment with the randomization. However, we accounted for such nonrandom dropouts with 2 sensitivity analyses, which yielded similar, if not stronger, results. Other participants dropped out for a variety of personal reasons, but since those who dropped out exercised less at baseline than those who did not dropout, it is possible that some of the dropouts felt overly burdened by the requirement to report exercise on a weekly basis and the follow-up assessments. In addition, we have no follow-up yet to determine whether improvements were sustained or whether participants would be inclined to continue regular Zumba practice in the long term. Furthermore, we only included women aged between 55 and 80 years. This heterogeneity may be one potential reason for our lack of significant findings as the adaptations to Zumba training may differ between younger and older participants. Finally, we did not investigate potential other factors that might impact the effect of Zumba on cognitive function, such as exercising in a group setting or having social interactions during exercise.
Conclusion
We observed that repeated practice of learning and inhibiting dance moves may have strengthened performance on visuospatial working memory and response inhibition tasks in the Zumba as compared to the control group, irrespective of APOE4 status. Given the limited sample size, lack of adjustment for multiple testing, lack of long-term follow-up, and only small improvement in scores, clinical relevance of the study findings cannot be determined. This pilot study at least leads to an empirically derived hypothesis that Zumba may have a potential to impact cognitive health. This hypothesis needs to be tested by a larger clinical trial. Therefore, the study findings need to be considered preliminary until confirmed by a larger study. Future studies could also test the benefit of Zumba for sedentary individuals or patients with MCI and perhaps use “real-world” outcome measures, such as a driving test.
Acknowledgments
The authors thank Marci Zomok, RN, for study coordination, and Jeanne Eilertsen and Jessie Jacobsen for assistance with psychometric testing.
Authors' Note: Janina Krell-Roesch is also affiliated with Institute of Sports and Sports Science, Karlsruhe Institute of Technology, Karlsruhe, Germany.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the Alzheimer’s Association (NIRG-09-131572), NIH APOE4 grant, “Brain Imaging, APOE and the Preclinical Course of Alzheimer’s Disease” (R01AG031581), Arizona Alzheimer’s Disease Core Center, and “the Arizona Alzheimer’s Research Consortium” (P30AG19610).
ORCID iD: Cynthia M. Stonnington  https://orcid.org/0000-0001-7843-4309
https://orcid.org/0000-0001-7843-4309
References
- 1. Young J, Angevaren M, Rusted J, Tabet N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2015;22(4):CD005381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Etnier JL, Salazar W, Landers DM, Petruzzello SJ, Han M, Nowell PM. The influence of physical fitness and exercise upon cognitive functioning: a meta-analysis. J Sport Exerc Psychol. 1997;19(3):249–277. [Google Scholar]
- 3. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–130. [DOI] [PubMed] [Google Scholar]
- 4. Smith PJ, Blumenthal JA, Hoffman BM. et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52(3):154–160. [DOI] [PubMed] [Google Scholar]
- 6. Gaertner B, Buttery AK, Finger JD, Wolfsgruber S, Wagner M, Busch MA. . Physical exercise and cognitive function across the life span: results of a nationwide population-based study. J Sci Med Sport. 2018;21(5):489–494. [DOI] [PubMed] [Google Scholar]
- 7. Etgen T, Sander D, Huntgeburth U, Poppert H, Forstl H, Bickel H. Physical activity and incident cognitive impairment in elderly persons: the INVADE study. Arch Intern Med. 2010;170(2):186–193. [DOI] [PubMed] [Google Scholar]
- 8. Ku PW, Stevinson C, Chen LJ. Prospective associations between leisure-time physical activity and cognitive performance among older adults across an 11-year period. J Epidemiol. 2012;22(3):230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498–504. [DOI] [PubMed] [Google Scholar]
- 10. Larson EB, Wang L, Bowen JD. et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144(2):73–81. [DOI] [PubMed] [Google Scholar]
- 11. Middleton LE, Manini TM, Simonsick EM. et al. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171(14):1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33(5):772–777. [DOI] [PubMed] [Google Scholar]
- 13. Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161(14):1703–1708. [DOI] [PubMed] [Google Scholar]
- 14. Krell-Roesch J, Pink A, Roberts RO. et al. Timing of physical activity, apolipoprotein e epsilon4 genotype, and risk of incident mild cognitive impairment. J Am Geriatr Soc. 2016;64(12):2479–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liang KY, Mintun MA, Fagan AM. et al. Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68(3):311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okonkwo OC, Schultz SA, Oh JM. et al. Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology. 2014;83(19):1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78(17):1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deeny SP, Poeppel D, Zimmerman JB. et al. Exercise, APOE, and working memory: MEG and behavioral evidence for benefit of exercise in epsilon4 carriers. Biol Psychol. 2008;78(2):179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Etnier JL, Caselli RJ, Reiman EM. et al. Cognitive performance in older women relative to ApoE-epsilon4 genotype and aerobic fitness. Med Sci Sports Exerc. 2007;39(1):199–207. [DOI] [PubMed] [Google Scholar]
- 20. Tolppanen AM, Solomon A, Kulmala J. et al. Leisure-time physical activity from mid- to late life, body mass index, and risk of dementia. Alzheimers Dement. 2015;11(4):434–443 e436. [DOI] [PubMed] [Google Scholar]
- 21. Rovio S, Kareholt I, Helkala EL. et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705–711. [DOI] [PubMed] [Google Scholar]
- 22. Etnier JL, Labban JD, Karper WB. et al. Innovative research design exploring the effects of physical activity and genetics on cognitive performance in community-based older adults. J Aging Phys Act. 2015;23(4):559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Solomon A, Turunen H, Ngandu T. et al. Effect of the apolipoprotein e genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75(4):462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pesce C. Shifting the focus from quantitative to qualitative exercise characteristics in exercise and cognition research. J Sport Exerc Psychol. 2012;34(6):766–786. [DOI] [PubMed] [Google Scholar]
- 25. Fabre C, Chamari K, Mucci P, Masse-Biron J, Prefaut C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. Int J Sports Med. 2002;23(6):415–421. [DOI] [PubMed] [Google Scholar]
- 26. Oswald WD, Gunzelmann T, Rupprecht R, Hagen B. Differential effects of single versus combined cognitive and physical training with older adults: the SIMA study in a 5-year perspective. Eur J Ageing. 2006;3(4):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gheysen F, Poppe L, DeSmet A. et al. Physical activity to improve cognition in older adults: can physical activity programs enriched with cognitive challenges enhance the effects? A systematic review and meta-analysis. Int J Behav Nutr Phys Act. 2018;15(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu C, Yi Q, Zheng X. et al. Effects of mind-body exercises on cognitive function in older adults: a meta-analysis. J Am Geriatr Soc. 2018;67(4):749–758. Epub 2018. [DOI] [PubMed] [Google Scholar]
- 29. Zhu Y, Wu H, Qi M. et al. Effects of a specially designed aerobic dance routine on mild cognitive impairment. Clin Interv Aging. 2018;13:1691–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brustio PR, Liubicich ME, Chiabrero M, Rabaglietti E. Dancing in the golden age: a study on physical function, quality of life, and social engagement. Geriatr Nurs. 2018;39(6):635–639. [DOI] [PubMed] [Google Scholar]
- 31. Luettgen M, Foster C, Doberstein S, Mikat R, Porcari J. Zumba(R): is the “fitness-party” a good workout? J Sports Sci Med. 2012;11(2):357–358. [PMC free article] [PubMed] [Google Scholar]
- 32. Barene S, Holtermann A, Oseland H, Brekke OL, Krustrup P. Effects on muscle strength, maximal jump height, flexibility and postural sway after soccer and Zumba exercise among female hospital employees: a 9-month randomised controlled trial. J Sports Sci. 2016;34(19):1849–1858. [DOI] [PubMed] [Google Scholar]
- 33. Delextrat AA, Warner S, Graham S, Neupert E. An 8-week exercise intervention based on zumba improves aerobic fitness and psychological well-being in healthy women. J Phys Act Health. 2016;13(2):131–139. [DOI] [PubMed] [Google Scholar]
- 34. Donath L, Roth R, Hohn Y, Zahner L, Faude O. The effects of Zumba training on cardiovascular and neuromuscular function in female college students. Eur J Sport Sci. 2014;14(6):569–577. [DOI] [PubMed] [Google Scholar]
- 35. Domene PA, Moir HJ, Pummell E, Knox A, Easton C. The health-enhancing efficacy of Zumba(R) fitness: an 8-week randomised controlled study. J Sports Sci. 2016;34(15):1396–1404. [DOI] [PubMed] [Google Scholar]
- 36. Cugusi L, Wilson B, Serpe R. et al. Cardiovascular effects, body composition, quality of life and pain after a Zumba fitness program in Italian overweight women. J Sport Med Phys Fit. 2016;56(3):328–335. [PubMed] [Google Scholar]
- 37. Krishnan S, Tokar TN, Boylan MM. et al. Zumba(R) dance improves health in overweight/obese or type 2 diabetic women. Am J Health Behav. 2015;39(1):109–120. [DOI] [PubMed] [Google Scholar]
- 38. Barene S, Krustrup P, Holtermann A. Effects of the workplace health promotion activities soccer and Zumba on muscle pain, work ability and perceived physical exertion among female hospital employees. PLoS ONE. 2014;9(12):e115059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Araneta MR, Tanori D. Benefits of Zumba Fitness(R) among sedentary adults with components of the metabolic syndrome: a pilot study. J Sport Med Phys Fit. 2015;55(10):1227–1233. [PubMed] [Google Scholar]
- 40. Delextrat A, Bateman J, Esser P, Targen N, Dawes H. The potential benefits of Zumba Gold(R) in people with mild-to-moderate Parkinson’s: feasibility and effects of dance styles and number of sessions. Complement Thr Med. 2016;27:68–73. [DOI] [PubMed] [Google Scholar]
- 41. Caselli RJ, Reiman EM, Osborne D. et al. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology. 2004;62(11):1990–1995. [DOI] [PubMed] [Google Scholar]
- 42. Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 43. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pietrzak RH, Maruff P, Mayes LC, Roman SA, Sosa JA, Snyder PJ. An examination of the construct validity and factor structure of the Groton Maze Learning Test, a new measure of spatial working memory, learning efficiency, and error monitoring. Arch Clin Neuropsychol. 2008;23(4):433–445. [DOI] [PubMed] [Google Scholar]
- 45. Fredrickson J, Maruff P, Woodward M. et al. Evaluation of the usability of a brief computerized cognitive screening test in older people for epidemiological studies. Neuroepidemiology. 2010;34(2):65–75. [DOI] [PubMed] [Google Scholar]
- 46. Delis DCKE, Kramer JH. Delis Kaplan Executive Function System (D-KEFS). San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 47. Lezak MD. Neuropsychological Assessment. 4th ed. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 48. Rey A. L’examen Clinique en Psychologie. Paris, France: Presses Universitaires de France; 1964. [Google Scholar]
- 49. Shapiro DM, Harrison DW. Alternate forms of the AVLT: a procedure and test of form equivalency. Arch Clin Neuropsychol. 1990;5(4):405–410. [PubMed] [Google Scholar]
- 50. Ware JE, Jr, Sherbourne CD, The MOS. 36-item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 51. Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–1141. [DOI] [PubMed] [Google Scholar]
- 52. Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. [DOI] [PubMed] [Google Scholar]
- 53. Ross RM, Murthy JN, Wollak ID, Jackson AS. The six minute walk test accurately estimates mean peak oxygen uptake. BMC Pulm Med. 2010;10(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, MI: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 55. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. [DOI] [PubMed] [Google Scholar]
- 56. Merom D, Grunseit A, Eramudugolla R, Jefferis B, McNeill J, Anstey KJ. Cognitive benefits of social dancing and walking in old age: the dancing mind randomized controlled trial. Front Aging Neurosci. 2016;8(3):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kim SH, Kim M, Ahn YB. et al. Effect of dance exercise on cognitive function in elderly patients with metabolic syndrome: a pilot study. J Sports Sci Med. 2011;10(4):671–678. [PMC free article] [PubMed] [Google Scholar]
- 58. Meng X, Li G, Jia Y. et al. Effects of dance intervention on global cognition, executive function and memory of older adults: a meta-analysis and systematic review. Aging Clin Exp Res. 2019. 10.1007/s40520-019-01159-w. [DOI] [PubMed] [Google Scholar]