Abstract
Background:
The authors examined associations between physical activity, cognitive function, activities of daily living, and behavioral and psychological dementia symptoms (BPSD) in severe and moderate dementia.
Methods:
A cross-sectional study was conducted to assess severe and moderate dementia groups according to the Clinical Dementia Rating. An actigraphy measured physical activity. Other measures included Mini-Mental State Examination, Cognitive Test for Severe Dementia, Hyogo Activities of Daily Living Scale, and Neuropsychiatric Inventory-Nursing Home.
Results:
Sixty-three participants were assessed (mean age = 89.3 ± 6.4). Physical activity was not associated with cognitive function among participants with severe dementia, although there was a trend-level association with cognitive function among those with moderate dementia. Physical activity was significantly associated with BPSD, specifically agitation/aggression symptoms, for participants with severe dementia, and there was a trend-level association with anxiety for participants with moderate dementia.
Conclusions:
Physical activity appears to be associated with BPSD among individuals in the advanced stages of dementia.
Keywords: behavioral and psychological symptoms of dementia, cognitive function, physical activity, sedentary, severe dementia
Physical activity has gained increasing focus as a nonpharmacological approach for managing symptoms of dementia. 1 Many studies have reported that more physically active older adults have diminished rates of cognitive decline and a lower incidence of dementia. 2,3 Hence, maintaining physical fitness well into late life is important. However, maintaining physical activity is difficult for the elderly patients, once they develop dementia. Patients with severe dementia, in particular, demonstrate progressive decline in motor functioning, 4 comorbidities, 5 cognitive impairment, limited activities of daily living (ADL), 6 and several additional behavioral and psychological symptoms of dementia (BPSD). 7 Therefore, most patients with severe dementia are quite sedentary. One study observed that among nursing home residents with dementia, individuals with severe dementia slept for an average of 14 hours daily. 8 Additionally, ability to engage in ordinary activities generally declines among individuals with dementia because of cognitive impairments 9 or lack of spontaneity. 10
However, since most previous studies on physical activity have mainly focused on individuals undergoing healthy aging or with only mild cognitive impairment, little is known about the effects of a sedentary lifestyle on dementia symptomology. 11 Research on physical activity in the context of severe dementia is particularly lacking, apart from a few intervention studies using observational rating methods. 12 Additionally, most of these limited previous studies examined severe dementia mixed with moderate dementia. 11,12 Another previous study suggested that people with dementia should be evaluated separately in each stage, since their clinical features vary according to the severity of dementia. 13
Thus, the present study quantitatively measured physical activity patterns using an actigraph and examined the associations between physical activity and cognitive function, ADL, and BPSD in patients with severe dementia, using patients with moderate dementia as a comparison group.
Materials and Methods
Participants
The current study was conducted at a 270-bed hospital for recuperation in Hyogo prefecture, Japan, from October 2015 to March 2018. Participants were evaluated with a standard clinical interview, completed a physical and neurological examination, and underwent a computerized tomography scan. The inclusion criteria were (1) diagnosis of dementia according to the Diagnostic and Statistical Manual of Mental Disorders-5 and (2) a Clinical Dementia Rating (CDR) 14 score of 2 (moderate dementia) or 3 (severe dementia). We excluded patients who had any of the following conditions: (1) additional diseases or comorbidities such as neuropsychiatric disorders or orthopedic disease that impair consciousness and/or physical activity; (2) a judgment made by the attending doctor that study participation would negatively influence the patient’s condition; and/or (3) use of prescribed antipsychotics, antidepressants, or hypnotics in the week prior to test administration.
A family member or caregiver provided written informed consent prior to participation. The ethics committee at Osaka Prefecture University approved the study protocol (2017-204).
Procedure
This study used a cross-sectional and observational design. All participants were assessed on physical activity, cognitive function, ADL, BPSD, and comorbidities. They were divided into severe and moderate dementia groups based on their CDRs. All examinations were conducted within 1 month to reflect conditions during the same period of time.
Clinical Assessments
Amount of physical activity was recorded using an actigraph device (Micro Motionlogger Watchware, version 1.94 in Ambulatory Monitoring, Inc). Actigraphy has been clinically useful for investigating physical activity in patients with dementia and provides objective quantitative information regarding sleep-related variables. 15 Prior studies have reported that 3 days of actigraphy data are needed to accurately estimate physical activity levels among older adults. 16 Thus, participants wore the actigraph on consecutive days for 1 week. It could be removed when bathing or undergoing examinations or medical treatments. 17 Any data from participants who wore the actigraph for fewer than 3 days were excluded. The actigraph was worn on the nondominant wrist; however, if paresis was present on the nondominant limb, the dominant hand was used. 18
The amount of daytime physical activity (9:00 to 16:59) and the total amount of daily physical activity were obtained from actigraphy data. Hourly mean physical activity was also calculated for each 24-hour period. 19
Cognitive function was assessed using the Mini-Mental State Examination (MMSE) 20 and the Cognitive Test for Severe Dementia (CTSD). 21 The MMSE is a widely used screening tool. The total score ranges from 0 to 30; lower scores indicate greater cognitive impairment. The CTSD is a reliable and valid test for severe and profound dementia. The total score ranges from 0 to 30; lower scores indicate greater cognitive impairment.
Activities of Daily Living was assessed using the Hyogo Activities of Daily Living Scale (HADLS). 22 The HADLS assesses the level of independence with basic and instrumental ADL (IADL). It consists of 18 items, including toileting, eating, dressing, grooming, personal hygiene, brushing, bathing, mobility, telephoning, shopping, preparing meals, cleaning, making one’s bed, cleaning up after meals, doing laundry, managing fire, handling switches, and managing finances. Since participants do not perform IADL while residing at a recuperation hospital, the IADL items were excluded from the present analyses. Thus, we assessed 8 basic ADL items that included toileting (1-7 points), eating (1-5 points), dressing (1-6 points), grooming (1-6 points), personal hygiene (1-6 points), brushing (1-6 points), bathing (1-7 points), and mobility (1-7 points). Total scores range from 8 to 50; higher scores indicate lower independence.
Behavioral and psychological dementia symptoms was assessed using the Neuropsychiatric Inventory-Nursing Home (NPI-NH) scale 23 that assesses 12 domains: delusions, hallucinations, agitation/aggression, depression, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behavior, night-time behavior, and eating behavior. A composite score from each domain is calculated by multiplying the frequency and severity within each domain as follows: 1-4 (4 = most frequent) and 1-3 (3 = most severe). Total scores range from 0 to 144; higher scores indicate increased neuropsychiatric symptomology.
Comorbidities were assessed using the Charlson Comorbidity Index (CCI) 24 that was employed for the assessment of severity. Charlson Comorbidity Index is a scale for predicting mortality by classifying or weighting comorbid conditions which has been widely utilized by health researchers to measure the burden of disease. Charlson Comorbidity Index has 19 items, and a higher score indicates severe comorbidities.
Statistical Analyses
The Mann-Whitney U test or χ2 test was employed to compare the severe and moderate dementia groups. Spearman rank correlation coefficients were employed to examine relationships among daytime physical activity (9:00 to 16:59), daily physical activity, and other variables (ie, age and MMSE, CTSD, HADLS, and NPI-NH scores) within each patient group. All analyses were conducted using IBM SPSS Statistics version 24. P values <.05 were considered statistically significant.
Results
Participant Characteristics
Table 1 shows participant characteristics. The total sample included 70 (53 women, 17 men) patients, all of whom wore the actigraph for 3 to 6 days. For the actigraph data, average values were estimated based on the days on which it was worn throughout each assessment period (9:00 to 16:59 and 9:00 to 8:59). Seven participants could not continuously wear the actigraph: 6 removed the device, and 1 participant declined because the device was too uncomfortable. Thus, a total of 63 participants completed the actigraphy assessment (mean age = 89.4 ± 6.4). A total of 43 participants had severe dementia and 20 had moderate dementia. The dementia diagnoses were Alzheimer disease (AD, n = 28), vascular dementia (n = 21), dementia with Lewy bodies (n = 1), frontotemporal dementia (n = 2), and others (n = 11, including mixed dementia, brain injury, and undiagnosed). The average CCI score was 2.7 ± 1.6 in the entire sample, indicating a medium level of comorbidities.
Table 1.
Participant Characteristics.
| Variables | Mean ± SD or n | P | |
|---|---|---|---|
| CDR3 (n = 43) | CDR2 (n = 20) | ||
| Age | 90.2 ± 6.4 | 87.5 ± 6.1 | .167a |
| Sex (male) | 8 | 6 | .663b |
| Dementia subtype (AD, VaD, DLB, FTD, others) | 19, 14, 1, 1, 8 | 9, 7, 0, 1, 3 | .924b |
| MMSE | 4.7 ± 3.6 | 12.4 ± 3.1 | <.000a |
| CTSD | 15.2 ± 7.3 | 26.3 ± 3.4 | <.000a |
| HADLS | 39.8 ± 4.0 | 32.3 ± 5.1 | <.000a |
| NPI-NH | 11.5 ± 9.1 | 10.0 ± 6.4 | .739a |
| CCI | 2.9 ± 1.6 | 2.5 ± 1.5 | .398a |
| Amount of daytime physical activity (counts/minute) | 85.1 ± 48.6 | 114.7 ± 34.3 | .004a |
| Daily amount of physical activity (counts/minute) | 73.5 ± 39.1 | 92.1 ± 27.9 | .028a |
Abbreviations: AD, Alzheimer disease; CCI, Charlson Comorbidity Index; CDR, Clinical Dementia Rating; CTSD, Cognitive Test for Severe Dementia; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; HADLS, Hyogo Activity of Daily Living Scale; MMSE, Mini-Mental State Examination; NPI-NH, Neuropsychiatric Inventory-Nursing Home; VaD, vascular dementia.
a Mann-Whitney U test.
b χ2 test.
Daily Patterns of Physical Activity
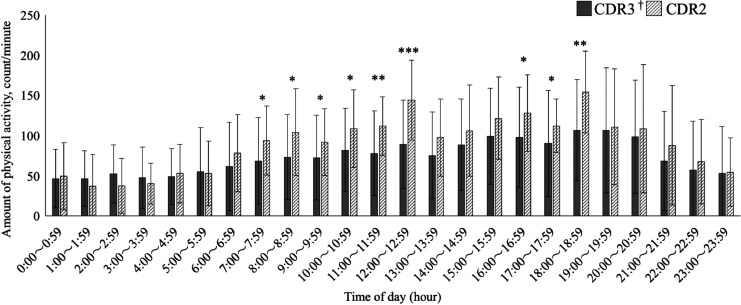
Figure 1 shows the pattern of hourly mean physical activity. For both groups, physical activity was most robust from 18:00 to 18:59 (106.7 ± 62.8 counts/minute and 154.3 ± 53.5 counts/minute, in the severe and moderate groups, respectively) and most sedentary from 1:00 to 1:59 (46.3 ± 34.6 counts/minute, 37.8 ± 40.8 counts/minute, respectively). However, physical activity significantly differed from 7:00 to 13:59 and 16:00 to 18:59. That is, participants with severe dementia were significantly less active than those with moderate dementia during most daytime periods, although they showed similar patterns of change in physical activity.
Figure 1.
Hourly mean amount of physical activity across dementia groups. Hourly mean physical activity was most robust from 18:00 to 18:59 and most sedentary from 1:00 to 1:59 for both groups. There were significant differences in physical activity from 7:00 to 13:59 and from 16:00 to 18:59. †CDR: Clinical Dementia Rating Mann-Whitney U test. *P < .05; **P < .01; ***P < .001.
Correlations of Physical Activity With Other Variables
Severe dementia group
Neither the amount of daytime physical activity nor of daily physical activity was correlated with age (ρ = −.072, P = .645; ρ = −.022, P = .889, respectively), the MMSE (ρ = .017, P = .914; ρ = .057, P = .716, respectively), the CTSD (ρ = −.001, P = .997; ρ = .075, P = .633, respectively), or the HADLS (ρ = −.155, P = .321; ρ = −.128, P = .365, respectively; Table 2).
Table 2.
Correlations Among Study Variables.
| Correlation coefficient (ρ) | Correlation coefficient (ρ) | |||
|---|---|---|---|---|
| Variables | Amount of Daytime Physical Activity | Daily Amount of Physical Activity | ||
| CDR3 | CDR2 | CDR3 |
CDR2 |
|
| MMSE | .017 | −.132 | .057 | −.298 |
| CTSD | −.001 | −.188 | .075 | −.411 |
| HADLS | −.155 | .084 | −.128 | .287 |
| NPI-NH | .355a | .319 | .411a | .421 |
Abbreviations: CDR, Clinical Dementia Rating; CTSD, Cognitive Test for Severe Dementia; HADLS, Hyogo Activities of Daily Living Scale; MMSE, Mini-Mental State Examination; NPI-NH, Neuropsychiatric Inventory-Nursing Home.
a Spearman correlation coefficient, P < .05.
By contrast, there were significant correlations between physical activity and BPSD. Specifically, the amount of daytime physical activity and daily physical activity was significantly correlated with the NPI-NH (ρ = .355, P = .0019; ρ = .411, P = .006, respectively; Table 2). In terms of subscale scores, both the amount of daytime physical activity and daily physical activity were significantly correlated with agitation/aggression (ρ = .479, P = .001; ρ = .456, P = .002, respectively). Daily physical activity was also significantly correlated with night-time behavior (ρ = .445, P = .003; Table 3).
Table 3.
Correlations Between Amount of Physical Activity and NPI-NH Subscore.
| Correlation coeffcient (ρ) | Correlation coefficient (ρ) | |
|---|---|---|
| Variables |
Amount of Daytime Physical Activity |
Daily Amount of Physical Activity |
| Delusions | −.075 | −.112 |
| Hallucinations | −.199 | −.199 |
| Agitation/aggression | .479a | .456a |
| Depression | −.044 | −.036 |
| Anxiety | −.195 | −.099 |
| Euphoria | .284 | .216 |
| Apathy | −.172 | −.071 |
| Disinhibition | .173 | .09 |
| Irritability | .126 | .115 |
| Aberrant motor behavior | .182 | .205 |
| Night-time behavior | .267 | .445a |
| Eating behavior | .075 | .012 |
Abbreviation: NPI-NH, Neuropsychiatric Inventory-Nursing Home.
a Spearman correlation coefficient, P < .01.
Moderate dementia group
Neither the amount of daytime physical activity nor of daily physical activity was correlated with age (ρ = −.225, P = .339; ρ = −.103, P = .665, respectively), the MMSE (ρ = −.132, P = .608; ρ = −.298, P = .202: respectively), or the HADLS (ρ = .084, P = .725; ρ = .287, P = .219, respectively). Regarding the relationships between physical activity and cognitive function, similar to the group with severe dementia, the amount of daytime physical activity was not correlated with the CTSD (ρ = −.188, P = .94). However, amount of daily physical activity had a negative trend-level correlation with the CTSD (ρ = −.411, P = .072; Table 2).
For the association between physical activity and BPSD, the daily amount of physical activity had a trend-level correlation with the NPI-NH, while amount of daytime physical activity did not (ρ = .421, P = .065; ρ = .319, P = .17, respectively; Table 2). In terms of subscale scores, amount of daily physical activity had a trend-level correlation with anxiety (ρ = .413, P = .071).
Overall, physical activity was not associated with cognitive function for severe dementia, while it had a trend-level association with cognitive function for moderate dementia. Physical activity was not associated with ADL independence in either group. Finally, physical activity was significantly associated with symptom severity within the agitation/aggression domain for the severe dementia group but only had a trend-level association with the anxiety domain for the moderate dementia group.
Discussion
The present study assessed physical activity among patients with moderate and severe dementia, examining associations between amount of physical activity and cognitive function, ADL, and BPSD. Two major findings emerged. First, physical activity was not associated with cognitive function for severe dementia. Second, physical activity was significantly associated with agitation/aggression symptoms among patients with severe dementia. Additionally, hourly activity values via actigraphy for most participants in the severe dementia group were lower than 100 counts/minute, which is considered sedentary, 19 suggesting that they went almost the whole day without engaging in any activity. People with severe dementia were significantly more sedentary than those with moderate dementia during most of the daytime period, in particular during the morning. Staff involved in the care for patients with severe dementia should consider how the patients spend their time during such periods. For instance, offering several types of activity programs during these periods may aid in improving the patients’ excessive inactivity.
Previous studies have reported a significant relationship between physical activity and cognitive function in healthy aging. 2,3 However, this study did not show such a relationship in the context of severe dementia. Patients with dementia exhibit decreased physical function and significant cognitive decline and tend to develop several comorbidities during the disease course. 25 The World Health Organization recommends that people aged 65 years and older should engage in at least 150 minutes of moderately intense (or 75 minutes of vigorously intense) aerobic physical activity throughout the week. 26 However, patients with severe dementia cannot achieve these physical activity levels. There are several studies reporting similar findings to ours. 27,28 A longitudinal study on AD samples 27 and a cross-sectional study on nursing home residents with moderate dementia 28 observed no association between physical activity level and cognitive function. These studies suggested that physical activity levels engaged in close to or after AD onset may not further affect the disease course in the face of accumulated AD pathology. 27 It is also possible that low physical activity levels provide no substantive benefit to cognitive function. 28 Therefore, any existing association between physical activity and cognitive function holds true only in healthy aging or mild dementia and not necessarily in severe dementia. Another interpretation of our findings is that some participants may have already exhibited a lower amount of physical activity, regardless of their dementia severity. This could be supported by a concept regarding frailty. In the older population, frailty is a prevalent clinical condition that includes unintentional weight loss, self-reported exhaustion, weakness, slow walking speed, and low physical activity. 29 Indeed, AD patients in even mild to moderate stages have also been reported to show clinical features of frail syndrome at a certain rate. 30 Concerning moderate dementia, the amount of physical activity had a negative trend-level association with cognitive function. Future studies should examine the relationship in detail, including the influence of BPSD.
Second, physical activity was not significantly associated with ADL in either group. One possible explanation for this finding is that patients who are past the moderate stage of dementia may not engage in higher levels of physical activity to engage more effectively in ADL, as they require much assistance with ADL. 31 In people with moderate and severe dementia, better ADL performances are largely dependent on the skills of caregivers, which induce positive attitudes from the patients toward the activities. 32 These attitudes may not lead to physical activity. Concerning the relationship between physical activity and BPSD, physical activity had a significant association with agitation/aggression symptoms in severe dementia and a trend-level association with anxiety symptoms in moderate dementia. These findings could be explained as follows. Selbaek and colleagues 33 reported that agitation symptoms worsen as dementia progresses. Relatedly, Moyle and colleagues measured physical activity among residents with dementia living in long-term care facilities and observed increased physical activity during the afternoon and early evening, suggesting that this increase in activity could reflect heightened agitation symptoms. 34 Similarly, physical activity in our samples was highest during the evening (18:00-18:59). Thus, activity among those with moderate and severe dementia is low; this includes few practical activities, owing to their limited functional capacity but many abnormal behaviors such as agitation/aggression. However, unexpectedly, there was no relationship found between the amount of physical activity and aberrant motor behavior in this study, which is one of the most common behavioral symptoms exhibited in severe dementia. 35 This result could be supported by the characteristics of aberrant motor behavior. In our study, almost no participants presented any disruptive behaviors such as wandering, which could substantially affect the amount of their physical activity. This lower development of disruptive behavior symptoms might be in part due to the hospital environment, where inpatients could not independently exit the hospital or ward.
This is the first study, to our knowledge, that quantitatively examined physical activity within the context of severe dementia. The present results suggest that potential relationships between physical activity and cognitive function, which previous studies have reported, may be irrelevant at severe stages of dementia. This result suggests that it may not be worthwhile to push patients to increase their physical activity once dementia symptoms have already reached a severe stage. It is necessary to consider other factors such as rest–activity rhythm, 36 and whether such activities are meaningful for the person 37 in examining physical activity in patients with severe dementia.
This study had several limitations. First, the patient sample was fairly small, making it difficult to adequately describe the associations of physical activity with cognitive function and BPSD in the group with moderate dementia. Additionally, we could not analyze the association between physical activity and types of dementia. Future studies with larger patient populations are warranted. Second, the HADLS may not have been sensitive enough to detect variability in ADL within the patient groups. Many HADLS items have exhibited floor effects among patients with severe dementia. 38 However, no currently available scale precisely measures the ADL of patients with severe dementia. Finally, the cross-sectional and correlational design precludes causal inferences. Therefore, longitudinal methods should be employed to further address temporal changes in these relationships and throughout disease progression.
In conclusion, physical activity was not associated with cognitive function but was related to agitation/aggression behaviors in patients with severe dementia. Thus interventions to improve the daily lives of such patients should focus not only on the amount but the quality of physical activity.
Acknowledgments
This study was supported by the Research Foundation for Dementia of Osaka (Daiki Ishimaru 2016).
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Daiki Ishimaru  https://orcid.org/0000-0002-9213-8220
https://orcid.org/0000-0002-9213-8220
Takashi Nishikawa  https://orcid.org/0000-0003-0151-660X
https://orcid.org/0000-0003-0151-660X
References
- 1. Rolland Y, Pillard F, Klapouszczak A, et al. Exercise program for nursing home residents with Alzheimer’s disease: a 1-year randomized, controlled trial. J Am Geriatr Soc. 2007;55(2):158–165. [DOI] [PubMed] [Google Scholar]
- 2. Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78(17):1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Middleton LE, Manini TM, Simonsick EM, et al. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171(14):1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zidan M, Arcoverde C, de Araújo NB, Vasques P, Rios A, Laks J, Deslandes A. Motor and functional changes in different stages of Alzheimer’s disease. Rev Psiquiatr Clin. 2012;39(5):161–165. [Google Scholar]
- 5. Koroukian SM, Schiltz NK, Warner DF, Stange KC, Smyth KA. Increasing burden of complex multimorbidity across gradients of cognitive impairment. Am J Alzheimers Dis Other Demen. 2017;32(7):408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Helvik AS, Engedal K, Benth JS, Selbæk G. A 52-month follow-up of functional decline in nursing home residents—degree of dementia contributes. BMC Geriatr. 2014;14:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Makimoto K, Kang Y, Kobayashi S, et al. Prevalence of behavioural and psychological symptoms of dementia in cognitively impaired elderly residents of long-term care facilities in East Asia: a cross-sectional study. Psychogeriatrics. 2019;19(2):171–180. doi:10.1111/psyg.12380. [DOI] [PubMed] [Google Scholar]
- 8. Fetveit A, Bjorvatn B. Sleep duration during the 24-hour day is associated with the severity of dementia in nursing home patients. Int J Geriatr Psychiatry. 2006;21(10):945–950. [DOI] [PubMed] [Google Scholar]
- 9. Cohen-Mansfield J, Marx MS, Regier NG, Dakheel-Ali M. The impact of personal characteristics on engagement in nursing home residents with dementia. Int J Geriatr Psychiatry. 2009;24(7):755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perugia G, Rodríguez-Martín D, Boladeras MD, Mallofré AC, Barakova E, Rauterberg M. Development of two measures of client engagement for use in home aged care. Am J Alzheimers Dis Other Demen. 2018;33:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burge E, Kuhne N, Berchtold A, Maupetit C, von Gunten A. Impact of physical activity on activity of daily living in moderate to severe dementia: a critical review. Eur Rev Aging Phys Act. 2012;9(1):27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bürge E, Berchtold A, Maupetit C, et al. Does physical exercise improve ADL capacities in people over 65 years with moderate or severe dementia hospitalized in an acute psychiatric setting? A multisite randomized clinical trial. Int Psychogeriatr. 2017;29(2):323–332. [DOI] [PubMed] [Google Scholar]
- 13. Herrmann N, Gauthier S, Lysy PG. Clinical practice guidelines for severe Alzheimer’s disease. Alzheimers Dement. 2007;3(4);385–397. [DOI] [PubMed] [Google Scholar]
- 14. Wakaho O, Rie N, Akira H, et al. Inter-rater reliability of the Japanese version of Clinical Dementia Rating (CDR) (in Japanese). Rhonen Seishin Igaku Zasshi. 2000;11(5):521–527. [Google Scholar]
- 15. Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139(6):1514–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kovach CR, Taneli Y, Dohearty P, Schlidt AM, Cashin S, Silva-Smith AL. Effect of the BACE intervention on agitation of people with dementia. Gerontologist. 2004;44(6):797–806. [DOI] [PubMed] [Google Scholar]
- 18. Goichot B, Vinzio S, Kaltenbach G. Marked decrease of rest–activity rhythm amplitude with time in non-demented disabled nursing home residents. Sleep Biol Rhythms. 2005;3(3):136–141. [Google Scholar]
- 19. Van Alphen HJ, Volkers KM, Blankevoort CG, Scherder EJ, Hortobágyi T, van Heuvelen MJ. Older adults with dementia are sedentary for most of the day. PLoS One. 2016;11(3):e0152457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 21. Tanaka H, Nagata Y, Uematsu M, et al. Development of the Cognitive Test for Severe Dementia. Dement Geriatr Cogn Disord. 2015;40(1-2):94–106. [DOI] [PubMed] [Google Scholar]
- 22. Nobutsugu H, Etsuro M, Hikari Y, Akitsugu T, Atsushi Y. A. Novel scoring system of activities of daily living for patients with Alzheimer’s disease: Hyogo Activities of Daily Living Scale (HADLS) (in Japanese). Shinkei Shinrigaku Zasshi. 1997;13(4):260–269. [Google Scholar]
- 23. Kazue S, Nobutsugu H, Kaoru T, Manabu I. Validity and reliability of the Japanese Version of the Neuropsychiatric Inventory-Nursing Home Version (NPI-NH) (in Japanese). Brain and Nerve. 2008;60(12):1463–1469. [PubMed] [Google Scholar]
- 24. Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5);373–383. [DOI] [PubMed] [Google Scholar]
- 25. Mitchell SL, Teno JM, Kiely DK. et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Health Organization. Global recommendations on physical activity for health. 2010. http://apps.who.int/iris/bitstream/handle/10665/44399/9789241599979_eng.pdf;jsessionid=38480668CEFA169147835792D8DF0334?sequence=1. Accessed July 4, 2018. [PubMed]
- 27. Scarmeas N, Luchsinger JA, Brickman AM, et al. Physical activity and Alzheimer disease course. Am J Geriatr Psychiatry. 2011;19(5):471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eggermont LH, Scherder EJ. Ambulatory but sedentary: impact on cognition and the rest–activity rhythm in nursing home residents with dementia. J Gerontol B Psychol Sci Soc Sci. 2008;63(5):279–287. [DOI] [PubMed] [Google Scholar]
- 29. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. [DOI] [PubMed] [Google Scholar]
- 30. Koch G, Belli L, Giudice TL, et al. Frailty among Alzheimer’s disease patients. CNS Neurol Disord Drug Targets. 2013;12(4):507–511. [DOI] [PubMed] [Google Scholar]
- 31. Carpenter GI, Hastie CL, Morris JN, Fries BE, Ankri J. Measuring change in activities of daily living in nursing home residents with moderate to severe cognitive impairment. BMC Geriatr. 2006;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tappen RM. Development of the refined ADL assessment scale for patients with Alzheimer’s and related disorders. J Gerontol Nurs. 1994;20(6);36–42. [DOI] [PubMed] [Google Scholar]
- 33. Selbaek G, Engedal K, Benth JŠ, Bergh S. The course of neuropsychiatric symptoms in nursing-home patients with dementia over a 53-month follow-up period. Int Psychogeriatr. 2014;26(1):81–91. [DOI] [PubMed] [Google Scholar]
- 34. Moyle W, Jones C, Murfield J. et al. Levels of physical activity and sleep patterns among older people with dementia living in long-term care facilities: A 24-h snapshot. Maturitas. 2017;102:62–68. [DOI] [PubMed] [Google Scholar]
- 35. Rozum WJ, Cooley B, Vernon E, Matyi J, Tschanz JT. Neuropsychiatric symptoms in severe dementia: associations with specific cognitive domains the Cache County Dementia Progression Study. Int J Geriatr Psychiatry. 2019;34(7):1087–1094. doi:10.1002/gps.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kodama A, Kume Y, Tsugaruya M, Ishikawa T. Deriving the reference value from the circadian motor active patterns in the “non-dementia” population, compared to the “dementia” population: What is the amount of physical activity conducive to the good circadian rhythm. Chronobiol Int. 2016;33(8):1056–1063. [DOI] [PubMed] [Google Scholar]
- 37. Regier NG, Hodgson NA, Gitlin LN. Characteristics of activities for persons with dementia at the mild, moderate, and severe stages. Gerontologist. 2017;57(5):987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hiroyuki T, Yuma N, Daiki I, Takashi N. The limitation of existing scales for assessing residual ADL of patients with severe dementia (in Japanese). Sagyoryoho. 2017;36:105–108. [Google Scholar]