Abstract
Alzheimer’s Disease (AD) is pathologically characterized by the accumulation of soluble oligomers causing extracellular beta-amyloid deposits in form of neuritic plaques and tau-containing intraneuronal neurofibrillary tangles in brain. One proposed mechanism explaining the formation of these proteins is impaired phagocytosis by microglia/macrophages resulting in defective clearance of soluble oligomers of beta-amyloid stimulating aggregation of amyloid plaques subsequently causing AD. However, research indicates that activating macrophages in M2 state may reduce toxic oligomers. NEU1 mutation is associated with a rare disease, sialidosis. NEU1 deficiency may also cause AD-like amyloidogenic process. Amyloid plaques have successfully been reduced using NEU1.Thus, NEU1 is suggested to have therapeutic potential for AD, with lysosomal exocytosis being suggested as underlying mechanism. Studies however demonstrate that NEU1 may activate macrophages in M2 state, which as noted earlier, is crucial to reducing toxic oligomers. In this review, authors discuss the potential therapeutic role of NEU1 in AD via immune system.
Keywords: Alzheimer’s, Neu1, neuroinflammation, immune system, pathophysiology
Introduction
Among neurodegenerative disorders, Alzheimer’s disease (AD) is considered to be the leading cause of dementia/cognitive decline. 1 In 2017, approximately 2.1 million Alzheimer’s patients over the age of 85 years were reported. 2 The hallmark neuropathological features of AD include extracellular amyloid deposition of the Aβ peptide processed from the amyloid precursor protein (APP) and intraneuronal neurofibrillary tangles (NFT). 3 -6 Even though more than 95% cases of the AD are sporadic, genetic factors such as APP, PSEN1/2, and APOE have also been associated with AD. Moreover, individuals with Down syndrome may develop dementia with AD-type neuropathological changes with younger age of onset than in patients without Down syndrome. 7 Various pathological mechanisms have been associated with the etiology of AD. 8
Neuroinflammation has been widely discussed as a causative mechanism 9 with microglia considered to be key players associated with AD but whether they possess a beneficial or deleterious role in its etiology has been a subject of discussion. 9 Multiple studies have tried to address the effects of microglia, considering them the perpetrators of inflammatory damage in neurodegenerative diseases and also playing a key role in the formation of amyloid deposits. 10 There are also studies that seem to suggest that despite enormous amounts of microglia becoming activated in the brain of AD patients, they fail to produce a phagocytic response to Aβ plaques. 11 Hence integrity of microglia/macrophages and phagocytic function seems to be a factor in the burden of amyloid clearance and thus may have a critical role in the pathogenesis of the disease. In fact, various therapeutic interventions that have been designed to enhance the phagocytotic role of microglia with the goal of reducing amyloid burden. 11 A well-regarded notion is the imbalance between production of amyloid neurotoxins and inadequate removal consequently leads to increased amyloid burden. Here it is worth mentioning that in addition to extracellular amyloid plaques and intraneuronal neurofibrillary tangles, 3,4 presence of soluble assemblies of Aβ oligomers in AD brain has been reported. 12 These soluble neurotoxins (known as ADDLs{Aß-derived diffuse ligands} and protofibrils) seem to likely precede the formation of insoluble fibrillar amyloid deposits and intraneuronal neurofibrillary tangles, thus leading to AD progression and cause behavioral symptoms 13 as well as early symptoms of cognitive impairment. 14 Thus, there are 2 main school of thoughts explaining AD pathogenesis, of which one supports “amyloid cascade theory” essentially stating that amyloid deposits accumulating in AD (Aß) possibly are the cause of cognitive decline. 5 However, results of recent studies support the ADDL hypothesis which suggests that a major factor in Alzheimer’s dementia is the neurological impact of soluble, globular oligomers of Aβ 1–42 (“ADDLs”) by causing specific aberrations in synaptic signaling. 13 In fact, these soluble oligomers have been considered a therapeutic target for AD as well. 12,13 Importantly, recent studies have established a new role monocytes and macrophages in restricting not only cerebral Aβ fibrils, but possibly soluble oligomers as well. 15
Interestingly NEU1, mammalian neuraminidase 1 which has been associated with Sialidosis, 16 has also been demonstrated to improve phagocytosis via receptor Fc gamma RI/CD64. 17 Our hypothesis therefore proposes NEU1 to be potentially therapeutic by enhancing phagocytosis in AD which may aid in reducing amyloid burden by decreasing the trafficking of soluble of Aβ. We also discuss the possible underlying mechanisms.
Background
Soluble Aβ Leads to Cognitive Decline and Pathological Progression in AD
Alzheimer’s disease (AD) being the most common cause of dementia, is neuropathologically characterized by amyloid beta Aβ accumulation in the form of diffuse and neuritic plaques, progressive deposition of tau positive intraneuronal neurofibrillary tangles, synaptic loss, and neurodegeneration. 9 The major component of neuritic plaques, β amyloid peptide (Aβ) is a product of proteolytic cleavage of the amyloid precursor protein (APP) 18 Thus, the abnormal processing of amyloid precursor protein (APP) leads to the formation of soluble Aβ. 12 The presence of elevated levels of these soluble Aβ oligomers have been attributed to cognitive impairment. 19 It has been said that soluble Aβ are present at an early stage and would precede malfunctions in memory-specific signal transduction, causing behavioral and cognitive decline, eventually causing aggregates of insoluble plaque and neurofibrillary tangles. 12,13,19 The accumulation of insoluble Aβ, in turn induces inflammatory mediators (e.g. cytokines) and free-radical production subsequently causing the death of neurons, resulting worsening of dementia/cognitive decline. 20,21 Some studies have identified intracellular oligomeric Aβ as the most neurotoxic form of Aβ and can be transferred by interconnected cells, which may also create a proinflammatory environment, potentially contributing pathological progression in AD. 22
Current available data strongly suggest that neuroinflammation of the CNS plays a crucial role in the development of several neurodegenerative diseases, including Alzheimer’s disease. 23 This neuroinflammation is a coordinated response between microglia and other cells of the CNS such as astrocytes, as well as peripheral immune cells infiltrating the CNS. Certain stimuli, including toxins, infections, trauma, or ischemia, may elicit a rapid activation of the immune system, referred to as acute neuroinflammation, characterized by microgliosis and the release of inflammatory mediators. When not regulated, this could lead to chronic neuroinflammation and subsequently neurodegeneration. 23 Inflammatory components that are thought to play a critical role in AD-associated neuroinflammation include microglia, astrocytes, the complement system, as well as cytokines and chemokines. 24
Under physiological conditions within the central nervous system (CNS), different types of macrophages including microglia, meningeal macrophages, perivascular (blood-brain barrier) and choroid plexus (blood-cerebrospinal fluid barrier) macrophages reside, provide immune surveillance of the brain and function to maintain homeostasis with a direct involvement in the development and resolution of neuroinflammatory processes. 24 -26
Neurotoxic Vs Protective Role of Microglia in AD Pathology
In contrast to their neuroprotective role in normal brain, the potential neurotoxic role of microglia particularly in AD has been discussed. Loss of blood-brain barrier (BBB) homeostasis occuring in the initial stage of AD causes production of proinflammatory cytokines and suppressors of cerebral blood flow by endothelial cells, which aggravates synapse destruction, accumulation and activation of microglia. These microglia in turn produce a variety of pro-inflammatory mediators as well as neurotoxins, potentially contributing to AD neuropathological changes. 27 -33
Another observation reported in the literature is that in situ, microglia tend to cluster at sites of Aβ plaques. 34,35 This clustering is thought to be a result of chemotactic signaling by Aβ itself as well as by numerous inflammatory mediators such as complement activation fragments, cytokines, and chemokines that are associated with Aβ in senile plaques. 35,36 The aggregation of activated microglia around Aß plaques led researchers to study the phagocytic mechanism of microglia in the AD brain. 37,38 It was reported that initially microglia are attracted to amyloid-β deposits, which they internalize and degrade, aiding clearance of amyloid-β from the brain. 39,40 Furthermore, various studies demonstrated microglial activation leading to amyloid reductions with both active and passive immunotherapy. 41 Thus, microglia may have a potentially beneficial role in the neurodegenerative disease process. 41,42 Nonetheless, as the disease progresses, microglia may lose this beneficial effect as they acquire a different phenotype due to chronic activation and continued production of pro-inflammatory mediators. 42,43
Impaired Phagocytosis in AD
Two main types of microglial activation have been discussed in the literature in relation to AD. These are named (i) classical activation (M1) and (ii) alternative activation (M2). The association between these states and AD neuropathological changes remains to be completely understood. 44 It has been suggested however that an M1-type response lowers amyloid load but may aggravate neurofibrillary tangle pathology. On the other hand, M2 is accompanied by elevated amyloid load and is further subdivided into alternative activation (M2a), type II alternative activation (M2b) or acquired deactivation (M2c). 44 It is noteworthy that various animal studies as well as human postmortem studies have reported that microglia in M2b state along with increased levels of expression of Fc-gamma receptor 1 (FcγRI), also known as CD64 are found in AD brains. 45 M2b type activation is thought to be induced by ligation of immunoglobulin Fc gamma receptors (FcγRs) (CD16, CD32 or CD64) via immune complexes on LPS or IL-1β-primed microglia/macrophages, causing downregulated expression of IL-12, increased IL-10 secretion and increased HLA-DR expression. 46 -49 FcγRI or CD64 seems to be a common marker expressed in postmortem AD brain as well as down syndrome AD brain. 50,51
CD64 has been described as a cell surface receptor, possessing a high affinity for the Fc portion of immunoglobulin (IgG), and able to trigger a monocyte/macrophage response. 52 Expression of CD64 reflects the presence of immunoglobulins in the brain and thus the involvement of systemic immunity. FcγRs are considered important for antibody-dependent cytotoxicity, antigen presentation via MHC, clearance of antibodies as well as phagocytosis. FcγRI (CD64) is found on macrophages, neutrophils, eosinophils and dendritic cells binds monomeric Fcγ-domain of IgG and leads through an Immunoreceptor tyrosine-based activation motif (ITAM) domain to the activation of phagocytosis and an inflammatory response. 52 Presence of CD64 in AD brain has been associated with presence of dementia/ cognitive decline. 48
It is noteworthy that FcR mediated microglial phagocytosis and its possible role in the therapy of AD has been previously examined when peripherally administered anti-Aβ antibodies were demonstrated to be capable of crossing the blood brain barrier, with subsequent binding to Aβ in plaques and trigger FcR-mediated microglial phagocytosis. 53 Active vaccination with Aβ42 in the AN-1742 trial showed evidence that the reduction of Aβ deposits was associated with microglial phagocytosis. Various other active and passive Aβ immunotherapy studies in animal models also suggested that FcR-mediated activation of microglia may be a central mechanism of reducing Aβ load in the brain although there are some studies that suggest a non-Fc-mediated mechanism may also be involved in amyloid-beta in vivo by immunotherapy. 54 Animals studies have elucidated that antibodies against Aβ -peptide trigger microglial cells to clear plaques through FcR mediated phagocytosis and subsequent peptide degradation. 55 Interestingly, under physiological conditions, CD64 is thought to increase the phagocytic activity of macrophages, monocytes. 17 Thus, it is plausible that CD64 can be a potential therapeutic target in AD. We suggest that Neu1 increases the phagocytotic activity of macrophages via CD64.
NEU1 and AD3.1 Link of NEU1 and Alzheimer Disease
NEU1 is the most abundant and ubiquitous of the 4 mammalian sialidases with a wide tissue distribution. Encoded by the gene for sialidase (neuraminidase 1) NEU1 plays a crucial role in lysosomal catabolism of sialylated glycoconjugates. 56,57 NEU1 essentially catalyzes the hydrolytic cleavage of terminal sialic acid residues from oligosaccharides and glycoproteins. 56 Deficiency of NEU1 is associated with sialidosis, a lysosomal storage disorder in which there is impaired processing/degradation of sialo-glycoproteins, and accumulation of oversialylated metabolites. 58 Sialidosis is divided into 2 main clinical types: Type I is a milder form of the disease, and Type II is further subdivided into 3 forms: congenital, infantile and juvenile. 59,60 Neu1 is expressed ubiquitously throughout the brain but more so in certain regions such as the CA3 region of the hippocampus and the cortex. 61,62 An MRI study involving 11 sialidosis patients reported decreased functional connectivity from the temporal and occipital lobes to the hippocampus and parahippocampus, while diffuse cortical atrophy with posterior focal lesions was also noticed, which may be linked with cortical blindness due to an altered neural network and a compromised visual pathway in the patients. 62 Nevertheless, since sialidosis is a rare disease, there is paucity of studies on humans, and researchers have had to rely on studying the mouse models of the diseases. In one animal study, Neu1−/− mice developed a certain pattern of disease in the brain, with vacuolated neurons present throughout the parenchyma (mainly in the olfactory bulb, the sub olfactory nucleus and the nuclei of the limbic system). Microglia and perivascular macrophages were noticed to be the most affected cells, often present in the proximity of degenerating neurons in the Neu1−/− mice. Also, numerous ballooned macrophage-like cells appeared to line neurons of the dentate gyrus [63]. Another study reported the occurrence of an AD-like phenotype in the sialidosis mice. It is indeed important to note that Neu1−/− mice showed a brain phenotype characterized by the presence of amyloid deposits primarily accumulated in the CA3 region of the hippocampus and resembling β-amyloid plaques like those seen in AD neuropathology, thus demonstrating decreased NEU1 enzyme activity as a risk factor for the development of AD. 61 -63 Additionally, it has been demonstrated that inactivation of the Neu1 gene in the transgenic 5XFAD mice overexpressing human mutant amyloid precursor protein (APP) increased the formation of the plaques while intracerebral injections of adeno-associated viruses (AAVs) expressing NEU1 and CTSA slowed down or even revert the amyloidogenic process, which indicates that NEU1 brain levels may pose a risk factor for developing Alzheimer disease in human. 62,64
Researchers established that in absence of Neu1, Aβ released via lysosomal exocytosis is a novel mechanism which leads to the extracellular deposition of this toxic peptide. 63
Evidence of NEU1 Being Able to Enhance Phagocytosis
In addition to its crucial role in the intralysosomal catabolism of glycoproteins and glycolipids, a growing body of literature suggest that Neu1 also possess a vital in immune system and is involved mechanisms associated with cellular signaling during the immune response. 64 It is reported to be essential for regulating numerous immune activities. 65 Studies suggest that Sialidase is overexpressed during the activation of
T cells, B cells, macrophages, and neutrophils on the surface of activated T cells and may have an effect on immune function. Also, endogenous sialidase activity has been reported to increase exponentially during activation in majority of immune cells including T cells, B cells, and monocytes, whereas sialylation of some of their surface molecules decreases. 65 -68 Animal studies report that sialidase deficiency subsequently leading to diminished stimulation of lymphocytes and macrophages Furthermore, Studies conducted on human subjects have attributed recurrent infections to sialidase deficiency in sialidosis patients, occurring due to decreased capacity of immune cells for cytokines and antibodies production. 64
Other studies have demonstrated activity of Neu1 sialidase in freshly isolated human monocytes to be increased 20 to 30-fold per cell as they differentiate into macrophages. An upregulation of expression of Neu1 has been reported during monocyte to macrophage differentiation, being targeted to the plasma membrane which in turn results in augmentation of the phagocytic capacity of these cells, hence indicative of a crucial role of Neu1 in immune activation and immunomodulation. Furthermore, in there work, Pshezhetsky and coworkers describes macrophages isolated from Neu1-deficient mice exhibited a reduction in phagocytosis. Also, the macrophages taken from the Neu1-deficient mice exhibited increased sialylation and impaired phosphorylation of FcR(FcRgamma 1/CD64) as well as markedly reduced phosphorylation of Syk kinase in response to treatment with IgG-opsonized beads indicating that the cell surface Neu1 activates the phagocytosis in macrophages and dendritic cells through desialylation of surface receptors, particularly via CD64, hence essential for their functional integrity. 17 Treatment of cells with exogenous Neu1 restored the phagocytic capability of macrophages. Hence the fact that FcRγ1/CD64 receptor has been demonstrated to be a substrate of NEU1, and NEU1 activation phagocytosis via CD64 receptor may create an anti-inflammatory environment since this bring microglia/macrophages to M2 state, which appears to be crucial in reducing the pathogenesis of neurodegeneration. 17,22
Combining these observations, makes it therefore plausible to hypothesize that in addition to Neu1’s role via lysosomal exocytosis, another pathogenic pathway exists. This pathway consists of immune activation and immunomodulation. 17,64,65 through which NEU1 may play a therapeutic role in AD.
A core problem in AD etiology is the imbalance between Aβ production and its removal. 69
Furthermore, an early elevation in soluble Aβ leads to neuronal loss, causing cognitive impairment, as well as promotes abnormal tau phosphorylation, perpetrates tauopathy along with plaque formation. 12 -14 Thus, controlling the trafficking of soluble oligomers may reduce the pathogenesis. 22 There is also evidence that microglia, instead of performing phagocytic activity adequately, often change the phenotype and hence may acquire a disastrous role. 11 However, studies suggest that stimulating microglia to M2 phenotype enhance their anti-inflammatory action can potentially slow down the disease progression by controlling the soluble Aβ oligomers. 22 Such microglia are characterized by various markers
including CD64. 44,47 Relying on the foundation established above, we present the
following hypotheses.
FcRγ1/CD64 is thought to be associated with increased phagocytotic activity. 70 -72 and with AD. We therefore hypothesize that phagocytotic activity in AD is compromised and enhancingFcRγ1/CD64 phagocytotic activity may enhance the anti-inflammatory characteristics of macrophages which in turn may help in reducing soluble Aβ oligomers. 22
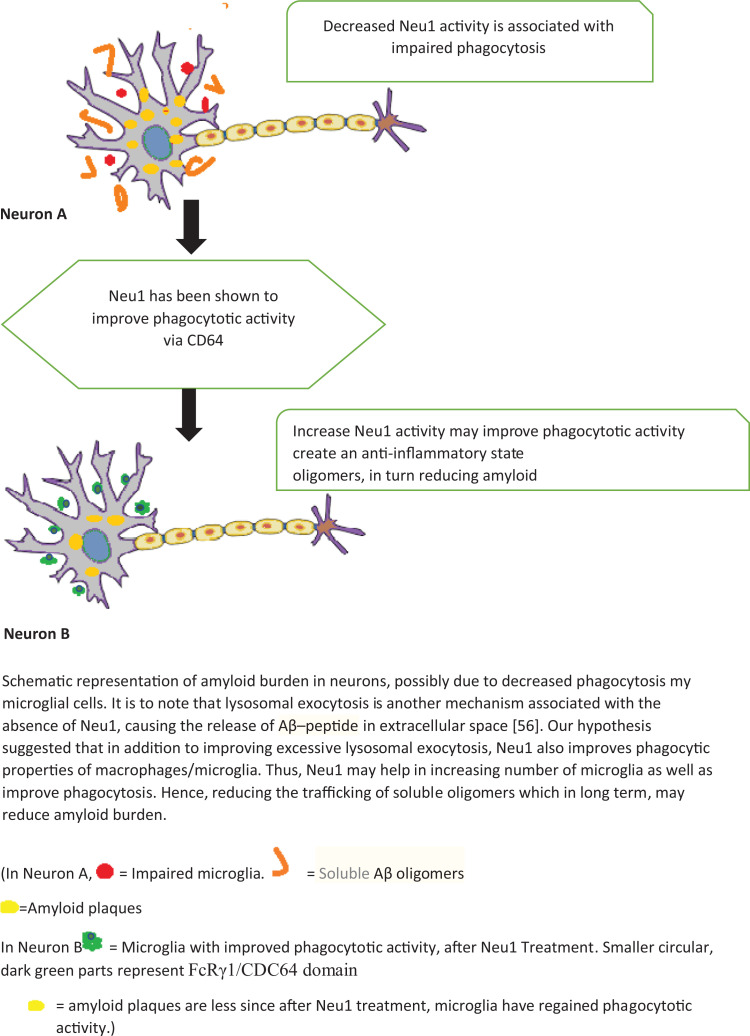
Neu1 has been demonstrated to increase the phagocytotic activity using FcRγ1/CDC64 receptor. 17 Thus, Neu1 may be used to increase phagocytosis, via FCR gamma one/CD64. Since macrophages associated with FCR gamma one/CD64 are in M2 state, thus perform an anti-inflammatory activity, which has been shown to reduce soluble oligomers of Aβ 22 and hence may be a potential therapeutic option. Figure 1
Figure 1.
Association between Neu1 and Alzheime1.
Via animal studies, it has been demonstrated that ultrastructural examination of Neu1− / − hippocampal region exhibit features associated with amyloid plaques, suggesting that such pathological changes result in some way from of Neu1 deficiency. In addition, Neu1− / − mice have been reported to suffer from profound systemic and neurological abnormalities. Not only are their brains smaller in size than their wild-type (WT) littermates, but also mapping of Neu1 expression revealed that this enzyme is present uniformly throughout the parenchyma and is especially abundant in the hippocampus. 62 -64
Furthermore, increased expression of CD64 has been reported in AD brains as well as Down syndrome brains. 49,50 This notion can be tested via immunohistochemistry in post-mortem brain sections from patients with AD, Down syndrome and control brains. We also recommend isolation of macrophages and studying sialyation pattern. Further testing may be possible by examining the macrophages in a Neu1− / − mouse model with the expectation to see altered sialyation of the cell surface proteins, with a possible effect on FcRI/CD64. Also, macrophages may show reduced induction of sialidase activity during differentiation. Furthermore, in order to detect phosphorylated FcR, macrophage lysates may be immunoprecipitated via the receptor using polyclonal anti-mouse FcRI/CD64 antibodies (RD Systems) specific against both alpha and gamma chains of the receptor. Also, the impaired phagocytosis in the Neu1-deficient cells can potentially be rectified by the treatment of cells with the exogenous enzyme Neu1. 17
Discussion
In their animal study, Annunziata et al. (2013) demonstrated that deficiency of the lysosomal sialidase NEU1 caused a spontaneous occurrence of an AD-like amyloidogenic process. 63 The authors suggested that the underlying mechanism involves NEU1 loss-of-function—accumulation and amyloidogenic processing of an over-sialylated amyloid precursor protein in lysosomes, and extracellular release of Aβ-peptides by excessive lysosomal exocytosis. 62,63 We propose another mechanism through which the loss of NEUI function may contributes to Alzheimer’s disease, and that is the immune system. NEUI deficiency has been demonstrated to result in impaired differentiation of macrophages, 17 as well as have a negative impact on their phagocytic activity, which in turn may contribute to AD disease. 16 Thus, in addition to lysosomal exocytosis, we advocate here for the investigation of the potential therapeutic role of Neu1 in AD via its role in immune system, in order to identify any additional pathological mechanism.
It is well-established that phagocytosis plays a vital role in the eradication of invading infectious agents. 23 The “professional” phagocytotic cells include monocytes, macrophages, and neutrophils. The entire process of phagocytosis is fairly complex and involves the steps of recognition of invading foreign particles by specific types of phagocytic receptors, which ultimately lead to internalization of the particles. 2,3 Fc gamma receptors (FCGRs) are considered to be the best studied phagocytic receptors which bind to Fc portion of immunoglobulin G (IgG). Antibodies are known to bind to specific antigen through their antigen binding (Fab) end and their constant (Fc) region binds to FCGRs on phagocytes. The clustering of FCGRs by IgG antibodies on the phagocyte commence to a various signal, subsequently causing reorganization of actin cytoskeleton and membrane remodeling, and in turn leading to the formation of a phagosome. Fc gamma receptors are classified into 3 classes: FCGRI, FCGRII and FCGRIII. Each class of these FCGRs consists of several individual isoforms. Among all these isoforms FCGRI, FCGRIIA and FCGRIIIA, are able to mediate phagocytosis. 70 -72
The significance of phagocytosis in the immune clearance of Aβ by macrophages of AD has been supported by numerous studies. 73 Moreover, FCR mediated microglial phagocytosis to treat AD pathology has been experimented on previously. 73 Also, another FcR knockout model specified the role of specific FcR components in the induction of MIP-1 and phagocytosis, thus demonstrating a pathway by which immune complexes can activate microglia. 74 It has therefore been suggested that cell surface Neu1 activates phagocytosis in macrophages and dendritic cells through desialylation of surface receptors, thus, contributing to their functional capacity. 17
Another important fact to consider is that animal studies have also provided evidence in support of peripheral blood macrophages and T-cells being able to invade the brain of aged APP23 mice. 75 In another mutant APP transgenic mouse model, bone-marrow derived microglia invaded the brain and reduced Aβ deposits. Of note, CNS infiltration of peripheral monocytes and the associated clearance of Aβ decreased with age in this APP mouse mode. 76 Hence it is conceivable that in addition to CNS based microglia, bone marrow-derived microglia also play a role in eliminating amyloid deposits. Furthermore, studies investigating phagocytosis by monocytes and macrophages isolated from the blood of age-matched control patients revealed that control monocytes showed exceptional differentiation into macrophages and intracellular phagocytosis of Aβ followed by Aβ degradation or export. AD monocytes on the other hand exhibited poor differentiation and suffered apoptosis. Additionally, macrophage phagocytosis has been reported to defective in AD. Monocytes of AD patients have been shown to be defective and result in incomplete/inadequate phagocytosis of Aβ. Thus, while normal human monocytes and macrophages appear to phagocytize, degrade and clear Aβ incredibly well, in AD monocytes and macrophages are defective and, instead of providing help, disrupt BBB, produce neurotoxic cytokines, invade and only ineffectively phagocytize Aβ deposits and suffer apoptotic cell death with release of Aβ. 11,15 Hence it can be assumed that the therapeutic strategies that enhance the functional integrity as well as improve the recruitment of macrophages could be potential tool for the elimination or at least reduction of senile plaques. 31 When Neu1-deficient immune cells exhibited a profound decline in their functional capacity all types of phagocytic function were affected while activity of Neu1 sialidase of freshly isolated human monocytes has been demonstrated to be increased 20 to 30-fold per cell as they differentiate into macrophages. Moreover, Neu1 deficiency may affect the ability of peritoneal, splenocyte as well as peripheral blood monocytes to engulf Gram-positive and Gram-negative bacteria as well as IgG-opsonized and non-opsonized particles and IgG-coated red blood cells, indicating that Neu1 deficiency negatively affect all types of phagocytosis. 17 We therefore hypothesize that NEU1 may improve the overall functional integrity of macrophages as well as microglia and serve as an effective therapeutic tool for AD.
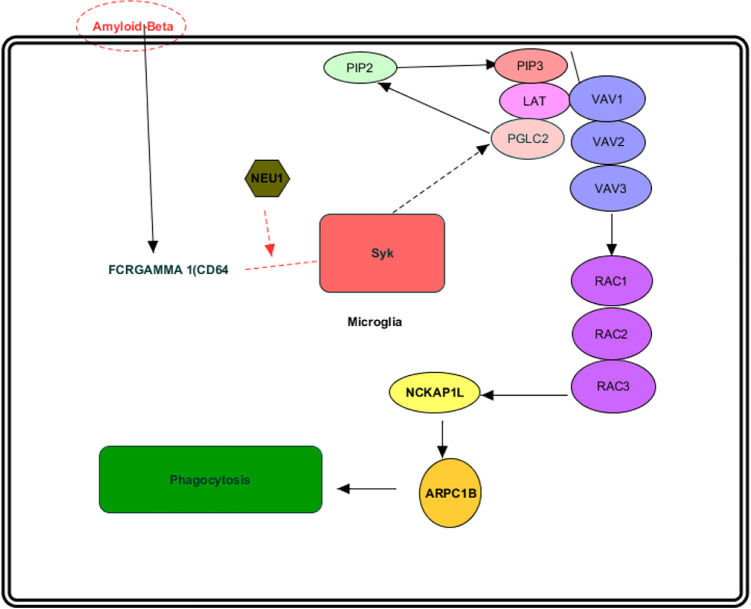
Although the complete understanding of the mechanism through which Neu1 upregulates phagocytosis is not yet achieved, so far there seems to be experimental evidence that absence of Neu1 results in increased sialylation of the cell surface proteins and hence may affect multiple receptors for phagocytosis including CD64 since it has been elucidated that the macrophages from the Neu1-deficient mice showed increased sialylation and impaired phosphorylation of FcR as well as markedly reduced phosphorylation of Syk kinase in response to treatment with IgG-opsonized beads. The signaling pathway that we propose is illustrated in Figure 2. The fact that treatment of the cells with purified mouse Neu1 can result in reduced surface sialylation and restored phagocytosis suggests that cell surface Neu1 activates phagocytosis in macrophages and dendritic cells through desialylation of surface receptors, and thus may contribute to their functional integrity. 17 We believe this also makes possible the notion that impaired macrophages in Alzheimer’s could be reactivated with NEU1.
Figure 2.
Therapeutic potential of Neu1 in Alzheimer’s disease via the immune system.
Optimal sialylation of cell surface glycoproteins may also be important for the interaction of macrophages with other cells (e.g. with T, B, and NK cells via sialic acid-binding immunoglobulin-like lectins). 17,61 It is therefore quite conceivable that if up-regulation of the NEU1 gene is functionally important for macrophage differentiation, then the primary or secondary deficiency of the Neu1 activity may affect functions of all monocyte-derived cells and cause defects.
While neurodegenerative disorders that mimic clinical features of Alzheimer’s disease, such as the more recently described Limbic-predominant Age-related TDP-43 Encephalopathy (LATE) has been recognized, Alzheimer’s remains the most common form of dementia. 77 The need for developing effective treatments for AD therefore imperative since it has been predicted that within the next 4 decades an increase number of patients will present with AD. 2 Currently, tremendous scientific ongoing efforts are being made, testing various type of therapies to find a suitable cure that for AD. 78 Among them, active immunotherapy (also known as vaccination) against amyloid β (Aβ) has been tried frequently. 78 Although successfully tested in model animals, human clinical trials of immunotherapy provided have shown at best minimal improvement in cognitive function, while causing several adverse effects. 12 Therapies targeting soluble oligomers of Aβ have provided promising results in reducing behavioral symptoms and cognitive declines, 12 and may be applied to clinical trials. Their curative capabilities and adverse effect profile are yet to be fully tested however. Meanwhile, another therapeutic approach that has been suggested and may be pursued in clinical trials is controlling and reducing intraneuronal trafficking of soluble oligomers. Since this may help in reduce not only soluble oligomers but may also prevent aggregation of amyloid plaques as well as tau pathology, thus may provide significant positive results. 78,22
It is noteworthy that studies have also demonstrated that activating macrophages in its M2 state to be a promising approach reducing the neurotoxicity of soluble oligomers. 22 In this study we have reviewed and discussed that Neu1 activates the macrophages in M2 17 state via CD64, creating an anti-inflammatory environment, thus it is plausible that NEU1 may be considered as an efficacious approach in reducing soluble oligomers trafficking which may not only reduce associated clinical symptoms, with subsequent less plaque formation and less tangles. lack of Neu1 is associated with AD like changes in brain, 63 and its administration does provide positive results via lysosomal exocytosis. 62,63 Although Neu1 has been demonstrated to activates macrophages in M2 state, 17 this remains to be tested in the context of AD. Perhaps this can be tested initially with animal models. Upregulation of Neu1 in animal AD models can be accomplished using the approaches previously described, i.e. pharmacologic chaperone-mediated therapy, and AAV-mediated gene therapy. 62,63 AAV-mediated gene therapy could be particularly a more suitable therapeutic strategy for AD because it has been successfully applied in clinical trials and preclinical studies. 62,63
Conclusion
In short, the beneficial role of NEU1 in AD brain via excessive lysosomal exocytosis has already been demonstrated and established previously. Moreover, activation oof macrophages in M2 stage has been illustrated to decrease the neurotoxicity of soluble oligomers, with a reduction of associated clinical symptoms and thus possess therapeutic potential for AD. Taken together, we propose that NEU1 may also play a critical role in reducing soluble oligomers by activating macrophages to M2 state via CD64, thereby further validating its curative potential for AD. Studies on animal models can be done to verify this hypothesis. The authors hope this will encourage future studies to test and confirm this hypothesis.
Footnotes
Authors’ Note: Sumit Das, MD, Consolato Sergi, MD, PhD, shared senior authorship.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Sumit Das  https://orcid.org/0000-0002-1826-9644
https://orcid.org/0000-0002-1826-9644
References
- 1. Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspect Med. 2011;1(1):a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chaudhary A, Maurya PK, Yadav BS, Singh S, Mani A. Current therapeutic targets for Alzheimer’s disease. J Biomed. 2018;3:74–84. [Google Scholar]
- 3. Selkoe DJ. Normal and abnormal biology of the beta-amyloid precursor protein. Ann Rev Neurosci. 1994;17(1):489–517. [DOI] [PubMed] [Google Scholar]
- 4. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. [DOI] [PubMed] [Google Scholar]
- 5. Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimers disease at 25 years. EMBO Mol Med. 2016;8:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Selkoe DJ. Amyloid β protein precursor and the pathogenesis of Alzheimer’s disease. Cell. 1989;58(4):611–612. [DOI] [PubMed] [Google Scholar]
- 7. Burger PC, Vogel FS. The development of the pathologic changes of Alzheimer’s disease and senile dementia in patients with Down’s syndrome. Am J Pathol. 1973; 73(2):457. [PMC free article] [PubMed] [Google Scholar]
- 8. Ruegsegger GN, Manjunatha S, Summer P, et al. Insulin deficiency and intranasal insulin alter brain mitochondrial function: a potential factor for dementia in diabetes. FASEB J. 2019;33(3):4458–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Eldik LJ, Carrillo MC, Cole PE, et al. The roles of inflammation and immune mechanisms in Alzheimer’s disease. Alzheimers Dement. 2016;2(2):99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wisniewski HM, Vorbrodt AW, Wegiel J, Morys J, Lossinsky AS. Ultrastructure of the cells forming amyloid fibers in Alzheimer disease and scrapie. Am J Med Genet. 1990;37(S7):287–297. [DOI] [PubMed] [Google Scholar]
- 11. Fiala M, Cribbs DH, Rosenthal M, Bernard G. Phagocytosis of amyloid-β and inflammation: two faces of innate immunity in Alzheimer’s disease. J Alzheimers Dis. 2007;11(4):457–463. [DOI] [PubMed] [Google Scholar]
- 12. Klein WL. Aβ toxicity in Alzheimer’s disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem Int. 2002;41(5):345–352. [DOI] [PubMed] [Google Scholar]
- 13. Richardson JC, Kendal CE, Anderson R, et al. Ultrastructural and behavioural changes precede amyloid deposition in a transgenic model of Alzheimer’s disease. Neuroscience. 2003;122(1):213–228. [DOI] [PubMed] [Google Scholar]
- 14. De Felice FG, Wu D, Lambert MP, et al. Alzheimer’s disease-type neuronal tau hyperphosphorylation induced by Aβ oligomers. Neurobiol Aging. 2008;29(9):1334–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zuroff L, Daley D, Black KL, Koronyo-Hamaoui M. Clearance of cerebral Aβ in Alzheimer’s disease: reassessing the role of microglia and monocytes. Cell Mol Life Sci. 2017;74(12):2167–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khan A, Sergi C. Sialidosis: a review of morphology and molecular biology of a rare pediatric disorder. Diagnostics. 2018;8(2):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seyrantepe V, Iannello A, Liang F, et al. Regulation of phagocytosis in macrophages by neuraminidase 1. J Biol Chem. 2010;285(1):206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120(4):545–555. [DOI] [PubMed] [Google Scholar]
- 19. Ledo JH, Azevedo EP, Clarke JR, et al. Amyloid-β oligomers link depressive-like behavior and cognitive deficits in mice. Mol Psychiatry. 2013;18(10):1053–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun X, Chen WD, Wang YD. β-Amyloid: the key peptide in the pathogenesis of Alzheimer’s disease. Front Pharmacol. 2015;6:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Santos LE, Beckman D, Ferreira ST. Microglial dysfunction connects depression and Alzheimer’s disease. Brain Behav Immun. 2016;55:151–165. [DOI] [PubMed] [Google Scholar]
- 22. Sackmann V, Ansell A, Sackmann C, et al. Anti-inflammatory (M2) macrophage media reduce transmission of oligomeric amyloid beta in differentiated SH-SY5Y cells. Neurobiol Aging. 2017;60:173–182. [DOI] [PubMed] [Google Scholar]
- 23. Mammana S, Fagone P, Cavalli E, et al. The role of macrophages in neuroinflammatory and neurodegenerative pathways of Alzheimer’s disease, amyotrophic lateral sclerosis, and multiple sclerosis: pathogenetic cellular effectors and potential therapeutic targets. Int J Mol Sci. 2018;19(3):831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Galea I, Palin K, Newman TA, Van Rooijen N, Perry VH, Boche D. Mannose receptor expression specifically reveals perivascular macrophages in normal, injured, and diseased mouse brain. Glia. 2005;49(3):375–384. [DOI] [PubMed] [Google Scholar]
- 25. Stollg G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58(3):233–247. [DOI] [PubMed] [Google Scholar]
- 26. Da Fonseca AC, Matias D, Garcia C, et al. The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci. 2014;8:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eikelenboom P, Veerhuis R. The role of complement and activated microglia in the pathogenesis of Alzheimer’s disease. Neurobiol Aging. 1996;17(5):673–680. [DOI] [PubMed] [Google Scholar]
- 29. Dickson DW, Lee SC, Mattiace LA, Yen SH, Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer’s disease. Glia. 1993;7(1):75–83. [DOI] [PubMed] [Google Scholar]
- 30. Halliday G, Robinson SR, Shepherd C, Kril J. Alzheimer’s disease and inflammation: a review of cellular and therapeutic mechanisms. Clin Exp Pharmacol Physiol. 2000;27(1-2):1–8. [DOI] [PubMed] [Google Scholar]
- 31. Rogers J, Strohmeyer R, Kovelowski CJ, Li R. Microglia and inflammatory mechanisms in the clearance of amyloid β peptide. Glia. 2002; 40(2):260–269. [DOI] [PubMed] [Google Scholar]
- 32. Griffin WS, Mrak RE. Interleukin-1 in the genesis and progression of and risk for development of neuronal degeneration in Alzheimer’s disease. J Leukoc Biol. 2002;72(2):233–238. [PMC free article] [PubMed] [Google Scholar]
- 33. Streit WJ. Microglia and Alzheimer’s disease pathogenesis. J Neurosci Res. 2004;77(1):1–8. [DOI] [PubMed] [Google Scholar]
- 34. D’Andrea MR, Cole GM, Ard MD. The microglial phagocytic role with specific plaque types in the Alzheimer disease brain. Neurobiol Aging. 2004;25(5):675–683. [DOI] [PubMed] [Google Scholar]
- 35. Frautschy SA, Cole GM, Baird A. Phagocytosis and deposition of vascular beta-amyloid in rat brains injected with Alzheimer beta-amyloid. Am J Pathol. 1992;140(6):1389. [PMC free article] [PubMed] [Google Scholar]
- 36. Weldon DT, Rogers SD, Ghilardi JR, et al. Fibrillar β-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo. J Neurosci. 1998;18(6):2161–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. D’Andrea MR, Nagele RG. Morphologically distinct types of amyloid plaques point the way to a better understanding of Alzheimer’s disease pathogenesis. Biotech Histochem. 2010;85(2):133–147. [DOI] [PubMed] [Google Scholar]
- 38. Koenigsknecht J, Landreth G. Microglial phagocytosis of fibrillar β-amyloid through a β1 integrin-dependent mechanism. J Neurosci. 2004;24(44):9838–9846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mandrekar S, Jiang Q, Lee CD, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia mediate the clearance of soluble Aβ through fluid phase macropinocytosis. J Neurosci. 2009;29(13):4252–4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu Z, Condello C, Schain A, Harb R, Grutzendler J. CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-β phagocytosis. J Neurosci. 2010;30(50):17091–17101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilcock DM, Munireddy SK, Rosenthal A, Ugen KE, Gordon MN, Morgan D. Microglial activation facilitates Aβ plaque removal following intracranial anti-Aβ antibody administration. Neurobiol Dis. 2004;15(1):11–20. [DOI] [PubMed] [Google Scholar]
- 42. Krabbe G, Halle A, Matyash V, et al. Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS One. 2013;8(4):e60921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Michaud JP, Rivest S. Anti-inflammatory signaling in microglia exacerbates Alzheimer’s disease-related pathology. Neuron. 2015;85(3):450–452. [DOI] [PubMed] [Google Scholar]
- 44. Wilcock DM. A changing perspective on the role of neuroinflammation in Alzheimer’s disease. Int J Alzheimers Dis. 2012;2012:495243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Walker DG, Lue LF. Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimers Res Ther. 2015;7(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peress NS, Fleit HB, Perillo E, Kuljis R, Pezzullo C. Identification of FcγRI, II and III on normal human brain ramified microglia and on microglia in senile plaques in Alzheimer’s disease. J Neuroimmunol. 1993;48(1):71–79. [DOI] [PubMed] [Google Scholar]
- 48. Minett T, Classey J, Matthews FE, et al. Microglial immunophenotype in dementia with Alzheimer’s pathology. J Neuroinflammation. 2016;13(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Colton CA, Mott RT, Sharpe H, et al. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006;3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wilcock DM, Hurban J, Helman AM, et al. Down syndrome individuals with Alzheimer’s disease have a distinct neuroinflammatory phenotype compared to sporadic Alzheimer’s disease. Neurobiol Aging. 2015;36(9):2468–7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Honeychurch J, Tutt AL, Valerius T, Heijnen IA, Van de Winkel JG, Glennie MJ. Therapeutic efficacy of FcγRI/CD64-directed bispecific antibodies in B-cell lymphoma. Blood. 2000;96(10):3544–3552. [PubMed] [Google Scholar]
- 53. Bard F, Cannon C, Barbour R, et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6(8):916–919. [DOI] [PubMed] [Google Scholar]
- 54. Mavoungou C, Schindowski K, Atta-ur-Rahman E. Immunotherapy with anti-Abeta monoclonal antibodies in Alzheimer’s disease: a critical review on the molecules in the pipelines with regulatory considerations. Front Clin Drug Res Alzheimer Disord. 2013;1:3–85. [Google Scholar]
- 55. Wilcock DM, Gordon MN, Ugen KE, et al. Number of A β inoculations in APP+ PS1 transgenic mice influences antibody titers, microglial activation, and congophilic plaque levels. DNA Cell Biol. 2001;20(11):731–736. [DOI] [PubMed] [Google Scholar]
- 56. d’Azzo A, Bonten E. Molecular mechanisms of pathogenesis in a glycosphingolipid and a glycoprotein storage disease. Biochem Soc Trans. 2010;38(6):1453–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bonten EJ, Annunziata I, d’Azzo A. Lysosomal multienzyme complex: pros and cons of working together. Cell Mol Life Sci. 2014;71(11):2017–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lowden JA, O’brien JS. Sialidosis: a review of human neuraminidase deficiency. Am J Hum Genet. 1979;31(1):1–18. [PMC free article] [PubMed] [Google Scholar]
- 59. Uhl J, Penzel R, Sergi C, Kopitz J, Otto HF, Cantz M. Identification of a CTL4/Neu1 fusion transcript in a sialidosis patient. FEBS Lett. 2002;521(1-3):19–23. [DOI] [PubMed] [Google Scholar]
- 60. Sergi C, Beedgen B, Kopitz J, et al. Refractory congenital ascites as a manifestation of neonatal sialidosis: clinical, biochemical and morphological studies in a newborn Syrian male infant. Am J Perinatol. 1999;16(3):133–141. [DOI] [PubMed] [Google Scholar]
- 61. Pshezhetsky AV, Ashmarina M. Keeping it trim: roles of neuraminidases in CNS function. Glycoconj J. 2018;35(4):375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. d’Azzo A, Machado E, Annunziata I. Pathogenesis, emerging therapeutic targets and treatment in sialidosis. Exp Opin Orphan Drug. 2015;3(5):491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Annunziata I, Patterson A, Helton D, et al. Lysosomal NEU1 deficiency affects amyloid precursor protein levels and amyloid-β secretion via deregulated lysosomal exocytosis. Nat Commun. 2013;4:2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Seyrantepe V, Poupetova H, Froissart R, Zabot MT, Maire I, Pshezhetsky AV. Molecular pathology of NEU1 gene in sialidosis. Hum Mutat. 2003;22(5):343–352. [DOI] [PubMed] [Google Scholar]
- 65. Landolfi NF, Leone J, Womack JE, Cook RG. Activation of T lymphocytes results in an increase inH-2-encoded neuraminidase. Immunogenetics. 1985;22(2):159–167. [DOI] [PubMed] [Google Scholar]
- 66. Landolfi NF, Cook RG. Activated T-lymphocytes express class I molecules which are hyposialylated compared to other lymphocyte populations. Mol Immunol. 1986;23(3):297–309. [DOI] [PubMed] [Google Scholar]
- 67. Cross AS, Wright DG. Mobilization of sialidase from intracellular stores to the surface of human neutrophils and its role in stimulated adhesion responses of these cells. J Clin Invest. 1991;88(6):2067–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Feng L, Seyrantepe V, Landry K, et al. Monocyte differentiation upregulates the expression of the lysosomal sialidase, neu1 and triggers its targeting to the plasma membrane via MHC class II-positive compartments. J Biol Chem. 2006;281(37):27526–27538. [DOI] [PubMed] [Google Scholar]
- 69. Huang H, Bihaqi SW, Cui L, Zawia NH. In vitro Pb exposure disturbs the balance between Aβ production and elimination: the role of AβPP and neprilysin. Neurotoxicology. 2011;32(3):300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282(5394):1717–1721. [DOI] [PubMed] [Google Scholar]
- 71. Okun E, Mattson MP, Arumugam TV. Involvement of Fc receptors in disorders of the central nervous system. Neuromol Med. 2010;12(2):164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. García-García E, Rosales C. Signal transduction during Fc receptor-mediated phagocytosis. J Leukoc Biol. 2002;72(6):1092–1108. [PubMed] [Google Scholar]
- 73. Fiala M, Lin J, Ringman J, et al. Ineffective phagocytosis of amyloid-β by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2005;7(3):221–232. [DOI] [PubMed] [Google Scholar]
- 74. Deane R, Sagare A, Hamm K, et al. IgG-assisted age-dependent clearance of Alzheimer’s amyloid β peptide by the blood–brain barrier neonatal Fc receptor. J Neurosci. 2005;25(50):11495–11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Song X, Shapiro S, Goldman DL, Casadevall A, Scharff M, Lee SC. Fcγ receptor I-and III-mediated macrophage inflammatory protein 1α induction in primary human and murine microglia. Infect Immun. 2002;70(9):5177–5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Stalder AK, Ermini F, Bondolfi L, et al. Invasion of hematopoietic cells into the brain of amyloid precursor protein transgenic mice. J Neurosci. 2005;25(48):11125–11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142(6):1503–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Marciani DJ. Promising results from Alzheimer’s disease passive immunotherapy support the development of a preventive vaccine. Research (Wash D C). 2019;2019:5341375. [DOI] [PMC free article] [PubMed] [Google Scholar]