Abstract
Hypertension increases the risk of cognitive impairment independent of detectable stroke or cerebral lesions. However, the principal pathophysiological basis of this increase has not been fully elucidated. The present study investigates the relationships among blood pressure, hippocampal subfields volume, and cognitive function in a relatively young non-stroke population. A total of 59 non-stroke non-dementia subjects (mean age, 57.2 ± 4.9 years) were enrolled. All subjects were subjected to complete assessment of vascular risk factors including 24-hour blood pressure monitoring, various neuropsychological tests, and 3D-T1 MR scan. Freesurfer V6.0 was used for segmentation of hippocampal subfields. Our analyses revealed that both 24-hour and daytime mean systolic blood pressure (SBP) were significantly associated with the low volume of the left DG. Higher coefficient of variation (CV) of daytime SBP was significantly associated with lower volume of the left Cornu Ammonis 4 and dentate gyrus (DG) region. Both higher CV of 24-hour mean SBP and daytime SBP were significantly associated with lower performance in both executive and linguistic function. The low volume of the left DG was significantly associated with the low performance in linguistic function. Our findings support that reduced DG volume and increased SBP variability associated with hypertension-related cognitive impairment.
Keywords: hypertension, blood pressure viability, hippocampal subfields volume, cognitive performance
Introduction
Previous studies have established that mid-life hypertension increases the risk of cognitive impairment and dementia in the late-life. 1 -3 Hypertension can lead to vascular cognitive impairment through stroke events or cerebral small vessel disease, 4,5 and also increase the risk of Alzheimer disease (AD) through degenerative pathological changes such as Aβ deposition and tau protein phosphorylation. 6
Increasing evidence suggests that long-term hypertension can lead to global or regional brain volume loss, including hippocampus, 7 but the conclusions are inconsistent. Studies have shown that higher diastolic blood pressure (DBP) and DBP variability, rather than systolic blood pressure (SBP), are associated with hippocampal shrinkage, 8 but other studies have come to different conclusions. 9 A 25-year follow-up study of young and middle-aged people showed that visit-to-visit blood pressure variability was associated with hippocampal volume loss, while cumulative exposure to SBP or DBP was not associated with hippocampal volume changes. 10 In particular, lower mean arterial pressure is associated with reduced hippocampal volume in elderly population. 11 There are several reasons for the inconsistency of those conclusions. The hippocampus is one of the most sensitive brain regions, and the influence of hypertension on hippocampal volume shows a spectrum of effects with age. 12 Additional reasons include, hypertension duration anti-hypertensive drug prescription, 13 use of ambulatory blood pressure monitoring and presence of ApoE4 positive gene can influence results.
The hippocampal formation is a heterogeneous structure, consisting of several histologically distinguishable modules, such as the Cornu Ammonis (CA) regions, dentate gyrus (DG), subiculum, and presubiculum. Subsequently, the sensitivity of different hippocampal subfields to insult are different. For example, CA1 is particularly sensitive to hypoxia, ischemia, and hyperglycemia, 14 while the presence of APOE4 is linked to a smaller volume of the CA3-DG region. 15 Thus, use of the total hippocampal volume may obscure the relationship between hypertension and hippocampal shrinkage.
In the present study, we use a novel volumetric analysis (Freesurfer version 6.0) to calculate the volume of bilateral hippocampal subfields automatically, and then investigate the effects of hypertension and its components on hippocampal subfields volume and cognitive function in a relatively young population.
Methods
Participants
In total, 59 stroke-free participants (28 men and 31 women; mean age, 57.2 ± 4.9 years) from memory clinic or physical examination center from January 2016 to December 2017 were consecutively enrolled in this study. All participants underwent a complete vascular risk factor assessment, cognitive tests, and 3-dimensional T1-weighted with T2 fluid attenuated inversion recovery MR scan. Participants with contraindications to MR imaging and/or a history of stroke or other central nervous system diseases (such as dementia, brain tumor) were excluded from this study. Vascular risk factors were determined based on the participants’ medical history and clinical examinations, according to the Framingham cardiovascular risk factors profile. 16 This study was approved by the medical ethics committee of Zhongnan Hospital, Wuhan University. Written informed consent was obtained from all participants (clinical research registration number: chiCTRRNC-12002205).
Blood Pressure and Components
All subjects completed 24-hour ambulatory blood pressure monitoring (Omron-HEM, OMRON Corporation, Japan). Blood pressure (BP) were measured every half an hour, and the night BP refers to the BP from evening to morning, 22:00 to 06:00. The mean BP was calculated, including 24-hour mean systolic blood pressure (SBP), 24-hour mean diastolic blood pressure (DBP), daytime mean SBP, daytime mean DBP, night mean SBP, night mean DBP and pulse pressure. Blood pressure coefficient of variation (CV, %) refers to the ratio of standard deviation to mean value of BP. In our sample, 41 participants (69.5%) reported a diagnosis of hypertension and 33 participants were taking anti-hypertensive medication. All participants were divided into 3 groups according to their medical history and whether their blood pressure reached the standard of systolic BP 140 mmHg or diastolic BP 90mmHg 17 : non-hypertension group, well-controlled hypertension group and poor-controlled hypertension group.
Neuropsychological Assessments
The cognitive statuses of all participants were assessed by 2 trained neurologists. Five cognitive scales were used to assess a full range of cognitive function, which included global cognitive function (Montreal Cognitive Assessment 18 ), memory (Rey Auditory Verbal Learning Test 19 ), executive function (Stroop Tests 20 and Trail Making Tests 21 ), and verbal fluency (Verbal Fluency Test 22 ). The Hamilton Anxiety Scale and Hamilton Depression Scale were used to exclude those with severe anxiety or severe depression. 23
MR Scan and Image Processing
MR scan and image processing have been reported in our previous study. 24 We obtained T1-weighted images using a single 3-Tesla MR scanner (MAGNETOM Trio, Siemens). Magnetization-prepared rapid gradient-echo imaging was conducted to acquire high-resolution 3-dimensional T1-weighted images according to the following protocol: repetition time = 1900 ms, echo time = 1.92 ms, inversion time = 900 ms, flip angle = 9°, thickness = 1.0 mm, field of view = 256 mm × 256 mm, and voxel size: 1.0 x 1.0 x 1.0mm3. A total of 176 images were collected sagittally from the whole brain. T2 fluid attenuated inversion recovery (FLAIR) sequence was obtained using the following parameters: repetition time = 7000 ms, echo time = 94 ms, inversion time = 2210 ms, flip angle = 130°, thickness = 6.0 mm, spacing between slices = 7.8 mm. White matter hyperintensities (WMH) were graded by the Fazekas Scale, and the number of lacunes was counted.
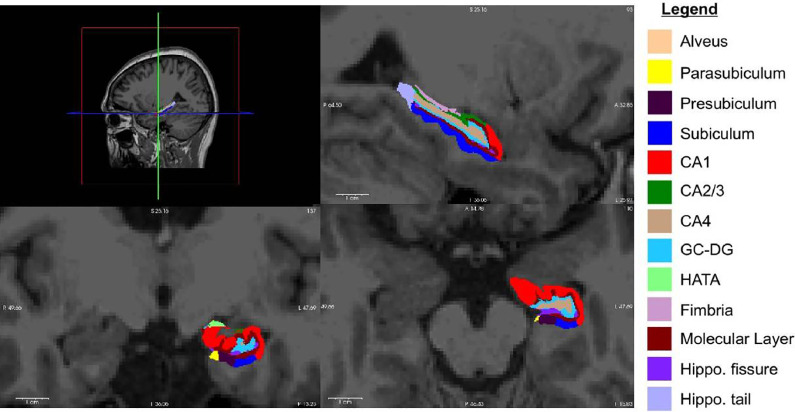
Volumetric analyses were performed on the 3-dimensional T1-weighted magnetization-prepared rapid gradient-echo images. Hippocampal subfield segmentation was performed using the FreeSurfer image analysis software version 6.0 (http://surfer.nmr.mgh.harvard.edu/), which is freely available for download online. Hippocampal subfields were divided as follows: CA1, CA2/3, CA4, fimbria, DG, hippocampal-amygdaloid transition region, tail, hippocampal fissure, molecular layer, parasubiculum, presubiculum, and subiculum (Figure 1). The total intracranial volume (ICV) was calculated on the T1-weighted images using SPM12. 25 Each hippocampal volume was adjusted for the ICV using the following covariance formula: adjusted hippocampal volume = raw hippocampal volume − b× (ICV − mean ICV) in which b is the slope of a regression of a region-of-interest volume of the ICV. 26 This approach yields a distribution that is more Gaussian than the distribution obtained using a ratio approach.
Figure 1.
Freesurfer V6.0 was used for hippocampal segmentation, including 13 subfields volumes.
Statistical Analysis
We used SPSS 19 (SPSS Science Inc., Chicago, IL, USA) to analyze the data. Normality was checked using the Shapiro–Wilk test. Group comparisons were conducted using analysis of variance (ANOVA) for continuous variables and chi-squared tests for categorical variables. Covariance analysis (ANCOVA) was used to analyze between-group differences for hippocampal subfields volume and cognitive performance in which age and sex were adjusted in model 1. Further adjustments followed for education, presence of ApoE4 positive status, Body Mass Index (BMI), history of diabetes and level of cholesterol in model 2. Pearson partial correlation analysis was used to correlate the BP components with cognitive performance and hippocampal subfields volume. The correlation analyses were adjusted for all covariates in model 2. In order to explore the mediating factors for the relationship between hypertension or its components and cognitive performance, we further conducted a mediating analysis. It was considered statistically significant when p-value was <0.05 in 2-side.
Results
Study Population
Table 1 presents the demographic and clinical data for all participants. No significant differences were identified among the groups for age, duration of education, physical activities and other main vascular risk factors. Sex distribution, BMI and presence of ApoE4 were significant differences among the groups. In particular, only 4 subjects had lacunae and only 3 subjects had WMHs Fezakes 2-3, and the presence of lacunes and WMHs were not significant differences among groups. The BP components were also compared among the groups, and the CV of night DBP were significant differences among groups.
Table 1.
Between-Group Differences in Demographic and Clinical Characteristics, as Evaluated by Analyses of Variance or Chi-Square Tests.
| Non-hypertension, n = 18 | Well-controlled hypertension, n = 20 | Poor-controlled hypertension, n = 21 | P value | |
|---|---|---|---|---|
| Age, y | 57.7 ± 4.8 | 58.1 ± 5.4 | 55.8 ± 4.5 | 0.275 |
| Sex (female, %) | 12 (66.7%) | 13 (65.0%) | 6 (28.6%) | 0.023 |
| Education, y | 13.5 ± 2.2 | 13.3 ± 1.9 | 14.9 ± 4.9 | 0.109 |
| History of diabetes, % | 0 | 2 (10.0%) | 2 (9.5%) | 0.389 |
| History of hyperlipemia, % | 13 (59.1%) | 12 (70.6%) | 15 (71.4%) | 0.546 |
| Total cholesterol, mmol/l | 5.2 ± 0.7 | 4.8 ± 1.0 | 4.6 ± 1.1 | 0.198 |
| BMI, kg/m2 | 23.4 ± 2.7 | 23.3 ± 2.1 | 25.2 ± 2.7 | 0.027 |
| Physical exercise, hour/week | 4.3 ± 3.4 | 4.7 ± 3.0 | 4.9 ± 3.9 | 0.843 |
| Presence of ApoE 4, % | 9 (50.0%) | 4 (20.0%) | 3 (14.3%) | 0.030 |
| Presence of lacunes, % | 0 (0) | 2 (10.0%) | 2 (9.5%) | 0.389 |
| Fezakes score 0-1 of WMH, % | 4 (22.2%) | 7 (35.5%) | 7 (33.3%) | 0.653 |
| Fezakes score 2-3 of WMH, % | 0 (0) | 1 (5.0%) | 2 (9.5%) | 0.402 |
| Hypertension duration, y | 0 | 12.8 ± 11.9 | 8.3 ± 8.7 | <0.001 |
| Use of anti-hypertension drugs | 0 | 19 (95.0%) | 14 (66.7%) | <0.001 |
| All day MSBP, mmHg | 124.2 ± 7.5 | 122.9 ± 8.4 | 146.8 ± 8.4 | <0.001 |
| All day SBP-CV, % | 11.3 ± 2.9 | 10.8 ± 3.1 | 11.7 ± 3.5 | 0.648 |
| Daytime MSBP, mmHg | 128.1 ± 7.8 | 124.7 ± 9.5 | 150.0 ± 10.0 | <0.001 |
| Daytime SBP-CV, % | 9.6 ± 1.6 | 10.2 ± 3.1 | 11.1 ± 3.9 | 0.322 |
| Night MSBP, mmHg | 115.9 ± 9.8 | 118.9 ± 9.9 | 139.6 ± 13.7 | <0.001 |
| Night SBP-CV, % | 11.6 ± 5.1 | 9.2 ± 3.8 | 9.9 ± 3.3 | 0.198 |
| All day MDBP, mmHg | 73.1 ± 7.3 | 76.2 ± 7.1 | 90.6 ± 5.7 | <0.001 |
| All day DBP-CV, % | 14.7 ± 2.8 | 12.0 ± 4.0 | 14.0 ± 3.9 | 0.071 |
| Daytime MDBP, mmHg | 75.8 ± 7.5 | 77.7 ± 7.3 | 92.6 ± 6.4 | <0.001 |
| Daytime DBP-CV, % | 13.1 ± 1.7 | 11.6 ± 4.4 | 13.8 ± 3.9 | 0.153 |
| Night MDBP, mmHg | 67.6 ± 8.1 | 72.5 ± 7.5 | 84.5 ± 6.1 | <0.001 |
| Night DBP-CV, % | 14.7 ± 5.5 | 10.2 ± 3.5 | 11.7 ± 5.1 | 0.016 |
Data are shown as mean ± SD. N (%): percentages are based on the individual categories. BMI: Body Mass Index, WMH: white matter hyperintensities, MSBP: Mean systolic blood pressure, SBP: Systolic blood pressure, CV: Coefficient of variation, MDBP: Mean diastolic blood pressure, DBP: Diastolic blood pressure.
BP Characteristics and Hippocampal Subfields Volume
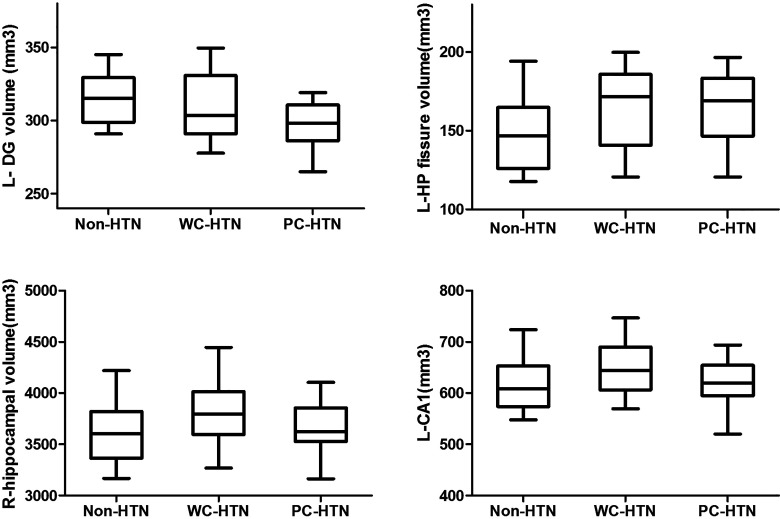
As shown in Figure 2 and Table 2, the volume of left DG, left hippocampal fissure, and right hippocampus were significantly different among the 3 groups in both model 1 and model 2 analysis. Compared with both hypertension groups, the left DG was significantly smaller and the left hippocampal fissure was significantly larger in non-hypertension group. Compared with non-hypertension or poor-controlled groups, the left CA1 volume was significantly larger in the well-controlled hypertension group in model 2 analysis.
Figure 2.
Between-group differences in hippocampal subfield volume. the volume of left DG, left hippocampal fissure, and right hippocampus were significantly different among the 3 groups. Compared with non-hypertension or poor-controlled groups, the left CA1 volume was significantly larger in the well-controlled hypertension group. DG, dentate gyrus; CA, cornu ammonis.
Table 2.
Between-Group Differences in Hippocampal Subfield Volume(mm3), as Evaluated by Analysis of Covariance.
| Non-hypertension, n = 18 | Well-controlled hypertension, n = 20 | Poor-controlled hypertension, n = 21 | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|---|
| F-value | P-value | F-value | P-value | ||||
| Left hippocampus | |||||||
| Total HPV | 3464.8 ± 251.8 | 3588.0 ± 277.5 | 3504.3 ± 233.1 | 1.461 | 0.241 | 2.065 | 0.138 |
| CA1 | 615.9 ± 50.9 | 649.1 ± 52.0 | 621.5 ± 43.1 | 2.907 | 0.063 | 4.027 | 0.024 |
| CA2+CA3 | 199.4 ± 25.3 | 208.5 ± 26.4 | 206.6 ± 19.9 | 0.739 | 0.482 | 0.997 | 0.376 |
| CA4 | 259.4 ± 24.7 | 260.2 ± 21.2 | 256.9 ± 16.8 | 0.189 | 0.828 | 0.243 | 0.785 |
| DG | 316.4 ± 25.2 | 306.2 ± 24.1 | 299.5 ± 21.4 | 3.201 | 0.048 | 3.573 | 0.035 |
| Hippocampal tail | 542.0 ± 84.1 | 563.0 ± 72.8 | 545.6 ± 65.3 | 0.514 | 0.601 | 0.245 | 0.784 |
| HATA | 56.6 ± 6.6 | 60.4 ± 6.1 | 57.8 ± 7.7 | 1.697 | 0.163 | 2.125 | 0.13 |
| Fimbria | 88.0 ± 15.7 | 88.5 ± 13.6 | 92.4 ± 17.3 | 0.282 | 0.756 | 0.256 | 0.775 |
| Hippocampal fissure | 147.5 ± 23.2 | 165.5 ± 24.8 | 168.3 ± 25.9 | 4.321 | 0.018 | 4.192 | 0.021 |
| Molecular layer | 572.2 ± 44.9 | 591.6 ± 48.5 | 572.2 ± 41.6 | 1.61 | 0.209 | 2.643 | 0.081 |
| Subiculum | 449.5 ± 36.7 | 466.8 ± 45.2 | 455.4 ± 50.3 | 0.918 | 0.406 | 1.389 | 0.259 |
| Parasubiculum | 61.0 ± 5.9 | 64.9 ± 11.5 | 65.2 ± 12.3 | 1.23 | 0.3 | 1.063 | 0.353 |
| Presubiculum | 316.4 ± 23.0 | 328.6 ± 32.6 | 330.1 ± 38.4 | 1.008 | 0.372 | 2.252 | 0.116 |
| Right hippocampus | |||||||
| Total HPV | 3619.0 ± 284.2 | 3816.9 ± 348.1 | 3673.1 ± 228.2 | 3.299 | 0.044 | 3.205 | 0.049 |
| CA1 | 649.2 ± 74.3 | 694.2 ± 83.9 | 667.7 ± 38.7 | 2.609 | 0.083 | 2.418 | 0.099 |
| CA2+CA3 | 219.7 ± 29.1 | 233.7 ± 30.5 | 235.9 ± 26.9 | 1.524 | 0.227 | 1.594 | 0.213 |
| CA4 | 270.0 ± 22.6 | 280.6 ± 26.6 | 280.4 ± 19.9 | 1.205 | 0.308 | 1.5 | 0.233 |
| DG | 315.4 ± 24.7 | 327.6 ± 29.5 | 326.1 ± 21.8 | 1.426 | 0.249 | 1.885 | 0.162 |
| Hippocampal tail | 586.3 ± 55.6 | 625.7 ± 70.8 | 578.1 ± 75.9 | 3.169 | 0.05 | 2.072 | 0.137 |
| HATA | 61.4 ± 7.0 | 65.7 ± 9.0 | 60.5 ± 7.5 | 2.355 | 0.105 | 2.804 | 0.07 |
| Fimbria | 84.3 ± 12.9 | 83.3 ± 13.3 | 81.9 ± 21.3 | 0.077 | 0.926 | 0.255 | 0.799 |
| Hippocampal fissure | 163.2 ± 23.2 | 177.1 ± 26.5 | 176.9 ± 27.9 | 1.638 | 0.204 | 2.332 | 0.108 |
| Molecular layer | 596.3 ± 54.1 | 627.5 ± 65.6 | 605.4 ± 39.8 | 2.509 | 0.091 | 2.554 | 0.088 |
| Subiculum | 463.9 ± 43.7 | 488.3 ± 45.7 | 464.6 ± 45.3 | 2.619 | 0.082 | 2.344 | 0.106 |
| Parasubiculum | 57.8 ± 9.5 | 63.3 ± 9.7 | 58.1 ± 12.4 | 1.363 | 0.265 | 1.273 | 0.289 |
| Presubiculum | 314.7 ± 22.8 | 327.1 ± 33.2 | 314.2 ± 44.4 | 1.099 | 0.34 | 1.363 | 0.265 |
Data are mean ± standard deviation. HPV: Hippocampal volume, CA: Cornu Ammonis, DG: Dentate gyrus, HATA: hippocampal-amygdaloid transition region.
Across all subjects, after adjusted for covariates fully in model 2, an increase of 24-hour mean SBP was significantly associated with the lower volume of the left DG (r = -0.297, p = 0.030), increased daytime mean SBP was significantly associated with lower volume of the left DG (r = -0.293, p = 0.036) and the left molecular layer (r = -0.268, p = 0.046). Increased CV of daytime SBP was significantly associated with lower volume of the left CA4 (r = -0.294, p = 0.036) and DG (r = -0.296, p = 0.035) regions (seen in supplementary materials 1 for detail data).
BP Characteristics and Cognitive Performance
As shown in Table 3, no significant between-group differences were identified for any of the cognitive tests in ANOVA, model 1 analysis. However, there were suggestive between-group differences for executive function (assessed by Trail Making Test B) (p = 0.067) and linguistic function (assessed by Verbal Fluency Test) (p = 0.063) in model 2 analysis.
Table 3.
Between-Group Differences in Cognitive Performance Score, as Evaluated by Analysis of Covariance.
| Non-hypertension, n = 18 | Well-controlled hypertension, n = 20 | Poor-controlled hypertension, n = 21 | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|---|
| F-value | P-value | F-value | P-value | ||||
| Moca | 25.8 ± 2.3 | 24.4 ± 2.6 | 25.0 ± 2.8 | 1.486 | 0.235 | 0.835 | 0.44 |
| Verbal Fluency Test | 46.4 ± 5.9 | 45.5 ± 9.7 | 43.6 ± 5.7 | 1.153 | 0.323 | 2.857 | 0.063 |
| RAVLT-immediated memory | 41.1 ± 9.4 | 36.9 ± 8.8 | 37.2 ± 7.1 | 1.278 | 0.287 | 0.891 | 0.417 |
| RAVLT-delayed memory | 8.7 ± 3.8 | 7.5 ± 2.9 | 7.2 ± 3.0 | 0.678 | 0.512 | 0.187 | 0.83 |
| Stroop A | 19.9 ± 6.5 | 22.9 ± 14.2 | 19.9 ± 8.0 | 0.457 | 0.635 | 0.665 | 0.519 |
| Stroop B | 17.9 ± 4.1 | 21.5 ± 7.9 | 22.4 ± 7.3 | 1.821 | 0.172 | 1.166 | 0.32 |
| Stroop C | 29.6 ± 9.2 | 31.7 ± 8.4 | 28.6 ± 8.8 | 0.822 | 0.445 | 0.31 | 0.735 |
| Trail Making Test A | 42.3 ± 13.8 | 38.5 ± 15.5 | 36.8 ± 8.1 | 0.755 | 0.475 | 1.429 | 0.249 |
| Trail Making Test B | 65.4 ± 23.8 | 71.8 ± 33.1 | 77.7 ± 28.1 | 1.366 | 0.264 | 2.309 | 0.067 |
Data are mean ± standard deviation. RAVLT: Rey Auditory Verbal Learning Test.
Among all subjects, after adjusted for fully covariates in model 2, higher CV of 24-hour SBP was significantly associated with lower performance in both Trail Making Test B (r = -0.267, p = 0.047) and Verbal Fluency Test (r = -0.272, p = 0.042), and higher CV of daytime SBP was significantly associated with lower performance in Trail Making Test B (r = -0.306, p = 0.022), Stroop C Test (r = -0.326, p = 0.014) and Verbal Fluency Test(r = -0.277, p = 0.039) (seen in supplementary materials 2 for detail data).
We further made partial correlation analyses between hippocampal subfields volume and cognitive performance. After adjusted for fully covariates in model 2, the reduction of the left DG volume was significantly associated with the low performance in Verbal Fluency Test (r = 0.279, p = 0.045). Mediating analysis indicated that the association between higher CV of 24-hour SBP and Verbal Fluency Test score was partially mediated by the left DG volume (β value decreased by 0.035).
Discussion
The main findings of this study are as follows: (1) the left DG volume was statistically significant showing a reduction and the left hippocampal fissure volume was statistically significant demonstrating an increase between hypertension subjects than those non-hypertension subjects. (2) Higher CV of daytime SBP was significantly associated with lower volume of the DG region. (3) Higher CV of 24-hour SBP and daytime SBP was both significantly associated with lower executive function and verbal fluency. (4) Low volume of left DG was significantly associated with low performance in linguistic function. These results suggest that executive and linguistic dysfunction, rather than memory dysfunction, may be the main involved cognitive domain of hypertension-related cognitive impairment, in which the variation of SBP and the left DG shrinkage may play an important role.
BP Characteristics and Cognitive Performance
A causal link between hypertension and cognitive impairment has been confirmed by a large number of studies, 2,27 however, the conclusion of the BP components that lead to cognitive impairment has inconsistencies, especially in relatively young elderly population. Our results showed that there was a suggestive between-group difference in executive and linguistic functions, while increased CV of 24-hour and daytime SBP was significantly correlated with executive and linguistic dysfunctions. A recent study has shown that elderly patients with well ambulatory BP control demonstrate higher BP variability but not average ambulatory BP level, were associated with cognitive impairment. 28 Collectively, our data indicated that the CV of SBP, rather than hypertension itself, is an important risk for cognitive impairment.
Previous studies have confirmed that elevated BP variability in patients with hypertension is an important risk factor for tissue and organ dysfunction, including cerebral white matter lesions 29 and hippocampal volume loss. 30 Stroke events and cerebral small vessel diseases are 2 important pathological bases for hypertension-related cognitive impairment. In our study population, stroke events are excluded, and the degree of cerebral small vessel disease is very mild (assessed by presence of lacuna and WMHs), which indicated that there may exist non-vascular pathological damage causing hypertension-related cognitive impairment.
BP Characteristics and the Hippocampal Subfields Volume
After all co-variates were controlled, the left DG and left hippocampal fissure volume in hypertension group were significantly larger than that in non-hypertension group, and the volume loss of the left DG region was associated with both daytime SBP and the CV of daytime SBP. Our results suggested that hypertension and BP variability may be associated with impaired structure of hippocampal subfields, and the volume loss of the left DG region may be an early imaging bio-marker.
There are several potential mechanisms that may underlie the observed hippocampal subfields volume and hypertension association. Firstly, hippocampal fissure is closely related to hippocampal neurogenesis. Previous studies have confirmed that subgranular areas of the subventricular area and DG region are the 2 main sites of neurogenesis in the brain, 31 and the hippocampal fissure may be the main source of neurogenic progenitor cells for CA1 region. 32 One of the prominent manifestations of hippocampal damage caused by chronic hypertension is the inhibition of hippocampal neurogenesis, 33 including less expression of brain-derived neurotrophic factor, an essential factor for neurogenesis. 34 Secondly, experimental and clinical studies have shown that hippocampal fissure enlargement is a very sensitive indicator for hippocampal shrinkage. 35 In AD patients, hippocampal fissure enlargement is significantly correlated with medial temporal lobe atrophy and with the degree of cognitive impairment. 36 In addition, because of their proximity, the expansion of the hippocampal fissure may also reflect the decrease in DG volume. Our findings strengthen the evidence that hypertension relate to cognitive impairment, and the volume loss of the left DG region may be an early imaging bio-marker in hypertension-related cognitive impairment.
One limitation of our study was the small number of participants, which may impede its generalisability. Nevertheless, this study clearly supports the view that for individuals with hypertension, the left DG and hippocampal fissure may be affected earlier. Additional studies, especially longitudinal studies, are needed to demonstrate the exact role of hippocampal fissure and its adjacent subfields in hypertension-related cognitive impairment.
Supplemental Material
supplementary_materials_1 for Low Hippocampal Dentate Gyrus Volume Associated With Hypertension-Related Cognitive Impairment by Huagang Li, Dong Sun, Dongwei Lu, Junjian Zhang and Junjie Zeng in American Journal of Alzheimer's Disease & Other Dementias
supplementary_materials_2 for Low Hippocampal Dentate Gyrus Volume Associated With Hypertension-Related Cognitive Impairment by Huagang Li, Dong Sun, Dongwei Lu, Junjian Zhang and Junjie Zeng in American Journal of Alzheimer's Disease & Other Dementias
Acknowledgments
I confirm that I have listed all coauthors contributed significantly to the work. We are very grateful to the patients who participated in this study, to the participant general practitioners, and their staff.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grant number: 81500935). This had no influence on study design and data collection, study conduct, analysis and interpretation of data, and in writing the manuscript.
ORCID iD: Dong Sun  https://orcid.org/0000-0003-4171-6681
https://orcid.org/0000-0003-4171-6681
Significance Statement: We investigate the role of hippocampal subfields volume in hypertension-related cognitive impairment.
Executive and linguistic dysfunction may be the main involved cognitive domain of hypertension-related cognitive impairment. Reduced left dentate gyrus volume and increased systolic blood pressure variability associated with hypertension-related cognitive impairment.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Unverzagt FW, McClure LA, Wadley VG, et al. Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology. 2011;77(19):1729–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gottesman RF, Schneider AL, Albert M, et al. Midlife hypertension and 20-year cognitive change: The Atherosclerosis Risk in Communities Neurocognitive Study. JAMA Neurol. 2014;71(10):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haring B, Omidpanah A, Suchy-Dicey AM, et al. Left ventricular mass, brain magnetic resonance imaging, and cognitive performance: results from the Strong Heart Study. Hypertension. 2017;70(5):964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williams OA, An Y, Beason-Held L, et al. Vascular burden and APOE epsilon4 are associated with white matter microstructural decline in cognitively normal older adults. Neuroimage. 2018;188:572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension. 2013;62(5):810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith EE, Muzikansky A, McCreary CR, et al. Impaired memory is more closely associated with brain beta-amyloid than leukoaraiosis in hypertensive patients with cognitive symptoms. Plos One. 2018;13(1):e0191345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beauchet O, Celle S, Roche F, et al. Blood pressure levels and brain volume reduction: a systematic review and meta-analysis. J Hypertens. 2013;31(8):1502–1516. [DOI] [PubMed] [Google Scholar]
- 8. McNeil CJ, Myint PK, Sandu AL, et al. Increased diastolic blood pressure is associated with MRI biomarkers of dementia-related brain pathology in normative ageing. Age Ageing. 2018;47(1):95–100. [DOI] [PubMed] [Google Scholar]
- 9. Ngwa JS, Fungwe TV, Ntekim O, et al. Associations of pulse and blood pressure with hippocampal volume by APOE and cognitive phenotype: the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Dement Geriatr Cogn Disord. 2018;45(1-2):66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yano Y, Reis JP, Levine DA, et al. Visit-to-visit blood pressure variability in young adulthood and hippocampal volume and integrity at middle age: the CARDIA study (Coronary Artery Risk Development in Young Adults). Hypertension. 2017;70(6):1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Foster-Dingley JC, van der Grond J, Moonen JE, et al. Lower blood pressure is associated with smaller subcortical brain volumes in older persons. Am J Hypertens. 2015;28(9):1127–1233. [DOI] [PubMed] [Google Scholar]
- 12. Ferreira D, Hansson O, Barroso J, et al. The interactive effect of demographic and clinical factors on hippocampal volume: a multicohort study on 1958 cognitively normal individuals. Hippocampus. 2017;27(6):653–667. [DOI] [PubMed] [Google Scholar]
- 13. Edwards JD, Ramirez J, Callahan BL, et al. Antihypertensive treatment is associated with MRI-derived markers of neurodegeneration and impaired cognition: a propensity-weighted cohort study. J Alzheimers Dis. 2017;59(3):1113–1122. [DOI] [PubMed] [Google Scholar]
- 14. Bartsch T, Dohring J, Rohr A, Jansen O, Deuschl G. CA1 neurons in the human hippocampus are critical for autobiographical memory, mental time travel, and autonoetic consciousness. Proc Natl Acad Sci U S A. 2011;108(42):17562–17567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mueller SG, Weiner MW. Selective effect of age, Apo e4, and Alzheimer’s disease on hippocampal subfields. Hippocampus. 2009;19(6):558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–318. [DOI] [PubMed] [Google Scholar]
- 17. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13–e115. [DOI] [PubMed] [Google Scholar]
- 18. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 19. Savage RM, Gouvier WD. Rey auditory-verbal learning test: the effects of age and gender, and norms for delayed recall and story recognition trials. Arch Clin Neuropsychol. 1992;7(5):407–414. [PubMed] [Google Scholar]
- 20. Treisman A, Fearnley S. The Stroop test: selective attention to colours and words. Nature. 1969;222(5192):437–439. [DOI] [PubMed] [Google Scholar]
- 21. Lu L, Bigler ED. Normative data on Trail Making Test for neurologically normal, Chinese-speaking adults. Appl Neuropsychol. 2002;9(4):219–225. [DOI] [PubMed] [Google Scholar]
- 22. Hecaen H, Ruel J. Verbal fluency test and sites of frontal lesions (author’s transl) [in French]. Rev Neurol (Paris). 1981;137(4):277–284. [PubMed] [Google Scholar]
- 23. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dong S, Dongwei L, Zhang J, et al. Individuals in the prediabetes stage exhibit reduced hippocampal tail volume and executive dysfunction. Brain Behav. 2019;9(8):e01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malone IB, Leung KK, Clegg S, et al. Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. Neuroimage. 2015;104:366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23(2):724–738. [DOI] [PubMed] [Google Scholar]
- 27. Nishtala A, Himali JJ, Beiser A, et al. Midlife hypertension risk and cognition in the non-demented oldest old: Framingham Heart Study. J Alzheimers Dis. 2015;47(1):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cho N, Hoshide S, Nishizawa M, Fujiwara T, Kario K. Relationship between blood pressure variability and cognitive function in elderly patients with good blood pressure control. Am J Hypertens. 2018;31(3):293–298. [DOI] [PubMed] [Google Scholar]
- 29. Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: the Honolulu-Asia Aging study. Stroke. 2002;33(1):26–30. [DOI] [PubMed] [Google Scholar]
- 30. Sabayan B, Wijsman LW, Foster-Dingley JC, et al. Association of visit-to-visit variability in blood pressure with cognitive function in old age: prospective cohort study. BMJ. 2013;347:f4600. [DOI] [PubMed] [Google Scholar]
- 31. Anacker C, Hen R. Adult hippocampal neurogenesis and cognitive flexibility—linking memory and mood. Nat Rev Neurosci. 2017;18(6):335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang L, Hernandez VS, Estrada FS, Lujan R. Hippocampal CA field neurogenesis after pilocarpine insult: the hippocampal fissure as a neurogenic niche. J Chem Neuroanat. 2014;56:45–57. [DOI] [PubMed] [Google Scholar]
- 33. Shih YH, Tsai SF, Huang SH, et al. Hypertension impairs hippocampus-related adult neurogenesis, CA1 neuron dendritic arborization and long-term memory. Neuroscience. 2016;322:346–357. [DOI] [PubMed] [Google Scholar]
- 34. Monnier A, Garnier P, Quirie A, et al. Effect of short-term exercise training on brain-derived neurotrophic factor signaling in spontaneously hypertensive rats. J Hypertens. 2017;35(2):279–290. [DOI] [PubMed] [Google Scholar]
- 35. Li Y, Yan J, Zhu X, et al. Increased hippocampal fissure width is a sensitive indicator of rat hippocampal atrophy. Brain Res Bull. 2018;137:91–97. [DOI] [PubMed] [Google Scholar]
- 36. Bastos-Leite AJ, van Waesberghe JH, Oen AL, et al. Hippocampal sulcus width and cavities: comparison between patients with Alzheimer disease and nondemented elderly subjects. AJNR Am J Neuroradiol. 2006;27(10):2141–2145. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary_materials_1 for Low Hippocampal Dentate Gyrus Volume Associated With Hypertension-Related Cognitive Impairment by Huagang Li, Dong Sun, Dongwei Lu, Junjian Zhang and Junjie Zeng in American Journal of Alzheimer's Disease & Other Dementias
supplementary_materials_2 for Low Hippocampal Dentate Gyrus Volume Associated With Hypertension-Related Cognitive Impairment by Huagang Li, Dong Sun, Dongwei Lu, Junjian Zhang and Junjie Zeng in American Journal of Alzheimer's Disease & Other Dementias