Abstract
Objective:
The present report aims to evaluate whether singing intervention can bring an immediate benefit that is greater than the one provided by painting intervention on pain and well-being.
Methods:
Fifty-nine mild patients with Alzheimer disease were randomized to a 12-week singing (n = 31) or painting group (n = 28). In the present analysis, the immediate evolution of pain and well-being was compared across sessions between the 2 groups using mixed-effects models.
Results:
We observed a significant improvement in well-being for both singing and painting groups immediately after sessions, compared to the assessment before the sessions. We did not observe this improvement across the sessions for pain intensity measurement.
Discussion:
Our results revealed that both painting and singing interventions provide an immediate benefit on the patients’ well-being.
Keywords: Alzheimer disease, music, pain, well-being, quality of life
Introduction
Alzheimer disease (AD) is a common disorder that principally concerns patients of 65 years and older, 1 with two-thirds of them being aged 75 years and older. 2 Patients with Alzheimer disease and related disorders (ADRDs) are more at risk of having physical pain because of the links between pain and cognition 3 but also because of their age and their frailty. In fact, these patients with AD often present multiple comorbidities that can induce chronic pain. 4 Pain in elderly is more persistent compared to younger patients. In a population of patients with ADRD at a moderate stage, a functional magnetic resonance imaging study showed a higher response to pain during a mechanical stimulation compared to control participants. 5 Otherwise, there is ample evidence that pain affects well-being. Pain interferes with activities of daily living, causing emotional distress and impacting psychological health. 6 So, this can accelerate the loss of autonomy associated with neurodegenerative disease per se. However, analgesic medications are not free of side effects, and they can increase the risk of confusion, falls, and cognitive decline. In addition, they can induce a physical and psychological dependence as well as difficulties to withdrawal. 7
The French Health Authority recommends combining nondrug therapies with standard treatments. Among them, musical intervention (MI) has already proven usefulness and effectiveness in certain domains such as affective symptoms 8 and cognition. 9 -11 In elderly patients, beneficial effects of MI have also been reported for chronic pain. 12,13 However, to our knowledge, the potential effects of MI on chronic pain have never been studied in elderly patients with AD.
The LACMé study aimed to determine the efficiency of singing intervention (SI) versus another artistic activity, namely painting intervention (PI) on chronic pain, mood, and cognition in patients with AD with moderate to severe chronic pain. Quality of life (QoL) was also measured as it is a core marker for the benefit of therapeutic strategies. 14,15
In a previous work, 16 we have reported the long-term results of the LACMé study, notably showing that after 12 weeks of either SI or PI, patients with mild AD had reduced chronic pain, improved mood, and QoL. Differential effects were shown with better memory performance with the SI and an improvement of depression with the PI, but there was no difference between the SI and PI for pain and QoL. However, these results did not provide information about the immediate benefit of these activities, leading us to report here the more detailed findings after each session.
The potential analgesic effect of music has been previously reported: The pleasurable character of the music-induced emotions can reduce the painful sensation due to an experimental stimulus. 17,18 Pleasant versus unpleasant music can modulate the activity of limbic areas and areas related to the reward system, 19,20 which are involved in painful perception as well as well-being. In addition, singing involves breathing and has an effect on endorphin production. 21 Finally, music can produce effects on heart rate and breathing rate depending on the tempo and then induces relaxation, mostly during pauses or slower rythms. 22 Singing intervention could therefore have an immediate relaxing transitory power that cannot be observed after a long time period from activities.
The present report was aimed to evaluate with an explanatory design whether SI can bring an immediate benefit that is greater than the one provided by PI on pain and well-being.
Materials and Methods
Study Design
The present analysis was based on data from the LACMé study, a multicenter randomized clinical trial with 2 parallel arms and a blinded end point evaluation. The LACMé design and global results about pre- and postintervention changes were disclosed in 2 previous papers. 16,23 This trial was registered at clinicaltrials.gov. (Identifier: NCT02670993).
Population
Participants of the LACMé study were recruited from 3 memory clinics in France. The inclusion criteria were as follows: 60 years and older and probable AD at mild stage, according to the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) 24 and the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association criteria. 25 The severity of cognitive disorders was assessed with the Mini-Mental State Examination (MMSE), 26 and only participants with an MMSE superior or equal to 20 were included. The included patients also presented chronic pain, assessed with Simple Verbal Scale 27 at moderate or severe stage (score superior or equal to 2/4 since more than 1 month) and were able to complete the clinical and neuropsychological evaluations. Psychotropic drugs, acetylcholinesterase inhibitors, or memantine had to be stabilized for more than 3 months, and pain medication for at least 1 month.
Ethics Approval
The study protocol was reviewed and approved by Saint Etienne University Ethics Committee (Comité de Protection des Personnes). This committee encompasses the ethical approval of all 3 sites of our data collection. All procedures were in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guidelines. All participants signed an informed consent.
Measures
We considered the type of group with the intervention modalities being painting and singing as well as the time of the evaluation, that is, before or after each session.
Outcomes
The differences in immediate changes before and after in well-being as measured by EVIBE (in French: Evaluation Instantanée du Bien-Etre) and pain as measured by Numeric Rating Scale (NRS) between SI group sessions and PI group sessions.
The immediate change in well-being and pain before and after SI and PI sessions in SI and PI groups.
Outcome Measures
The EVIBE scale and the NRS have been chosen because these scales were sufficiently sensitive and adapted to patients with cognitive impairment.
The NRS 28 was used as a measure of pain intensity at a given moment. It requires a lower degree of abstraction than the visual analog scale and can therefore be used in patients with early AD. 29 Participants had to select the number that indicates the intensity of the pain felt at the moment. The score varies from 0 (no pain) to 10 (the most severe pain one can imagine).
For the assessment of the immediate perceived well-being in patients with cognitive impairment, we used the EVIBE scale (in French: Evaluation Instantanée du Bien-Etre), 30 a Visual Analog Scale. On a graduated ruler, the patients had to answer the question “How do you feel now?.” The response varies from 0 (feeling bad) to 5 (feeling very good). To help the patient understand the instructions, pictograms representing simple facial expressions (joy, neutrality, and sadness) were proposed. The EVIBE scale can be used at severe stages of dementia and has already been used for measuring changes following a nondrug intervention. Furthermore, well-being measured by this scale was shown to be highly correlated with QoL.
Covariates
Sociodemographic and clinical assessment variables, including age, gender, and educational level, exploring global cognitive function, were collected at baseline and were used in the present analysis as covariates.
Randomization
All participants provided written inform consent. To ensure equal distribution between treatment groups, the randomization was made by a team member who had no contact with the patients. In each center (Lyon, Saint Etienne, see above), consented participants were randomly assigned to an SI or a PI group with a 1:1 ratio.
Blinding
All outcome measures were assessed by an independent psychologist in each center, who was different from the practitioner conducting SI or PI. The database and the statistical analysis were made by research team members. Both psychologists and the research team members were blind to the intervention type.
Intervention Conditions
The patients participated in 12 weekly 2-hour sessions during 3 months. They came to the hospital only for LACMé research. They had no other activities or contacts with other staff members.
Singing intervention groups
The SI was delivered by a professional choir conductor accompanied by a psychologist, whose role was to help the conductor to manage the patients if necessary (in particular welcome, departure, and possible difficulties with patients during the sessions). Before the intervention, the patients assigned to SI groups were asked to fulfill a questionnaire collecting their musical preferences among several songs. The choir conductor selected the songs according to the patients’ preferences. After a personalized welcome, the patients performed a body and voice warm-up before song learning. Four different songs previously chosen by the patients among a list of well-known songs were practiced across the different sessions. The songs were then worked by the patients with piano accompaniment made by the choir conductor.
Painting intervention groups
The PI was done by a painting teacher accompanied by a psychologist. After a personalized welcome in the first part of each session, paintings of professional painters were shown to the patients, generating discussions across the group. The second part of the session was devoted to the patients’ painting realization according to a predetermined theme.
Data Collection
The pain and well-being levels were collected by an independent psychologist before and after each session. The duration for these evaluations was approximately 5 minutes.
Data Analysis
The mean value of EVIBE and NRS before and then after the sessions was computed for each participant. The evolution of EVIBE and NRS scores across the sessions was then compared between SI and PI groups using mixed-effects models, including time as a fixed effect and participants as a random effect. The evolution of NRS and EVIBE was then compared across each session, between the 2 groups, with mixed-effects models. All statistical tests were 2 tailed. A P values inferior to .05 was considered as significant. Statistical analyses were performed with SPSS version 17 (SPSS Software, Chicago, Illinois).
Results
Population’s Characteristics
A total of 59 patients completed the baseline evaluation. Among them, 31 received SI and 28 PI. The demographic and clinical characteristics of the samples at baseline are summarized in Table 1. There was no imbalance in variables between groups with regard to demographic and severity of cognitive disorder.
Table 1.
Patients’ Baseline Characteristics.a
| Patients' Characteristics | Singing Intervention (N = 31) n (%) | Painting Intervention (N = 28) n (%) |
|---|---|---|
| Gender | ||
| Women | 23 (74.2) | 16 (57.1) |
| Education | ||
| <5 years | 2 (6.5) | 5 (17.9) |
| 5-6 years | 8 (25.8) | 8 (28.6) |
| 7-8 years | 10 (32.3) | 6 (21.4) |
| 9-11years | 5 (16.1) | 2 (7.1) |
| >11 years | 6 (19.4) | 7 (25.0) |
| Mean (SE) | Mean (SE) | |
| Age | 78.8 (7.43) | 80.2 (5.71) |
| MMSE | 25.07 (2.26) | 24.18 (2.72) |
| SVS | 2.06 (0.24) | 2.17 (0.38) |
Abbreviations: MMSE, Mini-Mental State Examination; SVS, Simple Verbal Scale.
aSVS varying from 0 to 4, with 0 indicating no pain, and 4 indicating very intense pain.
Pain and Well-Being Assessment
Table 2 shows the results of pain and well-being measures including means and standard deviations for NRS and EVIBE scales before and after sessions, and the results of the mixed models adjusted on age, sex, and educational level. The NRS scores decreased over time; however, the results did not reach the level of significance. No difference between groups or interaction time by group was observed in these analyses. The mean EVIBE score increased over time in both SI and PI. A trend toward significance was observed for the group factor. However, there was no interaction between time and group in this analysis.
Table 2.
Comparison of Singing Intervention and Painting Intervention on NRS and EVIBE Scale Measures for the Mean of 12 Sessions—Linear Mixed-Effect Models Adjusted on Age, Sex, and Education.a
| Scale | Singing Group, Mean (SD) | Painting Group, Mean (SD) | Time (T) | Group (G) | TxG | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | F | P | F | P | F | P | |
| NRS | 2.68 (2.3) | 2.39 (1.9) | 3.54 (2.3) | 2.88 (2.0) | 1.37 | .25 | 2.27 | .14 | 0.20 | .65 |
| EVIBE | 3.71 (0.7) | 4.30 (0.7) | 3.44 (0.7) | 3.90 (0.9) | 12.01 | .001 | 4.10 | .05 | 0.20 | .66 |
Abbreviations: EVIBE, Evaluation Instantanée du Bien-Etre; NRS, Numeric Rating Scale; SD, standard deviation.
aThe NRS assessing pain with range 0 to 10, from no pain (0) to the most severe pain one can imagine (10). EVIBE: The scale of instantaneous well-being with range 0 to 5, from feeling bad (0) to feeling very well (5). Bold values are statistically significant; Italic values show trend towards statistically significance.
Well-Being Assessment for Each Session
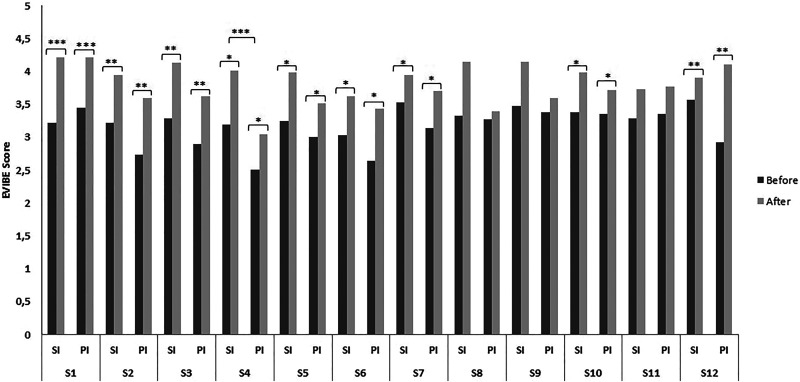
Figure 1 shows more precisely the evolution of EVIBE scores over time for each session in both SI and PI. The mean EVIBE score increased over time across each session in both SI and PI. The increase was significant for 9 sessions, and a trend toward increasing was observed in the 3 remaining sessions. There is not group effect except for session 4, for which there is a significant difference between groups. This analysis shows no interaction between time and group.
Figure 1.
EVIBE score by group and time. EVIBE: The Scale of instantaneous well-being with range 0 to 5, from feeling bad (0) to feeling very well (5) *P < .05, **P < .01, ***P < .0001. EVIBE indicates Evaluation Instantanée du Bien-Etre; PI, painting intervention; SI, singing intervention; Sn, number of session.
Discussion
This report is one of the few to assess the immediate benefit of artistic interventions over several sessions with a randomized trial. The findings reveal an improvement in well-being for both SI and PI groups after each session, compared to the assessment before the session. However, we did not observe this improvement across the sessions for pain intensity measurement. As in the previous study, we did not observe a stronger benefit of SI compared to PI for pain and well-being.
Our present findings confirm the usefulness of the EVIBE scale, allowing to reveal the patients’ well-being in the present moment. Moreover, this scale seems to be sensitive to change and to show a benefit on well-being after a nonpharmacological care activity. This scale is thus accessible to patients with cognitive disorders and has the advantage of being easy and fast to use.
The results of the EVIBE scale revealed a general benefit of artistic activity on patients’ well-being, regardless of the type of artistic practice. Similarly, previous researches have shown that artistic activities, whether musical or visual, can improve not only behavior and increase the sociability of patients with dementia 31 -36 but also well-being and QoL. 33,37 An improvement of about 0.5 points on the EVIBE scale is observed after the activities which seems to be clinically meaningful. However, as the EVIBE scale is recent, no relevance threshold has been determined.
When considering the evolution of pain judgments across the sessions, we can see a slight to moderate improvement, but the results were not significant. The painful intensity at the present moment measured with NRS scale may not be relevant for assessing change in painful feeling. Indeed, previous studies have shown that ratings of pain intensity remain often stable over time. A patient might judge pain with a relatively constant score even though an overall improvement is felt. 38
Otherwise, our previous results revealed an improvement in several measures including both usual and intense pain and pain repercussions. These results were revealed after the 12 sessions compared to the baseline both for SI and PI. Although a significant immediate benefit on pain could not be shown here, it nevertheless seems to be obtained in a longer term assessment. 16 Some assertions can explain the differences with our initial conclusions. Indeed, after the 12 sessions, we used a numerical scale and required participants to base their judgment on the maximum intensity of the last 7 days and not at the present time, as done in this study. The way the question is asked implies that the patient appeals more to his/her memory of pain than to the currently felt pain. This is also true for the other measures used after the 12 intervention sessions: the pain repercussions and the usual pain on the last 7 days. Memory of pain has been shown to be strongly linked to the emotional context and consequently to the feeling of well-being. 39 The results obtained after a long time period from the sessions are therefore difficult to dissociate from a more general improvement in well-being. Even if the interventions are more beneficial on the memory of pain than on the present pain per se, these results remain interesting as studies showed that this memory of pain can influence the chronicity of the pain afterward. 40
The present findings must be interpreted within the context of several limitations. First and foremostly, the nonsignificant results on the pain scale are probably due in part to the variability in pain scores between patients, as evidenced by the wide standard deviations observed in our results. Variability is also observed between baseline scores and the pain scores measured during the sessions, where some patients reported low pain levels. This may have limited statistical power over pain intensity measures. In the future, it would be interesting to propose a similar study with patients reporting higher pain scores but nevertheless comparable etiologies.
In addition, we did not include a control group with usual care or nonartistic activity. This kind of control group would have been interesting to support the hypothesis that artistic activities have a direct benefit on well-being rather than other activities. However, we chose to propose a painting group as a comparison group because the main objective of our work was to highlight the specificity of singing compared to another artistic activity. Indeed, unlike painting, a robust scientific literature shows the interest of music on different emotional and physiological parameters. We expected more profit with SI than with other art activities. This exploratory study shows an improvement in well-being but does not allow differentiating these results from a single placebo or a social effect. Moreover, the randomized controlled trial model is scientifically relevant but does not allow to adapt the groups according to patient’s preferences, which is usually done in routine care. Even though the interventions took into account their preferences they could have been more effective if the patients had been able to choose between SI and PI. Additional data are essential to determine whether the benefit is related to the artistic nature of these activities.
In conclusion, these exploratory results suggest that PI and SI provide an immediate benefit on the patients’ well-being. Immediate benefit is often sought in the management of dementia, as for example, in nursing homes or day care centers. These results encourage art therapy initiatives as a nonpharmacological method to instantly improve the well-being of patients with cognitive impairment.
Footnotes
Authors' Note: Pierre Krolak-Salmon is also affiliated with University Lyon, Lyon, France.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Elodie Pongan  https://orcid.org/0000-0001-8674-6361
https://orcid.org/0000-0001-8674-6361
References
- 1. Galimberti D, Scarpini E. Progress in Alzheimer’s disease. J Neurol. 2012;259(2):201–211. doi:10.1007/s00415-011-6145-3. [DOI] [PubMed] [Google Scholar]
- 2. Ramaroson H, Helmer C, Barberger-Gateau P, Letenneur L, Dartigues JF, PAQUID. Prevalence of dementia and Alzheimer’s disease among subjects aged 75 years or over: updated results of the PAQUID cohort [in French]. Rev Neurol (Paris). 2003;159(4):405–411. [PubMed] [Google Scholar]
- 3. Berryman C, Stanton TR, Bowering KJ, Tabor A, McFarlane A, Moseley GL. Do people with chronic pain have impaired executive function? A meta-analytical review. Clin Psychol Rev. 2014;34(7):563–579. [DOI] [PubMed] [Google Scholar]
- 4. Pickering G, Jourdan D, Eschalier A, Dubray C. Impact of age, gender and cognitive functioning on pain perception. Gerontology. 2002;48(2):112–118. doi:10.1159/000048937. [DOI] [PubMed] [Google Scholar]
- 5. Cole LJ, Farrell MJ, Duff EP, Barber JB, Egan GF, Gibson SJ. Pain sensitivity and fMRI pain-related brain activity in Alzheimer’s disease. Brain. 2006;129(Pt 11):2957–2965. [DOI] [PubMed] [Google Scholar]
- 6. Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: a world health organization study in primary care. JAMA. 1998;280(2):147–151. [DOI] [PubMed] [Google Scholar]
- 7. Ismail Z, Elbayoumi H, Fischer CE, et al. Prevalence of depression in patients with mild cognitive impairment: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(1):58–67. doi:10.1001/jamapsychiatry.2016.3162. [DOI] [PubMed] [Google Scholar]
- 8. Ueda T, Suzukamo Y, Sato M, Izumi SI. Effects of music therapy on behavioral and psychological symptoms of dementia: a systematic review and meta-analysis. Ageing Res Rev. 2013;12(2):628–641. doi:10.1016/j.arr.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 9. Optale G, Urgesi C, Busato V, et al. Controlling memory impairment in elderly adults using virtual reality memory training: a randomized controlled pilot study. Neurorehabil Neural Repair. 2010;24(4):348–357. doi:10.1177/1545968309353328. [DOI] [PubMed] [Google Scholar]
- 10. Särkämö T, Tervaniemi M, Laitinen S, et al. Cognitive, emotional, and social benefits of regular musical activities in early dementia: randomized controlled study. Gerontologist. 2014;54(4):634–650. doi:10.1093/geront/gnt100. [DOI] [PubMed] [Google Scholar]
- 11. Simmons-Stern NR, Budson AE, Ally BA. Music as a memory enhancer in patients with Alzheimer’s disease. Neuropsychologia. 2010;48(10):3164–3167. doi:10.1016/j.neuropsychologia.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krout RE. The effects of single-session music therapy interventions on the observed and self-reported levels of pain control, physical comfort, and relaxation of hospice patients. Am J Hosp Palliat Care. 2001;18(6):383–390. doi:10.1177/104990910101800607. [DOI] [PubMed] [Google Scholar]
- 13. McCaffrey R, Freeman E. Effect of music on chronic osteoarthritis pain in older people. J Adv Nurs. 2003;44(5):517–524. [DOI] [PubMed] [Google Scholar]
- 14. Hoe J, Katona C, Orrell M, Livingston G. Quality of life in dementia: care recipient and caregiver perceptions of quality of life in dementia: the LASER-AD study. Int J Geriatr Psychiatry. 2007;22(10):1031–1036. doi:10.1002/gps.1786. [DOI] [PubMed] [Google Scholar]
- 15. Mabire JB, Gay MC. Qualité de vie au cours des démences: définitions, difficultés et intérêt de son évaluation. Gériatr Psychol Neuropsychiatr Vieil. 2013;11(1):73–81. doi:10.1684/pnv.2013.0387. [DOI] [PubMed] [Google Scholar]
- 16. Pongan E, Tillmann B, Leveque Y, et al. Can musical or painting interventions improve chronic pain, mood, quality of life, and cognition in patients with mild Alzheimer’s disease? Evidence from a randomized controlled trial. J Alzheimers Dis. 2017;60(2):663–677. doi:10.3233/JAD-170410. [DOI] [PubMed] [Google Scholar]
- 17. Roy M, Peretz I, Rainville P. Emotional valence contributes to music-induced analgesia. Pain. 2008;134(1-2):140–147. doi:10.1016/j.pain.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 18. Roy M, Piché M, Chen JI, Peretz I, Rainville P. Cerebral and spinal modulation of pain by emotions. Proc Natl Acad Sci U S A. 2009;106(49):20900–20905. doi:10.1073/pnas.0904706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci U S A. 2001;98(20):11818–11823. doi:10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blood AJ, Zatorre RJ, Bermudez P, Evans AC. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci. 1999;2(4):382–387. doi:10.1038/7299. [DOI] [PubMed] [Google Scholar]
- 21. Dunbar RI, Kaskatis K, MacDonald I, Barra V. Performance of music elevates pain threshold and positive affect: implications for the evolutionary function of music. Evol Psychol. 2012;10(4):688–702. [PubMed] [Google Scholar]
- 22. Bernardi L, Porta C, Sleight P. Cardiovascular, cerebrovascular, and respiratory changes induced by different types of music in musicians and non-musicians: the importance of silence. Heart. 2006;92(4):445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rouch I, Pongan E, Leveque Y, et al. Personality modulates the efficacy of art intervention on chronic pain in a population of patients with Alzheimer’s disease. J Alzheimers Dis. 2018;63(2):617–624. doi:10.3233/JAD-170990. [DOI] [PubMed] [Google Scholar]
- 24. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 25. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi:10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Folstein MF, Folstein SE, McHugh PR. Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 27. Jensen M, Karow P. Self-report scales and procedures for assessing pain in adults . In: Handbook of Pain Assessment. New York: The Guilford Press; 1992:135–151. [Google Scholar]
- 28. Turk DC, Rudy TE, Sorkin BA. Neglected topics in chronic pain treatment outcome studies: determination of success. Pain. 1993;53(1):3–16. [DOI] [PubMed] [Google Scholar]
- 29. Wary B, Villard JF. Spécificités de l’évaluation de la douleur chez les personnes âgées. Psychol Neuropsychiatr Vieil. 2006;4(3):171–178. [PubMed] [Google Scholar]
- 30. Delphin-Combe F, Dauphinot V, Denormandie P, et al. The scale of instantaneous wellbeing: validity in a population with major neurocognitive disorders. Geriatr Psychol Neuropsychiatr Vieil. 2018;16(3):329–334. doi:10.1684/pnv.2018.0745. [DOI] [PubMed] [Google Scholar]
- 31. Guétin S, Portet F, Picot MC, et al. Effect of music therapy on anxiety and depression in patients with Alzheimer’s type dementia: randomised, controlled study. Dement Geriatr Cogn Disord. 2009;28(1):36–46. doi:10.1159/000229024. [DOI] [PubMed] [Google Scholar]
- 32. Holmes C, Knights A, Dean C, Hodkinson S, Hopkins V. Keep music live: music and the alleviation of apathy in dementia subjects. Int Psychogeriatr. 2006;18(4):623–630. doi:10.1017/S1041610206003887. [DOI] [PubMed] [Google Scholar]
- 33. Livingston G, Kelly L, Lewis-Holmes E, et al. Non-pharmacological interventions for agitation in dementia: systematic review of randomised controlled trials. Br J Psychiatry. 2014;205(6):436–442. doi:10.1192/bjp.bp.113.141119. [DOI] [PubMed] [Google Scholar]
- 34. Lord TR, Garner JE. Effects of music on Alzheimer patients. Percept Mot Skills. 1993;76(2):451–455. doi:10.2466/pms.1993.76.2.451. [DOI] [PubMed] [Google Scholar]
- 35. Raglio A, Bellelli G, Traficante D, et al. Efficacy of music therapy in the treatment of behavioral and psychiatric symptoms of dementia. Alzheimer Dis Assoc Disord. 2008;22(2):158–162. doi:10.1097/WAD.0b013e3181630b6f. [DOI] [PubMed] [Google Scholar]
- 36. Suzuki M, Kanamori M, Watanabe M, et al. Behavioral and endocrinological evaluation of music therapy for elderly patients with dementia. Nurs Health Sci. 2004;6(1):11–18. [DOI] [PubMed] [Google Scholar]
- 37. Rusted J. A multi-centre randomized control group trial on the use of art therapy for older people with dementia. Group Anal. 2006;39(4):517–536. [Google Scholar]
- 38. Feine JS, Lavigne GJ, Dao TT, Morin C, Lund JP. Memories of chronic pain and perceptions of relief. Pain. 1998;77(2):137–141. [DOI] [PubMed] [Google Scholar]
- 39. Laurent B. Memory of pain. Douleur Analg. 2011;24:S2–S13. doi:10.1007/s11724-011-0249-5. [Google Scholar]
- 40. Pickering G, Laurent B. Memory of pain. Douleur Analg. 2013;26(1):38–44. [Google Scholar]