Abstract
Tyrosine kinase receptor A (TrkA) plays an important role in the protection of cholinergic neurons in Alzheimer’s disease (AD). This study was designed to investigate whether β-hydroxybutyrate (BHB), an endogenous histone deacetylase (HDAC) inhibitor, upregulates the expression of TrkA by affecting histone acetylation in SH-SY5Y cells treated with amyloid β-protein (Aβ). The results showed that BHB ameliorated the reduction of cell vitality and downregulation of TrkA expression induced by Aβ. Furthermore, BHB inhibited the upregulation of HDAC1/2/3 expression and downregulation of histone acetylation (Ace-H3K9 and Ace-H4K12) levels in Aβ-treated cells. The expression of TrkA was upregulated in HDAC1- or 3-silenced SH-SY5Y cells. However, there was no significant difference in TrkA expression between the HDAC2 knockdown and control cells. In conclusion, this study demonstrates that BHB protects against Aβ-induced neurotoxicity in SH-SY5Y cells. The underlying mechanism of the effect may be associated with the upregulation of TrkA expression by inhibiting HDAC1/3.
Keywords: Alzheimer’s disease, β-hydroxybutyrate, tyrosine kinase receptor A, histonedeacetylases
Introduction
Alzheimer’s disease (AD) is a chronic neurodegenerative disease characterized by continuous exacerbation in cognitive, language, executive, and behavioral functions. 1 Its pathological hallmarks are extracellular plaques and intracellular neurofibrillary tangles, which consist of β-amyloid (Aβ) and hyperphosphorylated tau, respectively. 2,3 Excessive accumulation of Aβ leads to the formation of toxic oligomers that destroy synapses and neurons. 4 Accumulating evidence revealed that the progressive loss of memory and decline in cognitive function are due to the death of neurons, particularly cholinergic neurons, in brains affected by AD. 5 The effective protection of cholinergic neurons is of great importance to the prevention and therapeutics of AD.
Nerve growth factor (NGF) is the first identified neurotrophic factor participating in neuronal differentiation, growth, survival, and functional maintenance. 6 Nerve growth factor binds to 2 transmembrane receptors, high-affinity receptor tyrosine kinase receptor A (TrkA) and low-affinity receptor p75 neurotrophin receptor (p75NTR). The biological effects of NGF are mainly mediated by its high-affinity receptor TrkA, 7 while TrkA expression is decreased in cholinergic neurons of the striatum and basal forebrain in patients with AD. 8 The lack of neurotrophic support due to the reduction of TrkA may play an important role in cholinergic neurons death in patients with AD. 9 Therefore, upregulating TrkA expression is crucial to protect cholinergic neurons in AD. It has been documented that TrkA expression is regulated by histone acetylation modification. 10 Histone acetylation occurs at different lysine positions, especially histone 3 lysine 9 (H3K9) and histone 4 lysine 12 (H4K12) acetylations are implicated in cognition and significantly reduced in AD. 11 Histone acetylation is modulated by the opposing activities of histone acetyltransferases (HATs) and histone deacetylases (HDACs). There is increasing evidence that HDACs may be the potential targets for the treatment of AD. In mammals, HDACs are divided into 4 groups: zinc-dependent class I, II, and IV HDACs, and nicotinamide adenine dinucleotide-dependent class III HDACs (also known as sirtuins). Previous studies showed that the expression of TrkA is upregulated in both ΔNp73-overexpressed PC12 cells treated with a pan-HDAC inhibitor trichostatin A 10 and traumatic brain injury in rodent models exposed to another pan-HDAC inhibitor LB-205. 12 Iraci et al found that recruitment of class I HDAC1 induces a decrease in histone acetylation of the core promoters of TrkA in human TET-21/N cell line. 13 However, it is still unclear whether HDAC1 plays a role in the regulation of TrkA transcription by altering histone acetylation status in AD. Moreover, class I HDAC2 and 3 are known to be strongly associated with AD. More importantly, it remains unknown whether HDAC2/3 participates in regulating the expression of TrkA in AD.
β-Hydroxybutyrate (BHB), a major component of the ketone bodies, is an endogenous HDAC inhibitor. 14,15 In APP swe/PS1dE9 transgenic mouse model of AD, peripheral administration of BHB significantly reduces Aβ burden and greatly improves learning and memory ability. 16 β-Hydroxybutyrate also protects the cultured hippocampal neurons from Aβ1-42 toxicity. 17 However, the mechanism underlying the action of BHB against AD is not clarified. As an in vitro model, Aβ-treated SH-SY5Y cell line has been widely used to explore the mechanisms of action in AD. Therefore, the present study investigated the effects of BHB in SH-SY5Y cells exposed to Aβ. The results indicate that BHB upregulates TrkA expression by inhibiting HDAC1/3 to exert neuroprotective effects.
Materials and Methods
Reagents
β-Hydroxybutyrate was purchased from Shanghai Yingxin Laboratory Equipment Co, Ltd (Shanghai, China); all-trans retinoic acid (RA) was obtained from Sangon Biotech (Shanghai, China); rabbit anti-TrkA polyclonal antibodies (ab76291) were obtained from Abcam (Cambridge, UK); rabbit anti-Ace-H3K9 (ABE18) and rabbit anti-Ace-H4K12 (06-1352) polyclonal antibodies were obtained from Millipore (Billerica, USA); rabbit anti-HDAC1 (06-1352), rabbit anti-HDAC2 (sc-7899), rabbit anti-HDAC3 (sc-11417), and rabbit anti-β-actin (sc-10731) polyclonal antibodies were purchased from Santa Cruz Biotechnology (California, USA); rabbit anti-Histone H3.1 polyclonal antibodies (18-785-210201) were obtained from GenWay Biotech Inc. (San Diego, USA). The secondary antibodies used were conjugated to horseradish peroxidase, goat polyclonal anti-rabbit (31460; Thermo Fisher Scientific, USA). Cell culture media were from American Hyclone Inc. (USA). Aβ25-35 (toxic effect fragment of an Aβ peptide) was from American Peptide Inc. (USA), which was solubilized in sterile water at 1 mmol/L concentration and aggregated by in vitro incubation at 37°C for 7 days.
Cell Culture
Human neuroblastoma SHSY-5Y cells (KCB2006107 YJ; Chinese Academy of Sciences cell bank, Kunming, China) were grown in a mixture (1:1, vol/vol) of Dulbecco modified Eagle medium (DMEM) and Ham’s F12 medium (DMEM/F12) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were maintained at 37°C in a humidified incubator containing 95% air and 5% CO2. All-trans RA was used to differentiate SHSY-5Y cells. After 24 hours of plating, cells were incubated in medium containing all-trans RA (final concentration of 10 μmol/L) for 3 days.
Cell Viability Assay
Cell viability was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, differentiated SH-SY5Y cells were seeded in 96-well culture microplates at a density of 8 × 104/cm2 in 100 μL of antibiotic-free normal growth medium and incubated for 24 hours. Cells were treated with or without BHB (at a final concentration of 0, 5, 10, 20, 40, or 80 mmol/L) for 24 hours. Then, a 10 μL solution of MTT (5 μg/mL) was added to each well and cells were continuously incubated for 3 hours. The purple MTT formazan crystals were subsequently dissolved by adding a 100 μL solution of dimethyl sulfoxide to each well. Absorbance was measured at 490 nm with Microplate Reader (Biotek, USA). The experiment was performed in duplicate and repeated 3 times. Cells were pretreated with or without BHB (at a final concentration of 5 mmol/L). After 3 hours, the cells were exposed to Aβ (at a final concentration of 0, 10, 20, 40, or 80 μmol/L) and were continuously incubated for 24 hours. Then, the cell viability was determined by the above-mentioned MTT assay.
Cells Treatment for Quantitative Real-Time-Polymerase Chain Reaction and Western Blot Analyses
Differentiated SH-SY5Y cells were seeded in 6-well culture microplates at a density of 1 × 105 cells/well in 2 mL of antibiotic-free normal growth medium and incubated for 24 hours. The experiment was designed into 4 groups: control, BHB, Aβ, and Aβ + BHB. Cells in BHB group and Aβ + BHB group were pretreated with BHB (at a final concentration of 5 mmol/L); cells in other groups were incubated in BHB-free medium. Three hours later, 40 μL Aβ25-35 (1 mmol/L) was mixed quickly into the 2 mL medium in Aβ group and Aβ + BHB group, which Aβ25-35 at a final concentration of 20 μmol/L (no effect on cell viability), continuing to incubate cells for 24 hours. The cells were collected and used for quantitative real-time-polymerase chain reaction (qRT-PCR) and Western blot analyses. The experiments were performed in duplicate and repeated 3 times.
Quantitative Real-Time-Polymerase chain Reaction Analysis
Total messenger RNA (mRNA) from the collected cells was isolated using Trizol (CWBIO, Beijing, China). Reverse transcription and first-strand synthesis from each sample were carried out using the HiFiScriptcDNA Synthesis Kit (CWBIO). Resulting complementary DNAs were used as templates for qRT-PCR in the ABI 7500 Real-Time polymerase chain reaction (PCR) system (Applied Biosystems, Inc., Carlsbad, California) using Ultra SYBR Mixture (CWBIO). The PCR reaction was performed as reported before. 18 The primers were synthesized and purified by Shanghai Sangon Biotech with the following sequences: homo TrkA forward: 5′-AGGTTGAAGCCATTCTCCTG-3′, reverse: 5′-TCTCGGTGGTGAACTTACGG-3′ (145 bp product); homo HDAC1 forward: 5′-ACGACGGGGATGTTGGAAAT-3′, reverse: 5′-TGGCTTTGTGAGGGCGATAG-3′ (135 bp product); homo HDAC2 forward: 5′-AGGTTGAAGCCATTCTCCTG-3′, reverse: 5′-ATCCCAGCACTTTGGAAGG-3′ (179 bp product); homo HDAC3 forward: 5′-GAGGGATGAACGGGTAGACA-3′, reverse: 5′-CAGGTGTTAGGGAGCCAGAG-3′ (137 bp product); and β-actin forward: 5′-CATCCGTAAAGACCTCTATGCCAAC-3′, reverse: 5′-ATGGAGCCACCGATCCACA-3′ (171 bp product). The PCR reaction was amplified in the program including an initial denaturation step at 95°C for 30 seconds, followed by 40 cycles for denaturation at 95°Cfor 5 seconds, annealing at 60°C for 34 seconds, subsequent melting curves for 15 seconds at 95°C, 1 minute at 60°C, and 15 seconds at 95°C to ensure that only a single product was amplified. Absolute values from each sample were normalized to β-actin (constitutive gene) mRNA as a reference standard.
Western Blot Analysis
The collected cells were directly homogenized in radioimmunoprecipitation assay buffer containing 0.1% protease inhibitor (Amerso, USA). The lysates were centrifuged at 13 000 rpm for 10 minutes at 4°C, and the supernatants were used for protein analysis. The protein concentration was determined by the Bradford method with Coomassie Brilliant Blue (CBB G-250). Supernatants were mixed with β-mercaptoethanol (5%) and bromophenol blue (0.02%) and boiled for 5 minutes to denature the proteins. Equal amounts of soluble protein were separated by sodium dodecyl–polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride membrane. After blocking with blocking buffer for 1 hour, the membrane was, respectively, incubated with rabbit anti-TrkA (1:2000), anti-HDAC1 (1:2000), anti-HDAC2 (1:2000), anti-HDAC3 (1:2000), anti-Ace-H3K9 (1:200), anti-Ace-H4K12 (1:200), anti-Histone H3.1 (1:200), and anti-β-actin (1:1000) polyclonal antibodies overnight at 4°C, followed by incubation with goat anti-rabbit immunoglobulin G secondary antibody (1:8000) for 2 hours. Immunoreactive protein bands were detected by Gel Image System Ver. 4.00 (USA). The bands were quantified using the ImageJ software (Wayne Rasband, National Institutes of Health, Bethesda, Maryland). Variations in sample loading were normalized relative to β-actin or histone H3.1, which were used as reference standards.
Small-Interfering RNA Knockdown
HDAC1, 2, and 3 small-interfering RNA (siRNA) duplexes (Guangzhou RiboBio Co, Ltd, China) were used simultaneously (si-HDAC1-2-3) or singly (si-HDACs) to interfere with endogenous HDAC1, HDAC2, and HDAC3 expression. The siRNA oligos were as follows: HDAC1: 5′-CCGGTCATGTCCAAAGTAA-3′; HDAC2: 5′-TCCGTAATGTTGCTCGATG-3′; and HDAC3: 5′-GCATTGATGACCAGAGTTA-3′. In brief, differentiated SH-SY5Y cells were seeded in 6-well culture microplates at a density of 1 × 105 cells/well in 2 mL of antibiotic-free normal growth medium. The following day, cells were transfected with a solution of 50 nmol/L siRNA duplex and 12 μL of siRNA transfection reagent (Guangzhou RiboBio Co, Ltd) mixed in DMEM/F12 medium containing 10% FBS. The transfected cells were incubated at 37°C for 24 hours. The untreated (non-siRNA) and nonspecific siRNA treated (scrambled siRNA; Guangzhou RiboBio Co, Ltd) cells were used as controls. The interference efficiency was evaluated by qRT-PCR and Western blot analyses. In addition, the expression levels of TrkA mRNA and protein were investigated by the above methods. This experiment was performed in duplicate and repeated 3 times.
Statistical Analysis
All data are presented as the means ± standard deviation. One-way analysis of variance followed by post hoc Fisher least significant difference was used for data analyses in this study. The statistical analysis was performed with SPSS version 20.0 software. Probability values of less than .05 were considered to be statistically significant.
Results
β-Hydroxybutyrate Ameliorated the Decrease of Cell Viability in Aβ-Treated SH-SY5Y Cells
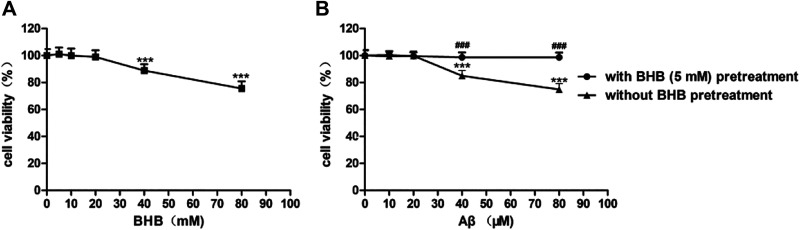
The results indicated that the cell viability was markedly decreased in cells treated with 40 or 80 mmol/L BHB (Figure 1A), and with 40 or 80 μmol/L Aβ (Figure 1B). After BHB pretreatment, the cell viability was significantly restored in cells exposed to 40 or 80 μmol/L Aβ (Figure 1B).
Figure 1.
The effects of β-hydroxybutyrate (BHB) on cell viability in Aβ-treated SH-SY5Y cells. The viability in cells (A) after treatment with different concentrations of BHB; with or without BHB pretreatment, the viability in cell (B) exposed to Aβ. (n = 6; mean ± standard deviation; one-way analysis of variance followed by least significant difference multiple comparison tests; ***P < .001 vs without BHB or Aβ treatment, ### P < .001 vs Aβ treatment).
β-Hydroxybutyrate Ameliorated the Decrease of TrkA Expression in Aβ-Treated SH-SY5Y Cells
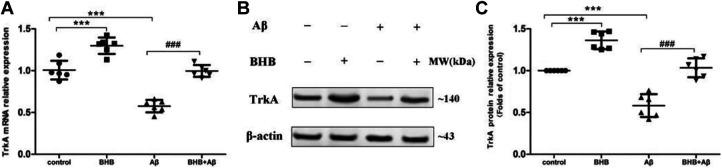
As shown in Figure 2, the expression levels of TrkA mRNA and protein were markedly reduced in cells exposed to Aβ and significantly increased in BHB-treated cells. Compared with the cells only treated with Aβ, the expression levels of TrkA mRNA and protein were significantly increased in cells treated with both BHB and Aβ.
Figure 2.
The effects of β-hydroxybutyrate (BHB) on the expression of TrkA in Aβ-treated SH-SY5Y cells. The cells in both BHB and Aβ + BHB groups were pretreated with BHB (at a final concentration of 5 mmol/L). Cells in both Aβ and Aβ + BHB groups were exposed to Aβ at a final concentration of 20 μmol/L (no effect on cell viability) for 24 hours. The expression levels of TrkA messenger RNA (A) and protein (B and C) in cells were analyzed by quantitative real-time-polymerase chain reaction and Western blot, respectively. (n = 6; mean ± standard deviation; one-way analysis of variance followed by least significant difference multiple comparison tests; ***P < .001 vs control, ### P < .001 vs Aβ treatment).
β-Hydroxybutyrate Alleviated the Decreases of Ace-H3k9 and Ace-H4k12 Levels and the Increase of HDAC1/2/3 Expression in Aβ-Treated SH-SY5Y Cells
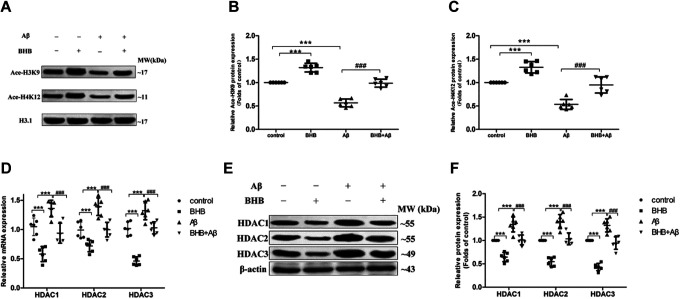
The levels of Ace-H3K9 and Ace-H4K12 were both dramatically declined in Aβ-treated cells and significantly upregulated in cells with BHB treatment; BHB pretreatment significantly reversed the decreases of Ace-H3K9 and Ace-H4K12 levels induced by Aβ (Figure 3A-C). As illustrated in Figure 3D-F, the expression levels of HDAC1/2/3 mRNA and protein were greatly upregulated in cells exposed to Aβ and significantly downregulated in cells with BHB treatment; BHB pretreatment significantly reversed the upregulation of HDAC1/2/3 expression induced by Aβ.
Figure 3.
The effects of β-hydroxybutyrate (BHB) on Ace-H3K9 and Ace-H4K12 levels and HDAC1/2/3 expression in Aβ-treated SH-SY5Y cells. The cells in both BHB and Aβ + BHB groups were pretreated with BHB (at a final concentration of 5 mmol/L). Cells in both Aβ and Aβ + BHB groups were exposed to Aβ at a final concentration of 20 μmol/L (no effect on cell viability) for 24 hours. The levels of Ace-H3K9 and Ace-H4K12 (A-C) in cells were analyzed by Western blot. The expression levels of HDAC1/2/3 messenger RNA (D) and protein (E and F) in cells were analyzed by quantitative real-time-polymerase chain reaction and Western blot, respectively. (n = 6; mean ± standard deviation; one-way analysis of variance followed by least significant difference multiple comparison tests; ***P < .001 vs control, ### P < .001 vs Aβ treatment).
Knockdown of HDAC1 or 3 But Not HDAC2 Upregulated the Expression of TrkA
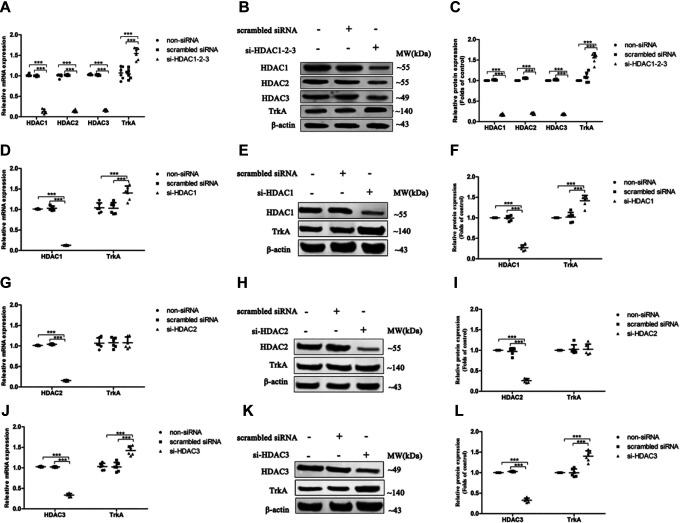
The results revealed that si-HDAC1-2-3 significantly upregulated the expression levels of TrkA mRNA and protein in cells compared with controls (Figure 4A-C). Furthermore, singly knockdown of HDAC1 or 3 markedly elevated TrkA mRNA and protein expression levels in cells (Figure 4D-F and J-L) compared with controls; however, there was no obvious difference in TrkA expression between HDAC2 knockdown cells and controls (Figure 4G-I).
Figure 4.
The expression of TrkA in SH-SY5Y cells with simultaneously or singly silenced HDAC1, 2, and/or 3. The small-interfering RNA (siRNA) duplex was used to simultaneously interfere with the expression of endogenous HDAC1, 2, and 3 (A-C) or singly interfere with the expression of endogenous HDAC1 (D-F), HDAC2 (G-I), or HDAC3 (J-L). The untreated (non-siRNA) and nonspecific siRNA-treated (scrambled siRNA) cells were used as controls. The relative expression levels of TrkA messenger RNA and protein in cells were analyzed by quantitative real-time-polymerase chain reaction and Western blot, respectively. (n = 6; mean ± standard deviation; one-way analysis of variance followed by least significant difference multiple comparison tests; ***P < .001 vs control).
Discussion
β-Hydroxybutyrate is a product of the metabolism of fatty acid that accumulates during prolonged exercise, ketogenic diets, calorie restriction, 14 acute fasting, 19 and intermittent fasting. 20 The concentration of BHB in blood can be increased to 1 to 2 mmol/L during fasting when the liver switches to fatty acid oxidation, 19,21 and to even higher concentrations during prolonged fasting (6-8 mmol/L). 22 It has been reported that BHB does not affect cell vitality at a concentration of 10 mmol/L in primary neurons. 23 Imamura et al found that pretreatment with 8 mmol/L BHB has a significant neuroprotective effect on differentiated SH-SY5Y cells. 24 The present study indicated that BHB, even at 20 mmol/L, had no effect on cell vitality in SH-SY5Y cells, and 5 mmol/L BHB pretreatment provided a significant protection against the reduction of cell vitality induced by Aβ, which is consistent with previous studies. 23,24 Furthermore, 1 mmol/L BHB (a dose is available in physiological level) had the same effect (data not shown).
Nerve growth factor is an important player in synaptic plasticity and cognitive function, and TrkA is known to be the functional receptor of NGF. In patients with AD, TrkA expression is reduced in the basal forebrain nuclei, which may impair the NGF-initiated cell signal transduction and, ultimately, the cell survival. 25 The data (not shown) from our study confirmed that the TrkA expression was decreased in the cerebral cortex and hippocampus of the AD model mice, and intermittent fasting, increase BHB level in blood, alleviated the decrease of TrkA expression. Moreover, in the present study, we demonstrated that BHB ameliorated the decrease of TrkA expression in SH-SY5Y cells treated with Aβ. Evidence showed that the SH-SY5Y cells can secrete neurotrophins such as NGF 1,26,27 Therefore, NGF/TrkA pathway may be involved in the protective actions of BHB against the reduction of cell viability induced by Aβ.
β-Hydroxybutyrate was reported to increase the expression of brain-derived neurotrophic factor (BDNF), a member of neurotrophic signaling, through inhibiting HDACs. 28 Currently, it remains unknown whether the HDAC inhibitor BHB ameliorates Aβ-induced reduction of TrkA expression by modulating histone acetylation. The present study confirmed that BHB could reverse the upregulation of HDAC1/2/3 expression and the downregulation of histone acetylation levels induced by Aβ in SH-SY5Y cells. In addition, in AD model mice, the HDACs expression and histone acetylation levels regulated by intermittent fasting (increasing the blood BHB level) were same to results in Aβ-induced SH-SY5Y cells with BHB treated. 29 A previous study showed that TrkA expression was repressed when HDAC1 bound to its promoter and restored when an HDAC inhibitor trichostatin A was used in neuroblastoma. 13 This study also showed that the expression of TrkA was upregulated in HDAC1-silenced cells. Furthermore, there was an elevation in TrkA expression in HDAC3-silenced cells. However, no difference was observed in TrkA expression between HDAC2-silenced and scramble-treated cells. These findings indicate that the upregulation of TrkA expression by BHB is mediated by lowering HDAC1 and 3, at least partly, but not HDAC2. Further study is also necessary to find out whether the acetylation level on the promoter of TrkA can be regulated in HDAC1-or 3-silenced cells. Interestingly, we found that the HDAC1/2/3 simultaneous knockdown resulted in very similar effect on TrkA transcript levels in comparison to HDAC1- or HDAC3-silenced cells, suggesting that although HDAC1/3 levels are changed upon BHB treatment, they rather act through unknown element that directly regulates TrkA mRNA levels. It could be also helpful to study a dose-dependent response to BHB in our model.
There are still several limitations in this study: other mechanisms of action may also participate in the protection of BHB against AD, such as preventing oxidative stress, 30 regulating metabolism, 31 or BDNF pathway. 32 In addition, the general problem is the transfer of data taken from AD models which need to be further investigated in humans.
Conclusions
Taken together, BHB possesses a protective effect against Aβ-induced neurotoxicity in SH-SY5Y cells. The mechanism underlying the effects may be associated with the upregulation of TrkA expression by inhibiting HDAC1/3. This study indicates that the endogenous HDAC inhibitor BHB has a potential application in AD therapeutics.
Supplemental Material
supplement for β-Hydroxybutyrate Ameliorates Aβ-Induced Downregulation of TrkA Expression by Inhibiting HDAC1/3 in SH-SY5Y Cells by Xinhui Li, Zhipeng Zhan, Jingzhu Zhang, Fuyuan Zhou and Li An in American Journal of Alzheimer"s Disease & Other Dementias
Acknowledgments
The authors wish to thank Ai-Ping Xing, Cong-Min Jiang, and Yan-Qiu Chen for their contributions to the project.
Footnotes
Author Contributions: The authors Xinhui Li, PhD Candidate, ZhipengZhan, PhD, contribute equally to this work.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by the Liaoning Provincial Natural Science Foundation of China [grant number L20170541014]
ORCID iD: Li An  https://orcid.org/0000-0002-8633-0136
https://orcid.org/0000-0002-8633-0136
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Tang LL, Wang R, Tang XC. Huperzine A protects SHSY5Y neuroblastoma cells against oxidative stress damage via nerve growth factor production. Eur J Pharmacol. 2005;519(1-2):9–15. [DOI] [PubMed] [Google Scholar]
- 2. Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368(9533):387–403. [DOI] [PubMed] [Google Scholar]
- 3. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. [DOI] [PubMed] [Google Scholar]
- 4. Ubhi K, Rockenstein E, Vazquez-Roque R, et al. Cerebrolysin modulates pronerve growth factor/nerve growth factor ratio and ameliorates the cholinergic deficit in a transgenic model of Alzheimer’s disease. J Neurosci Res. 2013;91(2):167–177. [DOI] [PubMed] [Google Scholar]
- 5. Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66(2):137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res Brain Res Rev. 2000;33(2-3):199–227. [DOI] [PubMed] [Google Scholar]
- 7. Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10(3):381–391. [DOI] [PubMed] [Google Scholar]
- 8. Boissiere F, Faucheux B, Ruberg M, Agid Y, Hirsch EC. Decreased TrkA gene expression in cholinergic neurons of the striatum and basal forebrain of patients with Alzheimer’s disease. Exp Neurol. 1997;145(1):245–252. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Q, Descamps O, Hart MJ, et al. Paradoxical effect of TrkA inhibition in Alzheimer’s disease models. J Alzheimers Dis. 2014;40(3):605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang J, Chen X. DeltaNp73 modulates nerve growth factor-mediated neuronal differentiation through repression of TrkA. Mol Cell Biol. 2007;27(10):3868–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang SC, Oelze B, Schumacher A. Age-specific epigenetic drift in late-onset Alzheimer’s disease. PLoS One. 2008;3(7):e2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu J, Frerich JM, Turtzo LC, et al. Histone deacetylase inhibitors are neuroprotective and preserve NGF-mediated cell survival following traumatic brain injury. Proc Natl Acad Sci U S A. 2013;110(26):10747–10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iraci N, Diolaiti D, Papa A, et al. A SP1/MIZ1/MYCN repression complex recruits HDAC1 at the TRKA and p75NTR promoters and affects neuroblastoma malignancy by inhibiting the cell response to NGF. Cancer Res. 2011;71(2):404–412. [DOI] [PubMed] [Google Scholar]
- 14. Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanegashima K, Sato-Miyata Y, Funakoshi M, Nishito Y, Aigaki T, Hara T. Epigenetic regulation of the glucose transporter gene Slc2a1 by beta-hydroxybutyrate underlies preferential glucose supply to the brain of fasted mice. Genes Cells. 2017;22(1):71–83. [DOI] [PubMed] [Google Scholar]
- 16. Yin JX, Maalouf M, Han P, et al. Ketones block amyloid entry and improve cognition in an Alzheimer’s model. Neurobiol Aging. 2016;39:25–37. [DOI] [PubMed] [Google Scholar]
- 17. Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL. D-beta-hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc Natl Acad Sci U S A. 2000;97(10):5440–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang R, Miao QW, Zhu CX, et al. Sulforaphane ameliorates neurobehavioral deficits and protects the brain from amyloid beta deposits and peroxidation in mice with Alzheimer-like lesions. Am J Alzheimers Dis Other Demen. 2015;30(2):183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cahill GF, Jr, Herrera MG, Morgan AP, et al. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966;45(11):1751–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang X, Liu Q, Zhou J, Wu X, Zhu Q. Beta hydroxybutyrate levels in serum and cerebrospinal fluid under ketone body metabolism in rats. Exp Anim. 2017;66(2):177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980;60(1):143–187. [DOI] [PubMed] [Google Scholar]
- 22. Cahill GF, Jr. Fuel metabolism in starvation. Annu Rev Nutr. 2006;26(1):1–22. [DOI] [PubMed] [Google Scholar]
- 23. Zhang J, Cao Q, Li S, et al. 3-Hydroxybutyrate methyl ester as a potential drug against Alzheimer’s disease via mitochondria protection mechanism. Biomaterials. 2013;34(30):7552–7562. [DOI] [PubMed] [Google Scholar]
- 24. Imamura K, Takeshima T, Kashiwaya Y, Nakaso K, Nakashima K. D-beta-hydroxybutyrate protects dopaminergic SH-SY5Y cells in a rotenone model of Parkinson’s disease. J Neurosci Res. 2006;84(6):1376–1384. [DOI] [PubMed] [Google Scholar]
- 25. Hock C, Heese K, Muller-Spahn F, Hulette C, Rosenberg C, Otten U. Decreased trkA neurotrophin receptor expression in the parietal cortex of patients with Alzheimer’s disease. Neurosci Lett. 1998;241(2-3):151–154. [DOI] [PubMed] [Google Scholar]
- 26. Croce N, Dinallo V, Ricci V, et al. Neuroprotective effect of neuropeptide Y against beta-amyloid 25-35 toxicity in SH-SY5Y neuroblastoma cells is associated with increased neurotrophin production. Neurodegener Dis. 2011;8(5):300–309. [DOI] [PubMed] [Google Scholar]
- 27. Tanaka K, Ogo H, Kaji H, et al. Dipeptidyl compounds ameliorate the serum-deprivation-induced reduction in cell viability via the neurotrophin-activating effect in SH-SY5Y cells. Neurol Res. 2012;34(6):619–622. [DOI] [PubMed] [Google Scholar]
- 28. Sleiman SF, Henry J, Al-Haddad R, et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. Elife. 2016;5:e15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang J, Li X, Ren Y, et al. Intermittent fasting alleviates the increase of lipoprotein lipase expression in brain of a mouse model of Alzheimer’s disease: possibly mediated by beta-hydroxybutyrate. Front Cell Neurosci. 2018;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maalouf M, Rho JM. Oxidative impairment of hippocampal long-term potentiation involves activation of protein phosphatase 2A and is prevented by ketone bodies. J Neurosci Res. 2008;86(15):3322–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Achanta LB, Rowlands BD, Thomas DS, Housley GD, Rae CD. Beta-hydroxybutyrate boosts mitochondrial and neuronal metabolism but is not preferred over glucose under activated conditions. Neurochem Res. 2017;42(6):1710–1723. [DOI] [PubMed] [Google Scholar]
- 32. Marosi K, Kim SW, Moehl K, et al. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J Neurochem. 2016;139(5):769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplement for β-Hydroxybutyrate Ameliorates Aβ-Induced Downregulation of TrkA Expression by Inhibiting HDAC1/3 in SH-SY5Y Cells by Xinhui Li, Zhipeng Zhan, Jingzhu Zhang, Fuyuan Zhou and Li An in American Journal of Alzheimer"s Disease & Other Dementias