Abstract
Aim:
Informal caregivers play a vital role in the care of people with Alzheimer’s Disease (AD), yet caregiving is associated with caregivers’ burden. The initial objective of the study was to develop a new outcome measure to assess quality of life (QoL) in AD caregivers.
Methods:
Informal (non-professional) caregivers providing 75% or more of the care activities for, and living in the same household as, a person with AD were invited to take part in the study. Qualitative interviews (N = 40) were conducted with AD caregivers in the UK and thematic analyses were applied to generate a pool of potential items. A draft questionnaire was produced and adapted for use in Italy, Spain, Germany and the US. In each of the 5 countries, cognitive debriefing interviews (N = 76) were conducted to determine the questionnaire’s face and content validity, followed by a postal validation survey (N = 268). The data from these surveys were combined to reduce the number of items and assess the new questionnaire’s psychometric properties.
Results:
Thematic analysis of the UK interview transcripts generated a draft questionnaire, which was successfully translated into each additional language. The items were well accepted and easy to complete. However, reanalysis of the qualitative interview data revealed that spousal and non-spousal caregivers identified different experiences of caregiving. A review of the item pool indicated that items were primarily targeted at spousal caregivers. Therefore, further analyses of the postal survey data included spousal caregivers only (n = 116). The results supported scaling assumptions (e.g., corrected item-total correlations ≥0.32), targeting (e.g., floor/ceiling effects <2.5%), internal consistency (α ≥0.93) and test-retest reliability (rs = 0.88) of the new questionnaire, according to classical test theory. Assessment of external construct validity yielded results in accordance with a priori expectations. QoL scores were most strongly related to scores on the emotional reactions sections of the Nottingham Health Profile and the General Well-Being Index. The new questionnaire was found to be capable of detecting meaningful differences between respondents; spousal caregivers had worse QoL when the person with AD was confused (p < .001), could not be left alone (p < .001), did not recognize the caregiver (p < .001), was incontinent (p < .05), and wandered around the house (p = .01).
Conclusions:
The Alzheimer’s Disease Patient Partners Life Impact Questionnaire (APPLIQue) is a questionnaire specific to spousal caregivers of people with AD. Data support its scaling assumptions and it exhibits excellent psychometric properties according to classical test theory. The questionnaire is recommended for use in intervention studies where the QoL of spousal caregivers is of interest.
Keywords: Alzheimer’s disease, quality of life, carer, international, outcome measure
Introduction
Dementia refers to cognitive decline that significantly interferes with an individual’s daily life. 1 An estimated 46.8 million people live with dementia worldwide and this number is expected to double every 20 years—reaching 131.5 million by 2050. 2 The financial impact of dementia is substantial, costing the global economy over $818 billion (£625 billion / €733 billion) each year. 3 Informal caregivers play a crucial role in preventing the institutionalization of people with dementia, saving the UK an estimated £6 billion ($7.8 billion / €7 billion) each year. 4 In 2010, the value of informal care in the United States was almost equal to the costs of direct medical and long-term care of dementia. 5 The most common cause of dementia is Alzheimer’s disease (AD), a chronic neurodegenerative disease. 6
The 1982 National Long-Term Care Survey and Informal Caregivers Survey provided data for the first national estimates of informal caregivers to noninstitutionalized disabled elders. 7 This survey found that 69% of informal caregivers were female. Zwannswijk and colleagues found a similar proportion (72%) in the Netherlands. Their sample also reported that 58% of respondents were spouses and that 94% were aged 65 years or older. 8
A recent systematic review of factors influencing the health-related quality of life (HRQL) of people with dementia found that those living at home reported better HRQL than those residing in care institutions. 9 Although beneficial to the individual, providing care at home is associated with a range of negative consequences for the caregiver, such as feelings of strain, burden and social isolation. 10 -12 Caregivers are also more likely to experience depression, have sleep problems, and are at higher risk of cardiovascular disease than the general population. 13 -15 All these factors are likely to have a profound impact on the quality of life (QoL) of the caregiver.
There is evidence that fewer symptoms in people with AD are associated with better mental functioning of the caregiver. 16 High burden, low life satisfaction and poor HRQL of caregivers is associated with an increased risk of the person with dementia becoming institutionalized. 17 Therefore, when determining the value of interventions for people with AD, it is important that the impact on the life of the caregiver is also considered.
The effect of caregiving has been recognized over the past few decades and self-reported outcome measures have been employed to determine this impact. Page et al. (2017) conducted a systematic review of measures used to evaluate the QoL of family caregivers of people with neurodegenerative diseases. 18 Although a wide range of questionnaires have been employed to determine this impact, they found that generic health status instruments such as the 36-item Short Form Survey (SF-36) 19 and the EuroQoL 5 dimensions questionnaire (EQ-5D) 20 were predominantly employed. By their nature, these and similar questionnaires miss many issues important to AD caregivers and it has been shown that they perform poorly when used with caregivers. 21,22 The review also concluded that the 7 caregiver-specific measures identified had several theoretical and psychometric shortcomings and that new measures were required. For example, the caregiver targeted quality of life measure (CGQOL) 23 which is intended for use with dementia caregivers, lacks a theoretical basis, is very long (80 items), has poor reproducibility and produces a profile of outcome scores rather than an index of impact.
While HRQL measures may be useful in evaluating clinical interventions, the true impact of a condition (or role) on an individual should be a holistic assessment of outcome that takes account of both clinical and non-clinical influences on life quality. 24 A widely implemented approach to such measurement in health research is the need-based QoL model. This conceptual model grew out of qualitative research into the impact of depression on the lives of patients. Interviewees described the impact of their depression in terms of needs that were not fulfilled. 25 The model postulates that life gains its quality from the ability of individuals to satisfy their basic human needs. 26 QoL is better when more needs are fulfilled and poorer when fewer needs are met. The need-based model has been applied successfully in the development of over 30 disease-specific outcome measures. 27 -29
The aim of the current study was to develop and validate a new questionnaire to assess the QoL of informal (non-professional) AD caregivers.
Method
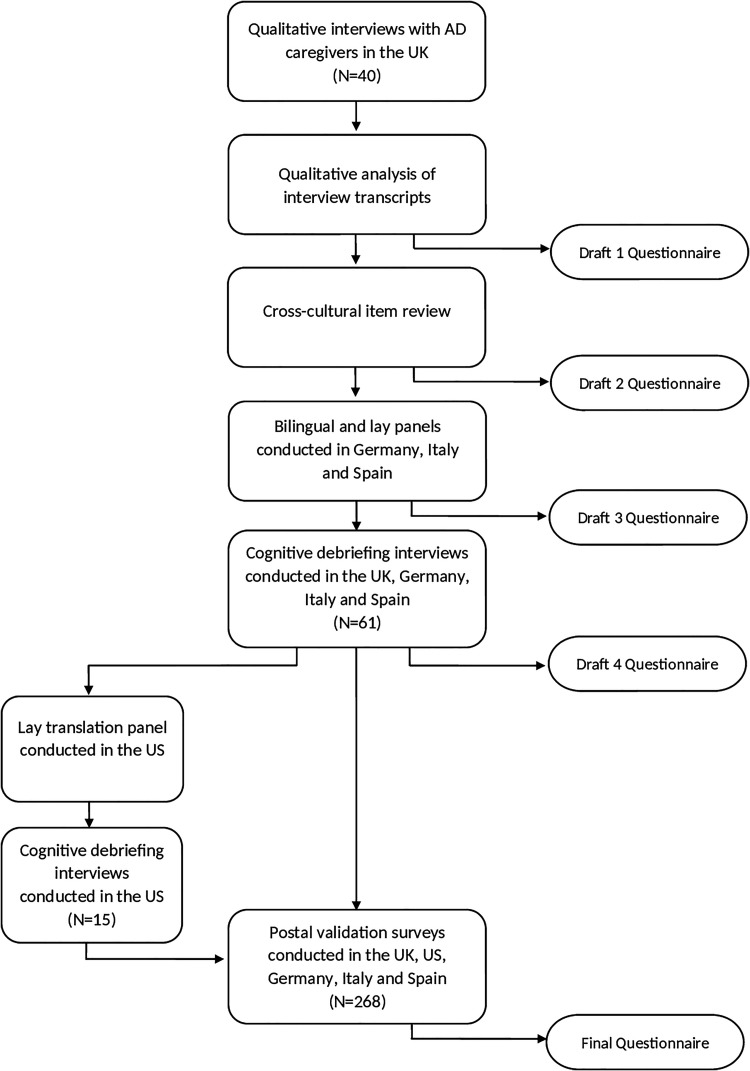
Item generation was conducted in the UK only, whereas the remaining stages were undertaken in the UK, Italy (Venice), Spain (Barcelona), Germany (Lübeck) and the US (San Francisco). Figure 1 summarizes the stages in the study.
Figure 1.
Flowchart illustrating the study stages involved in the development of a QoL questionnaire specific to AD caregivers.
Written informed consent was obtained according to the declaration of Helsinki, prior to caregivers’ inclusion in the study. The study was approved by the local ethics committees in each country.
Participants
Informal (non-professional) caregivers providing 75% or more of the care activities for, and living in the same household as, a person with AD were invited to take part. For each stage of the study, a different sample of AD caregivers was recruited.
Individuals were eligible to participate if they were: at least 18 years of age, a first language speaker in the representative country, capable of completing the study assessment independently and able to provide written informed consent. Individuals were ineligible to participate if they had any serious conditions known to be of a significant influence on caregiver’s QoL (and therefore likely to influence answers on a questionnaire) or were unable to provide written informed consent.
A broad range of participants representing different caregiver-patient relationships, living situations and disease severity of the people with AD were recruited.
Item Generation
Content for the questionnaire was derived from unstructured qualitative interviews conducted with the caregivers. Interviewees were recruited from the UK Alzheimer’s Disease Society (North West Region) and articles on the caring situation published in local newspapers (which included a call for volunteers). The face to face interviews were designed to explore the impact of caring on the caregiver, with emphasis on need fulfilment. Interviews took place either at the caregiver’s home or at another location, if preferred by the respondent. With permission, the interviews were audio-recorded and verbatim transcripts produced.
Using the needs-based model as a guide, thematic analysis was applied to the transcripts to generate a pool of potential questionnaire items. 30 These took the form of statements (derived from the interviews) with respondents asked to indicate whether the statement was “true” or “not true” for them at present. Items were removed from the pool if they were duplicated, idiosyncratic, ambiguous or poorly phrased. The draft questionnaire was designed to be self-completed by the AD caregiver (see “Draft 1 Questionnaire” in Figure 1).
Representatives from each of the participating countries met and agreed on a set of items that had cultural relevance to all countries and that appeared capable of translation with conceptual equivalence (see “Draft 2 Questionnaire” in Figure 1).
Translation
Items were translated into each European language using the dual-panel methodology. 31 This methodology involves conducting 2 independent translation panels; a bilingual panel followed by a lay panel. The purpose of the bilingual panel is to produce an initial translation of the questionnaire in the target language. This version is then presented to a lay panel of monolingual individuals of average educational level who assess the items and instructions for comprehensiveness and “naturalness” of language (see “Draft 3 Questionnaire” in Figure 1). As the panels are intended to identify the best translations rather than to check content validity, the panels did not include caregivers.
Field Testing for Face and Content Validity
Cognitive debriefing interviews (CDIs) were conducted with AD caregivers in the UK, Italy, Spain and Germany to assess the applicability, relevance and comprehensiveness of the questionnaire. Caregivers were recruited through psychiatric, geriatric or neurology clinics in their respective countries. All participants were asked to complete the questionnaire in the presence of an interviewer, who made note of any obvious difficulties or hesitation over items. Participants were then invited to comment on the questionnaire items and instructions. Specifically, they were asked whether they found the items and instructions suitable and if any important aspects of their experience had been omitted (see “Draft 4 Questionnaire” in Figure 1).
Postal Survey
A postal survey was conducted to identify the final version of the questionnaire and to test its psychometric properties.
Subsequent to the postal survey, the draft questionnaire was adapted into US English. It was only necessary to run a lay panel to check the wording. Cognitive debriefing interviews were also conducted with AD caregivers in the US. Consequently, it was possible to run postal surveys in the UK, US, Italy, Spain and Germany and to combine the data for analysis.
AD caregivers were recruited through clinicians in these countries. In the UK, caregivers who were members of AD Society branches also volunteered to participate. The survey pack consisted of a cover letter, the draft caregivers questionnaire, a demographic questionnaire, a comparator measure and a reply-paid envelope.
The General Well-Being Index (GWBI) 32 was used as the comparator scale in the UK and Italy (where validated versions were available), whereas the Nottingham Health Profile (NHP) 33 was used in Germany, Spain and the US. The GWBI is a 22-item measure of psychological well-being and comprises 22 items. Total scores range from 22-100, with higher scores indicating worse well-being. The NHP assesses perceived distress in 6 sections; energy level, pain, physical mobility, sleep, social isolation and emotional reactions. Each section of the NHP is scored 0 (no distress) to 100 (severe distress).
To determine reproducibility, AD caregivers were sent a second survey pack 2 weeks after returning the first. This contained a cover letter, the draft caregivers questionnaire, a reply paid envelope and an amended, shorter demographic questionnaire.
Identifying the Final Questionnaire
Survey data were analyzed according to Rasch Measurement Theory (RMT). 34 The results of these analyses are reported in detail elsewhere and are summarized below. 35 These analyses identified the final, unidimensional questionnaire (see “Final Questionnaire” in Figure 1).
Classical Psychometric Analyses
Further analyses were conducted according to Classical Test Theory (CTT) on the final version of the measure. These included tests of the scaling assumptions, targeting of items, internal consistency, reproducibility and external construct validity. 36 -39 Data for those participants who had missing responses were excluded from the statistical analyses, as the study was designed to validate the new questionnaire.
Data completeness was studied by calculating the percentage of missing data for items, which should be <10%. CTT scaling assumptions regarding the legitimacy of summing item scores into a total score assume that each item should contribute substantially to the total score (item-total correlations >0.3) and that items represent a common variable (supported by corrected item-total correlations >0.3-0.4). Item-total correlations were computed based on polyserial correlations (accounting for the ordinal nature of item level data). 40
Score distributions, skewness and floor-/ceiling effects were assessed as indices of targeting, i.e. how well scale scores accord with the sample levels of QoL. A well-targeted scale should have an average score close to the scale midpoint and span most of its potential range, without excess skewness (preferably between -1 and +1). Floor/ceiling effects are the proportions of people with the lowest (floor) and highest (ceiling) possible scores, respectively. Up to 15-20% floor/ceiling effects can be considered acceptable.
Internal consistency reliability was assessed by the polychoric based ordinal version of coefficient alpha. 41 The influence on alpha when deleting each item one at a time was also explored; an increased coefficient following item deletion suggests problems with, for example; construct conceptualization or multidimensionality. An alpha value at or above 0.80 is considered desirable for group level use of scales, whereas values of 0.90 or above have been suggested for use with individuals. In addition, the standard error of measurement (SEM) was calculated (SD x √(1-reliability)) as an estimator of score precision. To facilitate interpretation, SEM was also calculated as a percentage of the highest possible total scores.
Test-retest reliability (reproducibility) was assessed by administering the questionnaire to AD caregivers on 2 separate occasions, 2 weeks apart. Respondents reporting a change in their caregiving situation between administrations were removed from these analyses. These sets of scores were then correlated and compared using Spearman’s rank correlation coefficient and Wilcoxon signed-rank test, respectively. A high correlation (>0.85) indicates that scores have an acceptably low level of random error over time. 42
External construct validity was tested by convergent and known-group validity. Convergent validity was tested by correlating scores on the new caregivers questionnaire with other scores that tap into related constructs (in the present study, GWBI or NHP section scores). Spearman’s rank correlation coefficients were used. It was predicted that there would be low to moderate correlations between scores on the caregivers questionnaire and those from the NHP sections and GWBI. Correlations with energy level, social isolation, emotional reactions sections (NHP) and the GWBI scores were expected to be higher than correlations with the more physical sections of the NHP.
Known-group validity tests whether scores can distinguish between groups that are expected to differ. For the present study, caregivers were grouped by characteristics of the person they looked after. These were; level of confusion, whether they could be left alone, if they could recognize the caregiver, were incontinent, or were prone to wandering. Non-parametric tests for independent samples (Mann-Whitney U test for 2 groups and Kruskal-Wallis test for 3 or more groups) were employed to test these differences.
Psychometric analyses were performed using the SPSS 23.0 statistical package, and R version 3.4.0 (“psych” package version 1.7.5; www.r-project.org).
Results
Item Generation
Qualitative interviews were conducted with 40 AD caregivers in the UK, each lasting between 50 minutes and 3.5 hours. Demographic information of the interview sample is provided in Table 1. The sample included 28 (70%) females and ranged in age between 29 and 80 years. Twenty-nine (72.5%) of the participants were caring for a spouse.
Table 1.
Demographic Information of the Interview Sample (n = 40).
| Age of caregiver (years) | |
| Median | 63.5 |
| Range | 29–80 |
| Gender of caregiver (%) | |
| Male | 28 (70.0) |
| Female | 12 (30.0) |
| Age of patient (years) | |
| Median | 65 |
| Range | 54–92 |
| Duration of caregiving (years) | |
| Median | 4.5 |
| Range | 1–10 |
| Patient’s relationship to caregiver (%) | |
| Spouse | 29 (72.5) |
| Mother | 9 (22.5) |
| Father | 1 (2.5) |
| Sibling | 1 (2.5) |
The interviews covered issues such as relationships, autonomy and socialization. Interviewees also reported how they were affected by problems sleeping, having low energy levels, disruption of social life, emotional distress, poor health, difficulty undertaking household tasks and dealing with aggression / violence in some cases.
Thematic analysis of interview transcripts generated a pool of 665 potential items (statements). After removal of unsuitable and duplicated items, 86 remained. These were taken forward for review by the representatives from all 5 countries to assess cultural relevance, feasibility and suitability for translation. Following these discussions, 16 more items were removed (primarily alternative wordings for similar items), 5 were replaced with others from the original item pool and one new item (identified from the interview transcripts) was added, resulting in a pool of 71 items. Several alternative items describing similar issues were retained at this stage for consideration in the CDIs.
Table 2 describes the reasons for removal of items from the draft questionnaire at each stage.
Table 2.
Reasons for Modifications to the Draft Questionnaire at Each Stage.
| Stage | Number of potential items | Reasons for item removal/addition |
|---|---|---|
| Qualitative interviews | 665 | |
| Assessed other outcome constructs | ||
| Duplicated other items | ||
| Idiosyncratic | ||
| Poorly worded | ||
| Unsuitable for miscellaneous reasons | ||
| Cross-cultural review | 86 | |
| 16 items too similar | ||
| 5 items replaced with others from original item pool | ||
| 1 item added from interview transcript | ||
| Translation | 71 | |
| 8 items difficult to translate | ||
| Cognitive Debriefing Interviews | 63 | |
| Similarly worded item pairs | ||
| Problematic due to cultural and/or linguistic reasons | ||
| Postal survey | 30 | |
| 5 items removed due to RMT analyses (reported elsewhere) | ||
| Final APPLIQue | 25 |
Translation
The 71-items were presented for translation in each country. There were between 4 and 7 individuals in the 3 bilingual panels and between 6 and 8 individuals in the 4 lay panels. In general, the items were translated without problems. Conceptually equivalent translations were found for all items in Germany, but adequate equivalents could not be found for 8 items in Spanish and/or Italian. For example, the term “social life” is difficult to express in some languages where it is related to “high society.” Translations of anxious and stress became too severe when translated and the concept of “having nothing to look forward to” did not exist in Italian. The 8 difficult items were removed from all language versions, resulting in a 63-item draft questionnaire.
Field Testing for Face and Content Validity
Seventy-six CDIs were conducted with AD caregivers (23 in UK, 8 in Germany, 20 in Italy, 10 in Spain, 15 in the US). Most of the caregivers were female (70%), all but one of whom was currently or had recently provided care for a spouse. Participants considered the items to be highly appropriate to their caregiving situation. The questionnaire was judged as comprehensive by interviewees, with no aspects of their caregiving experience omitted. Where alternative items were included for assessment in the CDIs, the preferred items were retained. However, some individual items were highlighted as problematic in individual countries for cultural and/or linguistic reasons. These items were also removed from all language versions of the questionnaire, leaving a revised draft measure consisting of 30 items.
Postal Survey
A total of 268 AD family caregivers returned the first survey pack of the postal survey. Of these, 206 caregivers completed the survey pack at the second administration.
Initial RMT analyses identified that spousal and non-spousal caregivers responded in different ways to some of the items. 35 Subsequent reanalysis of the qualitative data suggested that spousal and non-spousal caregivers identified different experiences of caregiving. Many spousal caregivers believed that life without their partner was empty and without purpose. They were also more likely to have feelings of loss and bereavement than non-spousal caregivers. In contrast, non-spousal caregivers believed that their life would begin again if the person with AD died or went into institutional care. Most of the respondents (at all study stages of the study) were spousal caregivers and a review of the item pool indicated that items were primarily targeted at such respondents. Five items were removed from the draft questionnaire that were judged to have been more relevant to non-spousal caregivers—such as caring for other members of their family.
Non-spousal caregivers from the 5 countries were then removed, resulting in a sample of 116 spousal caregivers. Of these, 95 spousal caregivers completed the survey pack on both occasions. The reduced dataset (n = 116) was reanalyzed using RMT. The resulting questionnaire demonstrated good measurement properties with the spousal caregiver sample. 35 This final version was named the Alzheimer’s Patient Partners Life Impact Questionnaire (APPLIQue). Example items from the APPLIQue are shown in Table 3.
Table 3.
Example Items From the Final 25-item APPLIQue.
| Item |
| Organizing shopping is very difficult |
| There is no conversation between us |
| I have little freedom to do what I want to do |
| I feel that I’m losing my independence |
| It’s like being with a stranger |
Table 4 includes demographic and background information provided by spousal caregivers in the postal survey. No significant differences in demographic factors or APPLIQue scores were found between spousal caregivers who completed the postal survey on the first occasion only compared to those who completed it on both occasions.
Table 4.
Demographic and Background Information Provided by Spousal Caregivers in the Postal Survey (n = 116).
| Age of caregiver (years) | |
| Mean (SD) | 70.3 (9.6) |
| Duration of caregiving (years) | |
| Mean (SD) | 6.6 (9.0) |
| Gender of caregiver (%) | |
| Male | 28 (24.1) |
| Female | 88 (75.9) |
| Country (%) | |
| UK | 41 (35.3) |
| Germany | 12 (10.3) |
| Italy | 13 (11.2) |
| Spain | 11 (9.5) |
| US | 39 (33.6) |
| Perceived general health of caregiver (%) | |
| Excellent/very good | 13 (11.2) |
| Good/fair | 86 (74.1) |
| Poor | 15 (12.9) |
| Missing | 2 (1.7) |
| Is the patient confused? (%) | |
| Yes, all of the time | 29 (25.0) |
| Yes, most of the time | 44 (37.9) |
| Yes, sometimes | 39 (33.6) |
| No | 4 (3.4) |
| Is it possible to leave the patient alone? (%) | |
| Yes, as long as I need | 24 (20.7) |
| Yes, a short time | 45 (38.8) |
| No | 46 (39.7) |
| Missing | 1 (0.9) |
| Does the patient wander around house? (%) | |
| Yes | 63 (54.3) |
| No | 51 (44.0) |
| Missing | 2 (1.7) |
| Does the patient recognize you? (%) | |
| Yes, all the time | 61 (52.6) |
| Yes, most of the time | 32 (27.6) |
| Yes, sometimes | 14 (12.1) |
| No, never | 9 (7.8) |
| Is the patient incontinent? (%) | |
| Yes, all the time | 34 (29.3) |
| Yes, during the day | 16 (13.8) |
| Yes, only at night | 8 (6.9) |
| No | 54 (46.6) |
| Missing | 4 (3.4) |
| Comparator questionnaires | Median (IQR) |
| GWBI | 64.0 (57.0–77.0) |
| NHP | |
| Energy level | 33.3 (0.0–66.7) |
| Pain | 0.0 (0.0–34.4) |
| Sleep | 20.0 (0.0–80.0) |
| Emotional reactions | 33.3 (11.1–55.6) |
| Social isolation | 20.0 (0.0–40.0) |
| Physical mobility | 64.0 (0.0–25.0) |
IQR = Inter Quartile Range, GWBI = General Well-Being Index, NHP = Nottingham Health Profile.
Table 5 shows descriptive and psychometric statistics for the APPLIQue. Total APPLIQue scores range between 0 and 25, where higher scores indicate worse QoL.
Table 5.
Descriptive and Psychometric Data of the APPLIQue Among Spousal Caregivers of People With AD.
| Time 1 | Time 2 | |
|---|---|---|
| Data completeness | ||
| Missing item responses (min-max %)a | 1–6 | 1–7 |
| Scaling assumptions | ||
| Corrected polyserial item-total correlation (min-max)b | 0.36–0.78 | 0.32–0.80 |
| Targeting | ||
| Possible score range (midpoint) | 0–25 (12.5) | 0–25 (12.5) |
| Mean (SD) scorec | 13.4 (6.2) | 13.5 (5.9) |
| Median (q1-q3) scorec | 15 (8–18) | 14 (8.5–18) |
| Min-max scored | 0–25 | 0–23 |
| Floor/ceiling effects (%)e | 1 / 1 | 2.4 / 0 |
| Skewnessf | –0.25 | –0.27 |
| Reliability | ||
| Ordinal αg | 0.94 | 0.93 |
| Ordinal α when item deleted (min-max)h | 0.94–0.94 | 0.93–0.93 |
| SEM, ordinal α based (% of total score)i | 1.5 (6.1) | 1.6 (6.3) |
a Should be <10%.
b Should be >0.3 to support summation of raw item scores, and >0.3-0.4 to support a single underlying variable.
c Should be close to scale midpoint.
d Should span most of the scale’s score range.
e Should be <15-20%.
f Should be between -1 and +1.
g Should be ≥0.80.
h Should not increase compared to α for the total score.
i Should be less than half of the total score SD
APPLIQue, the Alzheimer’s Patient Partners Life Impact Questionnaire; SD, standard deviation; q1-q3, 1st-3 rd quartile (25th-75th percentile); SEM, standard error of measurement.
Data completeness was good (>93%), and corrected item-total correlations confirmed the legitimacy of summing items into a total score. Targeting was good with minimal floor and ceiling effects. In contrast, large floor effects were found for the NHP sections (energy level = 46%; pain = 57%; sleep = 26%; emotional reactions = 19%; social isolation = 48%; physical mobility = 52%), highlighting the limitations of this generic HRQL instrument.
Ordinal alpha coefficients for the APPLIQue were good (>0.92) and did not increase following item deletion. The SEM was low, representing about a quarter of the standard deviation and 6% of the range of total scores (Table 5). Test-retest reliability was assessed in spousal caregivers who reported no change in their caregiving situation between administrations (n = 59). Although 68 spousal caregivers provided complete data on the APPLIQue at both time points, 9 respondents were removed from the analysis of test-retest reliability due to a change in their caregiving situation. The median (q1-q3) scores at baseline and time 2 were 14.0 (7.0-17.0) and 13.5 (8.0-18.0), respectively (Wilcoxon signed-rank test, p = .38). Test-retest reliability (Spearman’s rho) was rs = 0.88.
Table 6 shows the correlations between APPLIQue scores and those on the NHP sections and GWBI. APPLIQue scores correlated moderately with GWBI scores, indicating a link between psychological well-being and need-based QoL in AD spousal caregivers. As expected, moderate correlations were also found between APPLIQue scores and the energy level, emotional reactions and social isolation section scores on the NHP. Weaker associations were found between APPLIQue scores and those for pain, physical mobility and sleep.
Table 6.
Correlation Coefficients Between APPLIQue Scores and NHP and GWBI Scores (Time 1).
| APPLIQue | |
|---|---|
| NHP | |
| Energy level | 0.59** |
| Pain | 0.39** |
| Emotional reactions | 0.74** |
| Sleep | 0.36* |
| Social isolation | 0.64** |
| Physical mobility | 0.27 |
| GWBI | 0.67** |
** Correlation is significant at p < .01.
* Correlation is significant at p < .05.
NHP = Nottingham Health Profile.
GWBI = General Well-Being Index.
Female spousal caregivers scored higher on the APPLIQue (p < .05). No statistically significant difference in APPLIQue scores was found between younger (below median age) and older (above median age) spousal caregivers (p = 0.16).
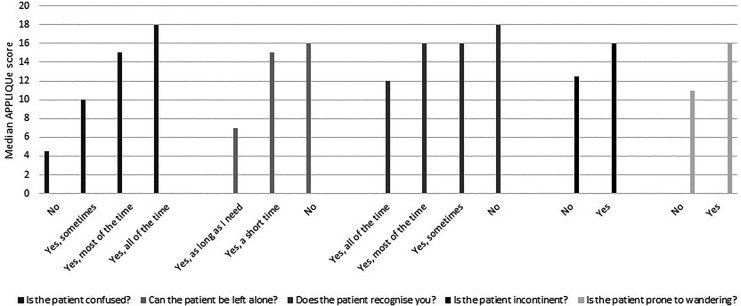
Figure 2 displays median APPLIQue scores by level of confusion, whether the person with AD could be left alone, whether they recognized the caregiver, whether they were incontinent and whether they were prone to wandering. Caregivers had worse QoL when the person with AD was confused (p < .001), could not be left alone (p < .001), did not recognize the caregiver (p < .001), was incontinent (p < .05), and wandered around the house (p = .01).
Figure 2.
Median APPLIQue scores by known groups.
Discussion
The present study describes the international development and validation of the APPLIQue; a QoL questionnaire specific to spousal caregivers of people with AD. The questionnaire is based on a clear conceptual model—the need-based model of QoL, and items were derived directly from caregivers, ensuring that all content was relevant. Translation into other languages was conducted using the dual-panel methodology. Research has demonstrated that this approach produces translations that are more acceptable to respondents than standard forward-backward translation. 43 Field-testing ensured that the items were well accepted and easy to complete. RMT and item-total correlations supported the scaling assumptions of the APPLIQue and reproducibility was good. 35 Unidimensionality of the scale was supported according to both RMT and CTT criteria, based on corrected item-total correlations. 35,36,39 External construct validity was supported by expected correlations with scores from comparator scales and the APPLIQue’s ability to differentiate caregivers according to the reported levels of dementia-related symptoms and behaviors of their spouses, suggesting that the QoL of caregivers worsens as AD symptoms progress. These findings support previous research demonstrating the negative impact of cognitive decline and behavioral disturbances in people with AD on their spousal caregiver. 44,45
Previous studies investigating the impact of caregiving on QoL have relied on generic health status questionnaires. By their nature, generic questionnaires typically include items that are not relevant, while missing key issues specific to the caregiver. In addition, the available caregiver-specific measures have poor psychometric properties and lack a clear theoretical basis. 18 Consequently, the APPLIQue represents important issues for spousal caregivers not covered by other measures. For example, the APPLIQue contains items relating to the inability of the caregiver to share their thoughts and feelings with their spouse. The questionnaire may well detect the benefits to the caregiver of clinical and non-clinical interventions aimed at people with AD, as well as interventions designed to support the caregiver, such as respite care. Furthermore, the development and validation of 5 different language versions of the APPLIQue increase its utility for multinational clinical trials. As care was taken in the development of the questionnaire to avoid potential translation difficulties, it is anticipated that the development of further language versions would prove feasible.
The original aim of the study was to create a QoL questionnaire specific to all AD caregivers. However, analysis of the qualitative interview data with caregivers revealed important differences between spousal and non-spousal caregivers, confirming previous findings. 46 Future research should focus on developing a questionnaire for non-spousal AD caregivers, for example adult children caregivers, based on the needs-based model of QoL.
Due to the original focus on all types of AD caregivers, 29 interviews were conducted with spousal caregivers. Despite this, similar sample sizes have been used in the development of other needs-based PROs. 28,29
The reduced sample size in the postal survey (n = 116) was a concern. Cultural differences in Germany, Spain and Italy resulted in relatively small samples of spousal caregivers. In these countries, a large proportion of individuals who took part in the postal survey were providing care for a parent or sibling and were therefore excluded from the analyses. The validation analyses were performed on the data from all 5 countries combined, to maximize the sample size and to strengthen the cultural validity of the measure. Again, similar sized samples have been demonstrated previously as producing stable conclusions regarding CTT-based reliability and validity. 47 Another potential limitation of the study was that most of the participants were female. This reflects the spousal caregiver population. 48 While female spousal caregivers scored higher on the APPLIQue, the RMT analyses did not detect differential item functioning related to gender. Previous research has suggested that female caregivers experience greater distress than male caregivers. 49
In conclusion, the APPLIQue should play an important role in assessing the impact of caregiving on the QoL of spousal caregivers. It should also be capable of showing how clinical and non-clinical interventions aimed at either the person with AD or the spousal caregiver influence the caregivers’ lives. The QoL of spousal caregivers is of paramount importance given the savings that accrue to health services, by caregivers preventing or delaying patients’ institutionalization. 2
Acknowledgments
The authors would like to thank all caregivers who participated in the study. The following researchers contributed to instrument development; Dr Thomas Kohlmann (Lübeck), Professor Mauro Niero (Venice), Dr Jordi Alonso (Barcelona) and Dr Meryl Brod (San Francisco). The study was supported by Kristianstad University, Kristianstad, Sweden.
Authors’ Note: Since completing the research, Matthew Rouse has moved to the MRC Cognition and Brain Sciences Unit at the University of Cambridge.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Stephen P. McKenna  https://orcid.org/0000-0003-4238-8333
https://orcid.org/0000-0003-4238-8333
Alice Heaney  https://orcid.org/0000-0002-4534-6705
https://orcid.org/0000-0002-4534-6705
References
- 1. Chertkow H, Feldman HH, Jacova C, Massoud F. Definitions of dementia and predementia states in Alzheimer’s disease and vascular cognitive impairment: consensus from the Canadian conference on diagnosis of dementia. Alzheimer’s Res Ther. 2013;5(suppl 1):S2. doi:10.1186/alzrt198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alzheimer’s Disease International. World Alzheimer Report 2015: the global impact of dementia: an analysis of prevalence, incidence, cost and trends. Published 2015. Accessed January 10, 2019. https://www.alz.co.uk/research/world-report-2015
- 3. Wimo A, Guerchet M, Ali GC, et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017;13(1):1–7. Accessed December 12, 2018. https://www.sciencedirect.com/science/article/pii/S1552526016300437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Age UK. (2016). Invisible army of oldest carers saving state billions. Published 2016. Accessed December 13, 2018. https://www.ageuk.org.uk/latest-news/archive/invisible-army-of-oldest-carers-saving-state-billions/
- 5. Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326–1334. doi:10.1056/NEJMsa1204629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alzheimer’s Association. 2017 Alzheimer’s disease facts and figures. Alzheimers Dement. 2017;13(4):325–373. doi:10.1016/j.jalz.2017.02.001 [Google Scholar]
- 7. Stone R, Cafferata GL, Sangl J. Caregivers of the frail elderly: a national profile. Gerontologist. 1987;27(5):616–626. doi:10.1093/geront/27.5.616 [DOI] [PubMed] [Google Scholar]
- 8. Zwaanswijk M, Peeters JM, Van Beek AP, Meerveld JH, Francke AL. Informal caregivers of people with dementia: problems, needs and support in the initial stage and in subsequent stages of dementia: a questionnaire survey. Open Nurs J. 2013;7:6–13. doi:10.2174/1874434601307010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jing W, Willis R, Feng Z. Factors influencing quality of life of elderly people with dementia and care implications: a systematic review. Arch Gerontol Geriatr. 2016;66:23–41. doi:10.1016/j.archger.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 10. Papastavrou E, Kalokerinou A, Papacostas SS, Tsangari H, Sourtzi P. Caring for a relative with dementia: family caregiver burden. J Adv Nurs. 2007;58(5):446–457. doi:10.1111/j.1365-2648.2007.04250.x [DOI] [PubMed] [Google Scholar]
- 11. Sanders S. Is the glass half empty or half full? Reflections on strain and gain in caregivers of individuals with Alzheimer’s disease. Soc Work Health Care. 2005;40(3):57–73. doi:10.1300/J010v40n03_04 [DOI] [PubMed] [Google Scholar]
- 12. Sherwood PR, Given CW, Given BA, Von Eye A. Caregiver burden and depressive symptoms: analysis of common outcomes in caregivers of elderly patients. J Aging Health. 2005;17(2):125–147. doi:10.1177/0898264304274179 [DOI] [PubMed] [Google Scholar]
- 13. Mausbach BT, Roepke SK, Ziegler MG, et al. Association between chronic caregiving stress and impaired endothelial function in the elderly. J Am Coll Cardiol. 2010;55(23):2599–2606. doi:10.1016/j.jacc.2009.11.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peng HL, Lorenz RA, Chang YP. Sleep quality in family caregivers of individuals with dementia: a concept analysis. Clin Nurs Res. 2016;25(4):448–464. doi:10.1177/1054773815610747 [DOI] [PubMed] [Google Scholar]
- 15. Pinquart M, Sörensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a meta-analysis. Psychol Aging. 2003;18(2):250–267. doi:10.1037/0882-7974.18.2.250 [DOI] [PubMed] [Google Scholar]
- 16. Markowitz JS, Gutterman EM, Sadik K, Papadopoulos G. Health-related quality of life for caregivers of patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2003;17(4):209–214. Accessed December 12, 2018. https://journals.lww.com/alzheimerjournal/Abstract/2003/10000/Health_Related_Quality_of_Life_for_Caregivers_of.3.aspx [DOI] [PubMed] [Google Scholar]
- 17. Luppa M, Luck T, Brähler E, König HH, Riedel-Heller SG. Prediction of institutionalisation in dementia. Dement Geriatr Cogn Disord. 2008;26(1):65–78. doi:10.1159/000144027 [DOI] [PubMed] [Google Scholar]
- 18. Page TE, Farina N, Brown A, et al. Instruments measuring the disease-specific quality of life of family carers of people with neurodegenerative diseases: a systematic review. BMJ Open. 2017;7(3):e013611. doi:10.1136/bmjopen-2016-013611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992;30(6):473–483. Accessed December 2, 2018. https://www.jstor.org/stable/3765916 [PubMed] [Google Scholar]
- 20. The EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi:10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 21. Bell CM, Araki SS, Neumann PJ. The association between caregiver burden and caregiver health-related quality of life in Alzheimer disease. Alzheimer Dis Assoc Disord. 2001;15(3):129–136. Accessed December 12, 2018. https://journals.lww.com/alzheimerjournal/Abstract/2001/07000/The_Association_Between_Caregiver_Burden_and.4.aspx [DOI] [PubMed] [Google Scholar]
- 22. Charlesworth G, Shepstone L, Wilson E, Thalanany M, Mugford M, Poland F. Does befriending by trained lay workers improve psychological well-being and quality of life for carers of people with dementia, and at what cost? A randomised controlled trial. Health Technol Assess. 2008;12(4):1–78. Accessed December 13, 2018. https://discovery.ucl.ac.uk/id/eprint/116694/1/FullReport-hta12040.pdf [DOI] [PubMed] [Google Scholar]
- 23. Vickrey BG, Hays RD, Maines ML, Vassar SD, Fitten J, Strickland T. Development and preliminary evaluation of a quality of life measure targeted at dementia caregivers. Health Qual Life Outcomes. 2009;7:56. doi:10.1186/1477-7525-7-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McKenna SP. The limitations of patient-reported outcome measurement in oncology. J Clin Pathways. 2016;2(7):37–46. Accessed December 17, 2018. https://www.journalofclinicalpathways.com/index.php/article/limitations-patient-reported-outcome-measurement-oncology [Google Scholar]
- 25. Hunt SM, McKenna SP. The QLDS: a scale for the measurement of quality of life in depression. Health Policy. 1992;22(3):307–319. doi:10.1016/0168-8510(92)90004-U [DOI] [PubMed] [Google Scholar]
- 26. McKenna SP, Wilburn J. Patient value: its nature, measurement, and role in real world evidence studies and outcomes-based reimbursement. J Med Econ. 2018;21(5):474–480. doi:10.1080/13696998.2018.1450260 [DOI] [PubMed] [Google Scholar]
- 27. McKenna SP, Doward LC. The needs-based approach to quality of life assessment. Value Health. 2004;7:S1–S3. Accessed December 13, 2018. https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1524-4733.2004.7s101.x [DOI] [PubMed] [Google Scholar]
- 28. Wilburn J, McKenna SP, Twiss J, Kemp K, Campbell S. Assessing quality of life in Crohn’s disease: development and validation of the Crohn’s Life Impact Questionnaire (CLIQ). Qual Life Res. 2015;24(9):2279–2288. doi:10.1007/s11136-015-0947-1 [DOI] [PubMed] [Google Scholar]
- 29. Wilburn J, McKenna SP, Heaney A, et al. Development and validation of the Parenteral Nutrition Impact Questionnaire (PNIQ), a patient-centric outcome measure for home parenteral nutrition. Clin Nutr. 2018;37(3):978–983. doi:10.1016/j.clnu.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 30. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. doi:10.1191/1478088706qp063oa [Google Scholar]
- 31. Hunt SM, Alonso J, Bucquet D, Niero M, Wiklund I, McKenna S. Cross-cultural adaptation of health measures. Health Policy. 1991;19(1):33–44. doi:10.1016/0168-8510(91)90072-6 [DOI] [PubMed] [Google Scholar]
- 32. Hunt SM, McKenna SP. A British adaptation of the General Well-being Index: a new tool for clinical research. J Med Econ. 1992;2:49–60. [Google Scholar]
- 33. Hunt SM, McEwen J, McKenna SP. Measuring health status: a new tool for clinicians and epidemiologists. J R Coll Gen Pract. 1985;35(273):185–188. Accessed December 12, 2018. https://bjgp.org/content/35/273/185.short [PMC free article] [PubMed] [Google Scholar]
- 34. Rasch G. Probabilistic Models for Some Intelligence and Achievement Tests. Danish Institute for Educational Research; 1960. [Google Scholar]
- 35. Hagell P, Rouse M, McKenna SP. Measuring the impact of caring for a spouse with Alzheimer’s disease: validation of the Alzheimer’s Patient Partners Life Impact Questionnaire (APPLIQue). J Appl Meas. 2018;19(3):271–282. Accessed December 11, 2018. https://pdfs.semanticscholar.org/731c/b92be9f96541d10c80508f39c009e879bd9c.pdf [PubMed] [Google Scholar]
- 36. Hobart J, Cano S. Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods. Health Technol Assess. 2009;13(12):1–177. doi:10.3310/hta13120 [DOI] [PubMed] [Google Scholar]
- 37. Nunnally JC, Bernstein IH. Psychometric Theory. 3rd ed. McGraw-Hill, Inc; 1994. [Google Scholar]
- 38. Saris-Baglama RN, Dewey CJ, Chisholm GB, Kosinski M, Bjorner JB, Ware JE. SF Health Outcomes™ Scoring Software User’s Guide. QualityMetric Inc; 2004. [Google Scholar]
- 39. Ware JE, Jr, Gandek B. Methods for testing data quality, scaling assumptions, and reliability: the IQOLA Project approach. J Clin Epidemiol. 1998;51(11):945–952. doi:10.1016/S0895-4356(98)00085-7 [DOI] [PubMed] [Google Scholar]
- 40. Olsson U, Drasgow F, Dorans NJ. The polyserial correlation coefficient. Psychometrika. 1982;47(3):337–347. doi:10.1007/BF02294164 [Google Scholar]
- 41. Gadermann AM, Guhn M, Zumbo BD. Estimating ordinal reliability for Likert-type and ordinal item response data: a conceptual, empirical, and practical guide. Pract Assess Res Evaluation. 2012;17(3):1–13. Accessed December 12, 2018. https://scholarworks.umass.edu/pare/vol17/iss1/3 [Google Scholar]
- 42. Weiner EA, Stewart B.J. Assessing Individuals: Psychological and Educational Tests and Measurements. Little Brown; 1984. [Google Scholar]
- 43. Hagell P, Hedin PJ, Meads DM, Nyberg L, McKenna SP. Effects of method of translation of patient-reported health outcome questionnaires: a randomized study of the translation of the Rheumatoid Arthritis Quality of Life (RAQoL) instrument for Sweden. Value Health. 2010;13(4):424–430. doi:10.1111/j.1524-4733.2009.00677.x [DOI] [PubMed] [Google Scholar]
- 44. Agüera-Ortiz L, Frank-García A, Gil P, Moreno A. Clinical progression of moderate-to-severe Alzheimer’s disease and caregiver burden: a 12-month multicenter prospective observational study. Int Psychogeriatr. 2010;22(8):1265–1279. doi:10.1017/S104161021000150X [DOI] [PubMed] [Google Scholar]
- 45. Germain S, Adam S, Olivier C, et al. Does cognitive impairment influence burden in caregivers of patients with Alzheimer’s disease? J Alzheimers Dis. 2009;17(1):105–114. doi:10.3233/JAD-2009-1016 [DOI] [PubMed] [Google Scholar]
- 46. Reed C, Belger M, Dell’Agnello G, et al. Caregiver burden in Alzheimer’s disease: differential associations in adult-child and spousal caregivers in the GERAS observational study. Dement Geriatr Cogn Dis Extra. 2014;4(1):51–64. doi:10.1159/000358234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hobart JC, Cano SJ, Warner TT, Thompson AJ. What sample sizes for reliability and validity studies in neurology? J Neurol. 2012;259(12):2681–2694. doi:10.1007/s00415-012-6570-y [DOI] [PubMed] [Google Scholar]
- 48. Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci. 2009;11(2):217–228. Accessed December 16, 2018. https://www.ncbi.nlm.nih.gov/pmc/articles/PmC3181916/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pöysti MM, Laakkonen ML, Strandberg T, et al. Gender differences in dementia spousal caregiving. Int J Alzheimers Dis. 2012. doi:10.1155/2012/162960 [DOI] [PMC free article] [PubMed] [Google Scholar]