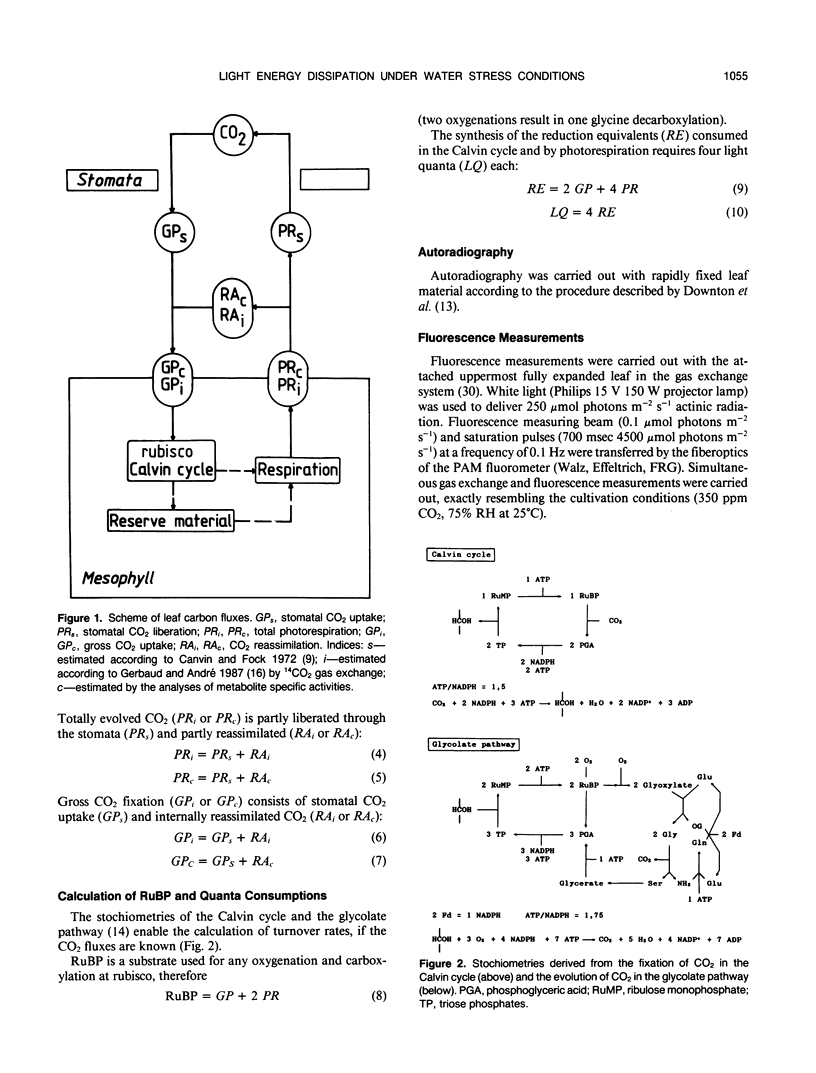
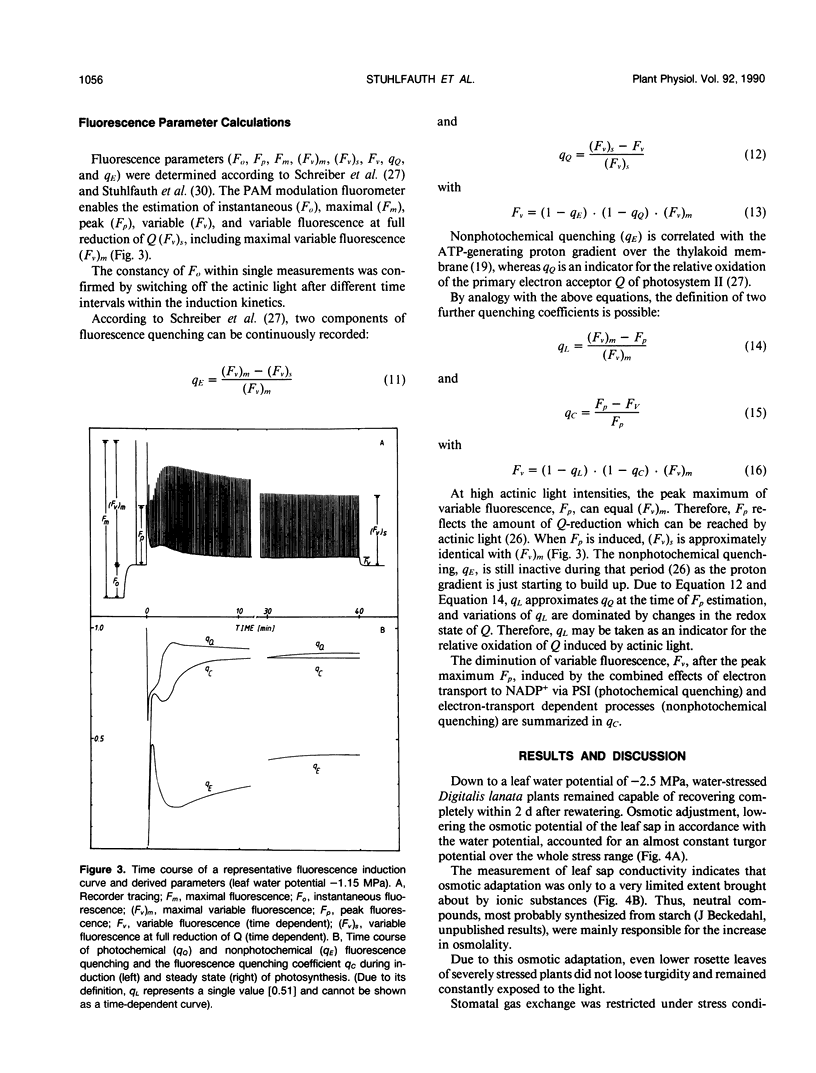
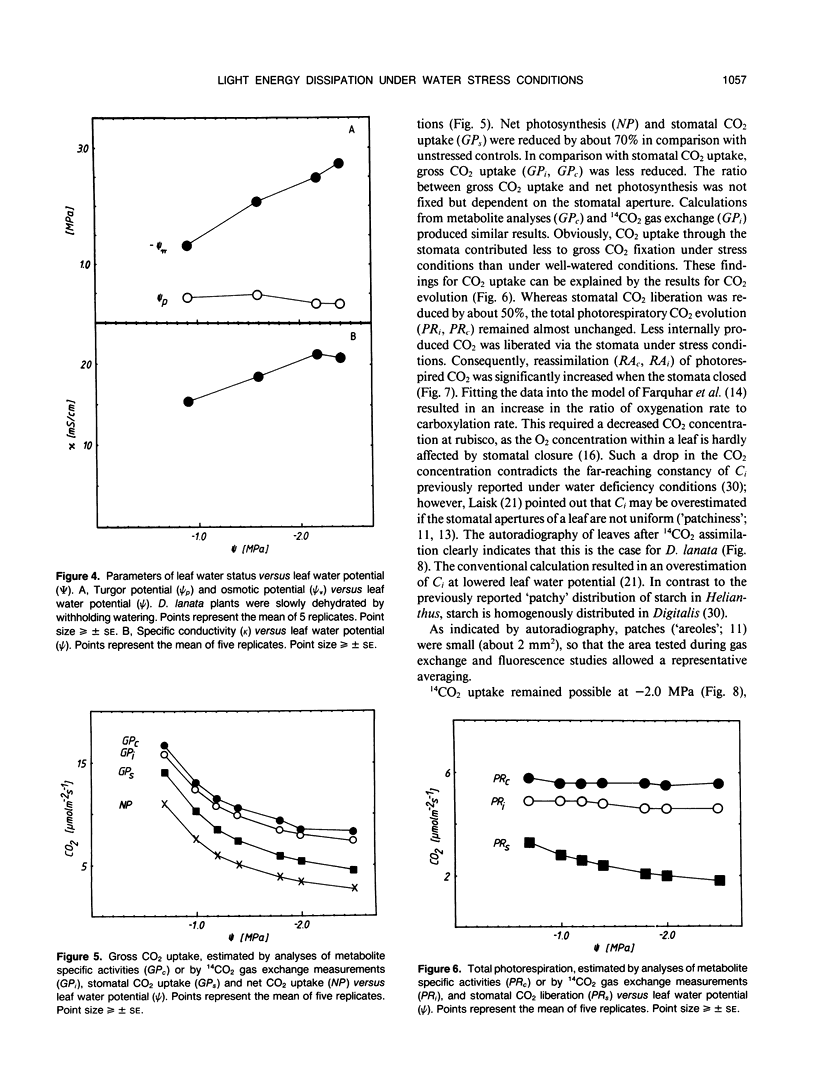
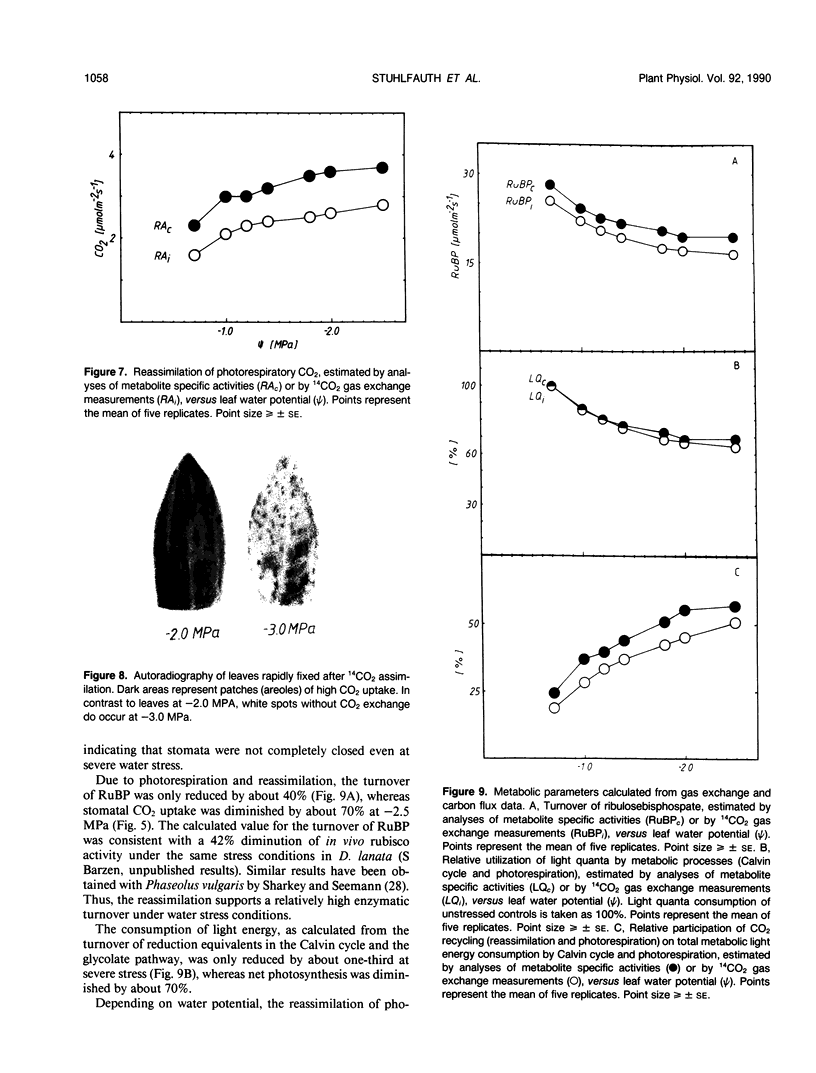
Abstract
Using 14CO2 gas exchange and metabolite analyses, stomatal as well as total internal CO2 uptake and evolution were estimated. Pulse modulated fluorescence was measured during induction and steady state of photosynthesis. Leaf water potential of Digitalis lanata EHRH. plants decreased to −2.5 megapascals after withholding irrigation. By osmotic adjustment, leaves remained turgid and fully exposed to irradiance even at severe water stress. Due to the stress-induced reduction of stomatal conductance, the stomatal CO2 exchange was drastically reduced, whereas the total CO2 uptake and evolution were less affected. Stomatal closure induced an increase in the reassimilation of internally evolved CO2. This `CO2 recycling' consumes a significant amount of light energy in the form of ATP and reducing equivalents. As a consequence, the metabolic demand for light energy is only reduced by about 40%, whereas net photosynthesis is diminished by about 70% under severe stress conditions. By CO2 recycling, carbon flux, enzymatic substrate turnover and consumption of light energy were maintained at high levels, which enabled the plant to recover rapidly after rewatering. In stressed D. lanata plants a variable fluorescence quenching mechanism, termed `coefficient of actinic light quenching,' was observed. Besides water conservation, light energy dissipation is essential and involves regulated metabolic variations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben G. Y., Osmond C. B., Sharkey T. D. Comparisons of Photosynthetic Responses of Xanthium strumarium and Helianthus annuus to Chronic and Acute Water Stress in Sun and Shade. Plant Physiol. 1987 Jun;84(2):476–482. doi: 10.1104/pp.84.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canvin D. T., Fock H. Measurement of photorespiration. Methods Enzymol. 1972;24:246–260. doi: 10.1016/0076-6879(72)24072-1. [DOI] [PubMed] [Google Scholar]
- Daley P. F., Raschke K., Ball J. T., Berry J. A. Topography of photosynthetic activity of leaves obtained from video images of chlorophyll fluorescence. Plant Physiol. 1989 Aug;90(4):1233–1238. doi: 10.1104/pp.90.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
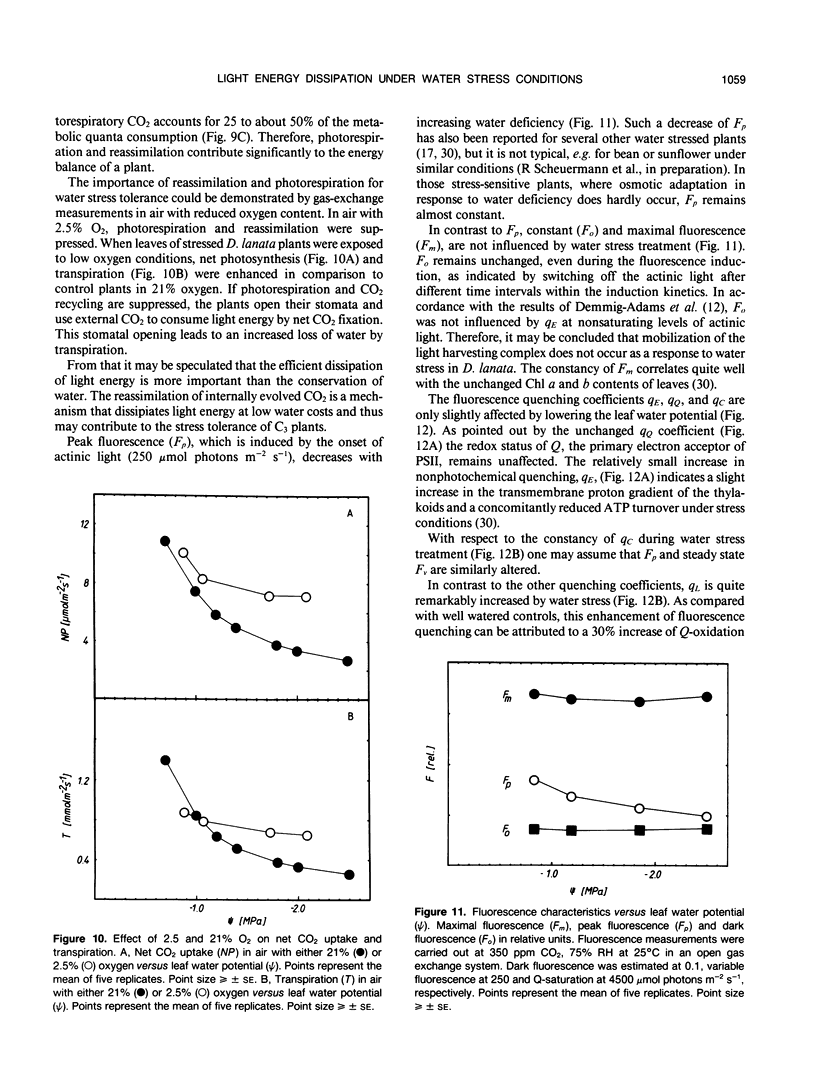
- Demmig-Adams B., Winter K., Krüger A., Czygan F. C. Light Response of CO(2) Assimilation, Dissipation of Excess Excitation Energy, and Zeaxanthin Content of Sun and Shade Leaves. Plant Physiol. 1989 Jul;90(3):881–886. doi: 10.1104/pp.90.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbaud A., Andre M. An evaluation of the recycling in measurements of photorespiration. Plant Physiol. 1987 Apr;83(4):933–937. doi: 10.1104/pp.83.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REINER J. M. The study of metabolic turnover rates by means of isotopic tracers. II. Turnover in a simple reaction system. Arch Biochem Biophys. 1953 Sep;46(1):80–99. doi: 10.1016/0003-9861(53)90171-4. [DOI] [PubMed] [Google Scholar]
- Sharkey T. D., Seemann J. R. Mild water stress effects on carbon-reduction-cycle intermediates, ribulose bisphosphate carboxylase activity, and spatial homogeneity of photosynthesis in intact leaves. Plant Physiol. 1989 Apr;89(4):1060–1065. doi: 10.1104/pp.89.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlfauth T., Sültemeyer D. F., Weinz S., Fock H. P. Fluorescence Quenching and Gas Exchange in a Water Stressed C(3) Plant, Digitalis lanata. Plant Physiol. 1988 Jan;86(1):246–250. doi: 10.1104/pp.86.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]



