Abstract
Purpose
To compare the efficacies of opioid-free anesthesia (OFA) and opioid-based anesthesia (OBA) in laparoscopic cholecystectomy (LC).
Patients and Methods
A total of 150 patients who underwent 3-port LC procedures were randomly divided into an OFA group with esketamine, dexmedetomidine and lidocaine intravenous combined with local anesthetic incision infiltration or an OBA group with remifentanil combined with local anesthetic incision infiltration. The primary outcome was the consumption of rescue analgesics within 24 hrs after surgery. Secondary outcomes included time to LMA removal, time to orientation recovery, time to unassisted walking, sleep quality on the night of surgery, time to first flatus, hemodynamics during induction of general anesthesia, postoperative pain level on the visual analog scale (VAS), incidence of postoperative nausea and vomiting (PONV) and global satisfaction score (GSS) within 24 hrs after surgery.
Results
Both the consumption of rescue analgesics and the time to first flatus in the OFA group were significantly lower than those in the OBA group (P < 0.001 and P = 0.029, respectively). However, the time to LMA removal and the time to orientation recovery were significantly longer in the OFA group than in the OBA group (P < 0.001). In addition, the VAS scores at 2 hrs and 8 hrs after surgery and HR at laryngeal mask airway insertion in the OFA group were significantly lower than those in the OBA group (P = 0.002 and P = 0.001, and P =0.016, respectively).
Conclusion
OFA may be beneficial for patients undergoing LC in that it could decrease the dosage of postoperative analgesics and pain intensity and even shorten the time to first flatus after surgery.
Keywords: opioid-free anesthesia, laparoscopic cholecystectomy, esketamine, dexmedetomidine
Introduction
At present, opioids have been most commonly used for the treatment of perioperative pain, and an increasing number of related studies have emphasized their association with side effects, opiate tolerance or withdrawal, and opioid-induced hyperalgesia.1 All these opioid-related adverse events can cause significant distress and interfere with postoperative recovery. They are also associated with greater postoperative pain and opioid consumption.2 Most specifically, pain and postoperative nausea and vomiting (PONV) are important contributors to readmission.3
Over the years, opioids have been deemed essential for intraoperative anesthesia. However, we are gradually realizing that opioids, such as remifentanil, may not be ideal pain relievers and may even have the opposite effect, thereby increasing postoperative pain. Additionally, research has also revealed that opioids are deficient in that they lead to poor postoperative outcomes during the perioperative period.4 Current research evidence suggests that OFA is not inferior to opioid-inclusive protocols and that OFA could improve perioperative outcomes. Most importantly, OFA has had good effects on perioperative outcomes.1
A systematic review revealed that various analgesic methods were recommended to relieve postoperative pain in laparoscopic cholecystectomy (LC) patients.5 Postoperative pain after LC may be one important reason for prolonged admissions or readmissions.3 Pain relief is essential as LC is gradually becoming a day surgery. A prospective cohort study demonstrated that the surgical opioid-avoidance protocol (SOAP) was as effective as the non-SOAP protocol in controlling daily maximum pain scores and that pain control was equivalent in patients of different races.6 Surgeons are striving to reduce the incidence of opioid overprescription.7 Anesthesiologists also have a responsibility to reduce unnecessary use of opioids during the perioperative period.
We hypothesized that an opioid-free anesthesia (OFA) protocol consisting of intravenous use of esketamine, dexmedetomidine, and lidocaine combined with local anesthetic incision infiltration as an opioid substitute would lead to lower postoperative pain scores, decreased use of rescue analgesics, and decreased incidence of opioid-related adverse events after LC.
Materials and Methods
Patients and Study Design
The study was conducted at the Third Affiliated Hospital of Anhui Medical University (The First People’s Hospital of Hefei) and approved by our Ethics Committee (No. 2021–48). We registered in the trial prospectively in the Chinese Clinical Trial Registry (ChiCTR1900022993, principal investigator: Jun-Ma Yu; registration: https://www.chictr.org.cn/showprojEN.html?proj=38738; date of registration: May 6, 2019), and written informed consent was provided from each patient before participation in the study. All research protocols were carried out in accordance with the Declaration of Helsinki.
Exclusion criteria: patients who were aged < 18 years or > 65 years; patients with a body mass index (BMI) ≥ 30 kg/m2; patients with hepatic or renal disease, coagulopathy, a history of alcohol or drug abuse, an American Society of Anesthesiologists (ASA) physical status ≥ III, or a basal heart rate (HR) ≤ 50 beats/min; patients who were pregnant; patients who had a past medical history of chronic pain; patients who should not take NSAIDs; patients with allergies to related medication; and patients with communication disorders.
Randomization and Blinding
Patients who underwent three-hole LC by an older surgeon were randomly divided into 2 groups by a computer-generated randomization table: the opioid-free anesthesia (OFA) group and the opioid-based anesthesia (OBA) group. An anesthesia nurse who was not involved in the study kept the group allocation and study number in sealed envelopes. Before the induction of general anesthesia, the anesthesia nurse opened the sealed opaque envelopes and prepared the experimental drugs according to the randomization sequence. Then, the anesthetists who performed perioperative anesthesia management administered the corresponding drugs. All the participants in the current study were blinded to the treatment group assignment.
Anesthesia
Electrocardiography (ECG), HR, blood oxygen saturation (SpO2), noninvasive blood pressure, respiratory rate (RR), and BIS value were routinely monitored in all patients. The participants in the OFA group received an infusion of dexmedetomidine 0.6 µg/kg at a constant rate for 10 min (the participants in the OBA group received an infusion of the same dose of normal saline). Then, anesthesia was induced with a fixed protocol of propofol 2–3 mg/kg, lidocaine 1.5 mg/kg (followed by intravenous infusion at 2 mg·kg−1·h−1 continuously but terminated when the gallbladder was extracted), and cisatracurium besilate 0.2 mg/kg. Moreover, anesthesia in the OBA group was induced with a fixed protocol of 2–3 mg/kg propofol, 1 µg/kg remifentanil (followed by continuous intravenous infusion of 0.1–0.3 µg·kg−1·min−1), and 0.2 mg/kg cisatracurium besilate. Then, in either group, a continuous intravenous infusion of 3–12 mg·kg−1·h−1 of propofol was administered, and the BIS value was maintained between 40 and 60. After 3 minutes of assisted breathing, a laryngeal mask airway (LMA) was lubricated with dyclonine hydrochloride mucilage and inserted for mechanical ventilation (followed by continuous inhalation of sevoflurane 1% with 100% O2 until gallbladder removal), and end-tidal CO2 was kept at 35–45 mmHg for all patients. Then, patients in the OFA group received a preincisional infiltration of 0.4 μg/kg dexmedetomidine mixed with 0.5% ropivacaine, but the OBA group received 1 μg/kg dexmedetomidine mixed with 0.5% ropivacaine, with a total volume of 30 mL.8 A single dose of 0.3 mg/kg esketamine was injected approximately 2 minutes before incision infiltration in the OFA group. Added injection dosages of muscle relaxants were 0.05 mg/kg cisatracurium besilate in 40th min for patients operating time might last for more than 1 hr. When the mean arterial pressure (MAP) was > 20% higher than the baseline value, 1 mg/kg propofol was administered intravenously, and esketamine 0.15 mg/kg was administered immediately if the above did not occur in the OFA group. An intravenous injection of ondansetron (4 mg) was administered after the beginning of surgery.
The intra-abdominal pressure of the CO2 pneumoperitoneum was maintained at 12 mmHg during laparoscopy. At the end of the operation, CO2 was expelled possibly through the open incision by manual compression of the abdomen. All patients received flurbiprofen axetil at 1.5 mg/kg for preventive analgesia 10 min before anesthesia induction.9
Postoperative Management
The LMA was removed through a standardized protocol (expired tidal volume of more than 6 mL/kg and respiration rate more than 12 beats/min) after surgery when all patients were transferred to the postanesthesia care unit (PACU). Then, patient-controlled intravenous analgesia (PCIA) composed of 10 mg butorphanol with a total volume of 100 mL was used as rescue analgesia within 24 hrs. A rescue analgesia dose of 2.5 mL was administered each time with an interval of 15 minutes, without any background infusion.
Outcomes
An anesthesiologist blinded to the treatment group assignment completed the remaining part of the study. The primary outcome was the use of rescue analgesic within 24 hrs after surgery recorded from the effective pressing frequency of the PCIA. The secondary outcomes included the postoperative pain level on the visual analog scale (VAS) at 2 hrs, 8 hrs, and 24 hrs after surgery, the time to LMA removal (time from the end of operation to when the patients could open their eyes), time to orientation recovery (time from the end of operation to when patients could follow instructions to time, place and person), time to unassisted walking (time from the end of operation to when patients could walk independently without assistance), sleep quality (patient awoke because of pain on the first postoperative night or not), time to first flatus, incidence of PONV and Global Satisfaction Score (GSS)9 within 24 hrs; and HR and MAP prior to intravenous administration (T0), LMA insertion (T1), pneumoperitoneum established (T2), and gallbladder extraction (T3).
Sample Size Estimation and Statistical Analyses
To calculate the sample size, the consumption of butorphanol in our pilot study as a rescue analgesic within 24 hrs after 3-port LC for the OBA group and OFA group within 24 hrs was 2.4 ± 0.2 mg and 2.2 ± 0.3 mg, respectively (n = 9 in each group). A sample size of 122 (61 patients per group) was needed to achieve 95% power and an α-error of 5% using PASS 15.0. To allow for potential dropouts, the sample size was increased by 20%, and 75 patients were included in each group.
SPSS 20.0 statistical analysis software was used for statistical analysis of the data. The measurement data are expressed as the mean ± SD or mean ± SEM. Nonnormally distributed data are represented by the median and interquartile range (IQR), and categorical variables are summarized as numbers and percentages. Parametric data were compared by analysis of variance and post hoc testing. Categorical variables were assessed using χ2 or Fisher’s exact test. Statistical significance was defined as a P value < 0.05.
Results
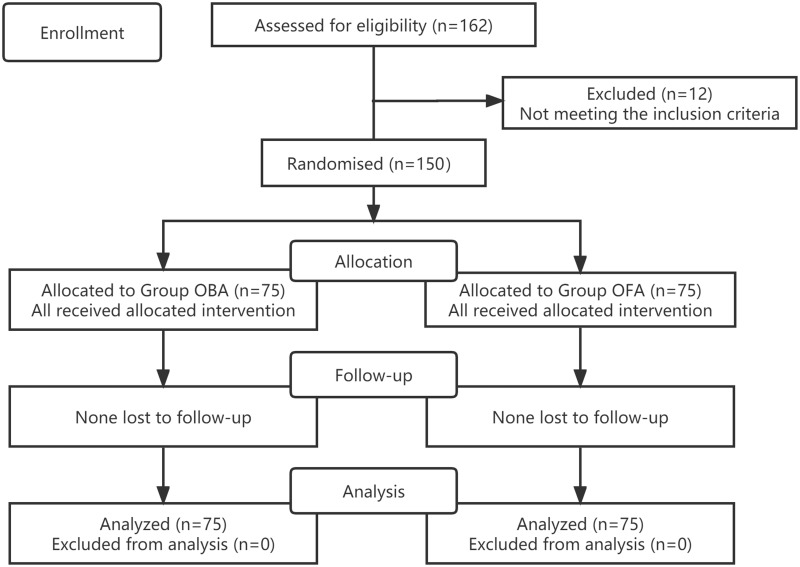
A total of 162 patients were recruited, but 12 of them were excluded for not meeting the inclusion criteria. One hundred and fifty patients were finally enrolled in the study (Figure 1). The clinical baseline data were similar for the two groups (Table 1).
Figure 1.
CONSORT flow diagram. OBA opioid-based anesthesia, OFA opioid-free anesthesia.
Notes: Adapted from Schulz KF, Altman DG, Moher D, CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010;7(3): e1000251. Copyright: © 2010 Schulz et al. Creative Commons Attribution License.10
Table 1.
Clinical Baseline Characteristics and Perioperative Data in Two Groups (n=75)
| OBA Group (n=75) | OFA Group (n=75) | P-value | |
|---|---|---|---|
| Age, years | 47.7±9.9 | 47.6±10.2 | 0.575 |
| Sex, n (%) | 0.736 | ||
| Male | 27 (36) | 29 (38.7) | |
| Female | 48 (64) | 46 (61.3) | |
| BMI, kg/m2 | 24.7±3.3 | 24.2±3.6 | 0.399 |
| ASA physical status, n (%) | 0.597 | ||
| I | 7 (9) | 9 (12) | |
| II | 68 (91) | 66 (88) | |
| Duration of surgery, min | 49.7±11.1 | 51.5±11.5 | 0.346 |
| Duration of anesthesia, min | 73.7±10.6 | 74.6±10.4 | 0.597 |
| Disease types, n (%) | 0.927 | ||
| Gallstones with cholecystitis | 57 (72) | 58 (72) | |
| Gallstone | 14 (22.7) | 14 (24) | |
| Gallbladder polyps | 4 (5.3) | 3 (4) |
Notes: Data are presented as mean (SD) or number (percentage).
Abbreviations: OBA, opioid-based anesthesia; OFA, opioid-free anesthesia; BMI, body mass index; ASA, American Society of Anesthesiologists.
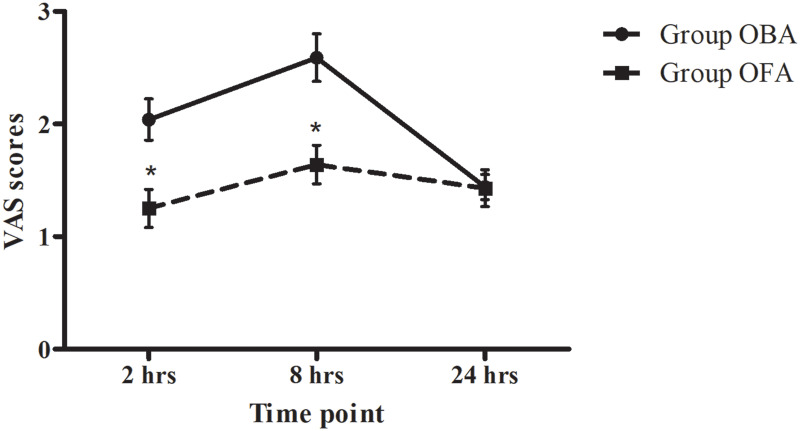
Both the use of rescue analgesics within 24 hrs and the time to first flatus after surgery were significantly lower in the OFA group than in the OBA group (P < 0.001 and P = 0.029, respectively). The VAS scores at 2 hrs and 8 hrs after surgery in the OFA group were significantly lower than those in the OBA group (P = 0.002 and P = 0.001, respectively, Figure 2). However, both the time to LMA removal and the time to orientation recovery were significantly longer in the OFA group than in the OBA group (P < 0.001, Table 2), but there was no difference in the time to unassisted walking between the two groups (P = 0.272). There was no significant difference in the time to waking, incidence of PONV or GSS between the two groups (Table 2).
Figure 2.
Pain scores at 2 hrs, 8 hrs, and 24 hrs after surgery. Data are given as the mean ± SEM; OBA opioid-based anesthesia, OFA opioid-free anesthesia, VAS visual analog scale from 0 to 10. *P < 0.05, OFA vs OBA at the same time point.
Table 2.
Observed Data for Perioperative Period in Two Groups (n=75)
| OBA Group (n=75) | OFA Group (n=75) | P-value | |
|---|---|---|---|
| Butorphanol (mg) | 2.5 (2.25–2.75) | 2.25 (1.75–2.5)* | < 0.001 |
| Time of LMA removed (min) | 6 (5–8) | 12 (10–14)* | < 0.001 |
| Recovery time of orientation (min) | 9 (7–10) | 27 (25–29)* | < 0.001 |
| Time to unassisted walking (h) | 3 (2–4) | 3 (2–5) | 0.272 |
| Waking up | 0.414 | ||
| Yes, n(%) | 9 (12) | 6 (8) | |
| No, n(%) | 66 (88) | 69 (92) | |
| Time to first flatus (h) | 18 (16–20) | 16 (13–20)* | 0.029 |
| Incidence of PONV, n (%) | 11 (14.7) | 10 (13.3) | 0.814 |
| GSS | 3 (3–4) | 3 (3–4) | 0.978 |
Notes: Data are in median (IQR), or number (proportion). *P < 0.05 vs OBA group.
Abbreviations: OBA, opioid-based anesthesia; OFA, opioid-free anesthesia; LMA, laryngeal mask airway; PONV, postoperative nausea and vomiting; GSS, global satisfaction score; IQR, interquartile range.
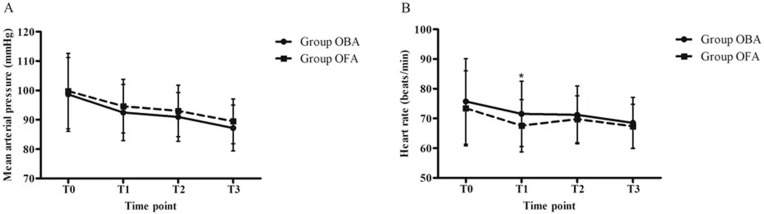
Hemodynamics were stable in the two groups (Figure 3A and B) except for HR, which was lower at T1 in the OFA group than in the OBA group (P =0.016, B in Figure 3).
Figure 3.
Hemodynamics during the perioperative period ((A) - mean arterial pressure, and (B) - heart rate). Data are given as the mean ± SD; T0 prior to intravenous administration, T1 LMA insertion, T2 pneumoperitoneum establishment, T3 gallbladder extraction. *P < 0.05, OFA vs OBA at the same time point.
No supplementary propofol or esketamine was administered.
Discussion
The present study shows that OFA provides satisfactory postoperative pain relief within 8 hrs after operation and reduces both the use of rescue analgesics within 24 hrs and the time to first flatus after surgery, with no obvious adverse reactions for patients undergoing LC compared to the OBA group. However, the time to LMA removal and the time to orientation recovery were slightly longer in the OFA group, but there was no difference in the time to unassisted walking between the two groups.
For moderate to severe pain, opioids remain the main treatment for intraoperative and postoperative analgesia.11 Short-acting remifentanil was recommended to be continuously infused to maintain general anesthesia and decrease the convalescence time.12 However, remifentanil-induced hyperalgesia (RIH) is an important adverse reaction in that it upregulates T-type calcium channel-dependent burst firing in glutamatergic neurons in the ventral posterolateral nucleus neurons to activate hindlimb primary somatosensory cortex glutamatergic neurons.13 Moreover, the use of opioids has increased the prevalence of drug abuse and addiction and the incidence of mortality. However, these opioids were mainly administered in healthcare facilities.11 Thus, the efficacy of OFA needs to be measured in different procedures, and the indications and contraindications need to be expanded.14 In this study, the dose of rescue analgesics, VAS scores within 8 hrs and time to first flatus after surgery were significantly lower in the OFA group than in the OBA group, possibly because remifentanil was not yet in use. Fletcher et al2 confirmed that high intraoperative doses of remifentanil may slightly increase pain intensity during the first postoperative 24 h. However, the dose of remifentanil was not recorded for the OBA group in our study, which could be a future research direction. In addition, the incidence of PONV, a common side effect of opioids, was not significantly different between the two groups, probably because of the use of ondansetron.
Dexmedetomidine has minimal adverse effects during the intraoperative period and can reduce surgical stress responses without compromising hemodynamic stability.15 Moreover, patients who received a lidocaine bolus, followed by continuous infusion, had higher patient satisfaction without evidence of significant side effects and had better recovery.16,17 In addition, studies revealed that dexmedetomidine had an intraoperative analgesic efficacy that was equal to that of fentanyl but was not as effective as remifentanil.18,19 Incisional pain after LC is usually more intense than both visceral and shoulder pain on the day of the operation, so incision pain relief should be the goal.20 In another study, pain was relieved by incisional infiltration of ropivacaine combined with dexmedetomidine.8 An incision infiltration of ropivacaine mixed with dexmedetomidine at the abdominal wall reduced both postoperative pain and analgesic requirements and enhanced both postoperative analgesic effects and sleep quality. In addition, patients treated with ropivacaine expressed more satisfaction than those treated with peripheral nerve blocks.8,9 All these findings were confirmed by the administration of no supplementary propofol or esketamine during the surgery process in the present study. Therefore, we used incisional infiltration of ropivacaine combined with dexmedetomidine as described above and obtained satisfactory results. To unify the dosage, a total dose of 1 µg/kg dexmedetomidine was used. Importantly, pretreatment with dexmedetomidine can induce some adverse reactions, such as bradycardia and hypotension;21 however, no relevant changes were observed except for a slightly decreased HR at T1 when compared to the OBA group in the present study.
Compared to ketamine, esketamine has a shorter recovery and orientation recovery time,22,23 thereby confirming its popularity in the clinic, and it was reported that both male and female Chinese patients may not require a dose adjustment, which presents potential clinical advantages.24 Esketamine could prevent RIH by antagonizing NMDA receptors without increasing the incidence of adverse reactions25 and even reducing the incidence of chronic or mild postoperative pain.26 It has also been used in OFA as an assistant drug in patients undergoing laparoscopic surgery in recent years.27–29 Moreover, in our study, the application of esketamine mainly alleviated pain from incision infiltration and achieved a better analgesic effect. However, as in previous studies, the present study found that the time to LMA removal and the time to orientation recovery were slightly longer, which may be because of the effect of esketamine alone or its combined effect with dexmedetomidine. However, there was no difference in the time to unassisted walking between the two groups, which would not delay the release from the hospital.
Of course, the present study has several weaknesses. First, similar to past studies, the present study did not compare any stress-related serological inflammatory factors despite showing favorable results. Second, we found that compared to the patients in the OBA group, more patients in the OFA group needed oral cavity suction after LMA removal; unfortunately, we did not count the volume of sputum. Third, butorphanol, a type of opioid, was used for postoperative management, which may have influenced our results. Fourth, more convenient analgesic methods should be used to replace PCIA in future studies, as LC is an ambulatory surgery in most hospitals, although patients are routinely hospitalized for up to 48 hours after surgery in our clinic. Finally, all surgical procedures were not performed by the same surgeon, which may have influenced the intensity of the patients’ pain during the perioperative period; however, all the older surgeons in our study were quite skilled in performing LC.
Conclusion
We achieved favorable results in the present study based on our OFA protocol. However, although removal of the LMA and the time to orientation recovery were slightly longer, we obtained satisfactory postoperative pain relief and reduced both the use of rescue analgesics and the time to first flatus. It is vital that there was no difference in the time to unassisted walking, one of the most important factors affecting the length of hospital stay for day surgery, between the two groups.
Acknowledgments
We would like to thank American Journal Experts (AJE) for English language editing. Jun-Ma Yu and Ye Zhang are cocorresponding authors.
Funding Statement
This work was supported by the Applied Medicine Research Project Fund of Hefei Health Committee (grant number: 2019-ZC-2) and the Research Project of Anhui Health Committee (grant number: 145).
Data Sharing Statement
All necessary data supporting our findings have been presented within the manuscript. The datasets used and/or analyzed during the current study are available for anyone who wishes to access them for reasonable request. The data will be accessible from the corresponding author.
Ethics Approval and Informed Consent
The authors declared that all research protocols were carried out in accordance with the Declaration of Helsinki. The study was conducted at the Third Affiliated Hospital of Anhui Medical University (The First People’s Hospital of Hefei) and approved by our Ethics Committee (No. 2021-48).
Consent for Publication
All the authors have read this article and agreed to publish it.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Adams TJ, Aljohani DM, Forget P. Perioperative opioids: a narrative review contextualising new avenues to improve prescribing. Br J Anaesth. 2023;130(6):709–718. doi: 10.1016/j.bja.2023.02.037 [DOI] [PubMed] [Google Scholar]
- 2.Fletcher D, Martinez V. Opioid-induced hyperalgesia in patients after surgery: a systematic review and a meta-analysis. Br J Anaesth. 2014;112(6):991–1004. doi: 10.1093/bja/aeu137 [DOI] [PubMed] [Google Scholar]
- 3.Rosero EB, Joshi GP. Hospital readmission after ambulatory laparoscopic cholecystectomy: incidence and predictors. J Surg Res. 2017;219:108–115. doi: 10.1016/j.jss.2017.05.071 [DOI] [PubMed] [Google Scholar]
- 4.Bugada D, Lorini LF, Lavand’homme P. Opioid free anesthesia: evidence for short and long-term outcome. Minerva Anestesiol. 2021;87(2):230–237. doi: 10.23736/S0375-9393.20.14515-2 [DOI] [PubMed] [Google Scholar]
- 5.Barazanchi AWH, MacFater WS, Rahiri JL, et al. Evidence-based management of pain after laparoscopic cholecystectomy: a PROSPECT review update. Br J Anaesth. 2018;121(4):787–803. doi: 10.1016/j.bja.2018.06.023 [DOI] [PubMed] [Google Scholar]
- 6.Votta-Velis G, Daviglus ML, Borgeat A, et al. Surgical opioid-avoidance protocol: a postoperative pharmacological multimodal analgesic intervention in diverse patient populations. Reg Anesth Pain Med;2023. rapm-2022–103864. doi: 10.1136/rapm-2022-103864 [DOI] [PubMed] [Google Scholar]
- 7.Melucci AD, Dave YA, Lynch OF, et al. Predictors of opioid-free discharge after laparoscopic cholecystectomy. Am J Surg. 2023;225(1):206–211. doi: 10.1016/j.amjsurg.2022.07.027 [DOI] [PubMed] [Google Scholar]
- 8.Yu JM, Sun H, Wu C, Dong CS, Lu Y, Zhang Y. The Analgesic Effect of Ropivacaine Combined With Dexmedetomidine for Incision Infiltration After Laparoscopic Cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2016;26(6):449–454. doi: 10.1097/SLE.0000000000000325 [DOI] [PubMed] [Google Scholar]
- 9.Wu L, Wu L, Sun H, Dong C, Yu J. Effect of ultrasound-guided peripheral nerve blocks of the abdominal wall on pain relief after laparoscopic cholecystectomy. J Pain Res. 2019;12:1433–1439. doi: 10.2147/JPR.S203721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulz KF, Altman DG, Moher D. CONSORT Statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7(3):e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kharasch ED, Brunt LM. Perioperative Opioids and Public Health. Anesthesiology. 2016;124(4):960–965. doi: 10.1097/ALN.0000000000001012 [DOI] [PubMed] [Google Scholar]
- 12.Horosz B, Nawrocka K, Malec-Milewska M. Anaesthetic perioperative management according to the ERAS protocol. Anaesthesiol Intensive Ther. 2016;48(1):49–54. doi: 10.5603/AIT.2016.0006 [DOI] [PubMed] [Google Scholar]
- 13.Jin Y, Mao Y, Chen D, et al. Thalamocortical circuits drive remifentanil-induced postoperative hyperalgesia. J Clin Invest. 2022;132(24):e158742. doi: 10.1172/JCI158742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavand’homme P, Estebe JP. Opioid-free anesthesia: a different regard to anesthesia practice. Curr Opin Anaesthesiol. 2018;31(5):556–561. doi: 10.1097/ACO.0000000000000632 [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Wang B, Zhang LL, et al. Dexmedetomidine Combined with General Anesthesia Provides Similar Intraoperative Stress Response Reduction When Compared with a Combined General and Epidural Anesthetic Technique. Anesth Analg. 2016;122(4):1202–1210. doi: 10.1213/ANE.0000000000001165 [DOI] [PubMed] [Google Scholar]
- 16.Kim DH, Park JY, Yu J, et al. Intravenous Lidocaine for the Prevention of Postoperative Catheter-Related Bladder Discomfort in Male Patients Undergoing Transurethral Resection of Bladder Tumors: a Randomized, Double-Blind, Controlled Trial. Anesth Analg. 2020;131(1):220–227. doi: 10.1213/ANE.0000000000004405 [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Sun J, Zhang X, Wang G. The Effect of Lidocaine on Postoperative Quality of Recovery and Lung Protection of Patients Undergoing Thoracoscopic Radical Resection of Lung Cancer. Drug Des Devel Ther. 2021;15:1485–1493. doi: 10.2147/DDDT.S297642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jebaraj B, Ramachandran R, Rewari V, et al. Feasibility of dexmedetomidine as sole analgesic agent during robotic urological surgery: a pilot study. J Anaesthesiol Clin Pharmacol. 2017;33(2):187–192. doi: 10.4103/0970-9185.209753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortinez LI, Hsu YW, Sum-Ping ST, et al. Dexmedetomidine pharmacodynamics: part II: crossover comparison of the analgesic effect of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101(5):1077–1083. doi: 10.1097/00000542-200411000-00006 [DOI] [PubMed] [Google Scholar]
- 20.Bisgaard T, Klarskov B, Rosenberg J, Kehlet H. Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain. 2001;90(3):261–269. doi: 10.1016/S0304-3959(00)00406-1 [DOI] [PubMed] [Google Scholar]
- 21.Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta-analysis. Intensive Care Med. 2010;36(6):926–939. doi: 10.1007/s00134-010-1877-6 [DOI] [PubMed] [Google Scholar]
- 22.Engelhardt W, Stahl K, Marouche A, Hartung E. Recovery time after (S)-ketamine or ketamine racemate. Recovery time after short anesthesia in volunteers. Anaesthesist. 1998;47(3):184–192. doi: 10.1007/s001010050546 [DOI] [PubMed] [Google Scholar]
- 23.Pfenninger EG, Durieux ME, Himmelseher S. Cognitive impairment after small-dose ketamine isomers in comparison to equianalgesic racemic ketamine in human volunteers. Anesthesiology. 2002;96(2):357–366. doi: 10.1097/00000542-200202000-00022 [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Huang J, Yang S, et al. Pharmacokinetics and Safety of Esketamine in Chinese Patients Undergoing Painless Gastroscopy in Comparison with Ketamine: a Randomized, Open-Label Clinical Study. Drug Des Devel Ther. 2019;13:4135–4144. doi: 10.2147/DDDT.S224553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren YL, Yuan JJ, Xing F, Zhu LN, Zhang W. Effects of Different Doses of Esketamine on Pain Sensitivity of Patients Undergoing Thyroidectomy: a Randomized Controlled Trial. Pain Ther. 2023;12(3):739–750. doi: 10.1007/s40122-023-00488-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan H, Chen W, Chen Y, et al. Opioid-Free Versus Opioid-Based Anesthesia on Postoperative Pain After Thoracoscopic Surgery: the Use of Intravenous and Epidural Esketamine. Anesth Analg. 2023;137(2):399–408. doi: 10.1213/ANE.0000000000006547 [DOI] [PubMed] [Google Scholar]
- 27.Massoth C, Schwellenbach J, Saadat-Gilani K, et al. Impact of opioid-free anaesthesia on postoperative nausea, vomiting and pain after gynaecological laparoscopy - A randomised controlled trial. J Clin Anesth. 2021;75:110437. doi: 10.1016/j.jclinane.2021.110437 [DOI] [PubMed] [Google Scholar]
- 28.Chen HY, Meng XY, Gao H, et al. Esketamine-based opioid-free anaesthesia alleviates postoperative nausea and vomiting in patients who underwent laparoscopic surgery: study protocol for a randomized, double-blinded, multicentre trial. Trials. 2023;24(1):13. doi: 10.1186/s13063-022-07003-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, He W, Liu X, Lv F, Li Y. Application of opioid-free general anesthesia for gynecological laparoscopic surgery under ERAS protocol: a non-inferiority randomized controlled trial. BMC Anesthesiol. 2023;23(1):34. doi: 10.1186/s12871-023-01994-5 [DOI] [PMC free article] [PubMed] [Google Scholar]