Abstract
Dechlorination of tetrachloroethene, also known as perchloroethylene (PCE), was investigated in an upflow anaerobic sludge blanket (UASB) reactor after incorporation of the strictly anaerobic, reductively dechlorinating bacterium Dehalospirillum multivorans into granular sludge. This reactor was compared to the reference 1 (R1) reactor, where the granules were autoclaved to remove all dechlorinating abilities before inoculation, and to the reference 2 (R2) reactor, containing only living granular sludge. All three reactors were fed mineral medium containing 3 to 57 μM PCE, 2 mM formate, and 0.5 mM acetate and were operated under sterile conditions. In the test reactor, an average of 93% (mole/mole) of the effluent chloroethenes was dichloroethene (DCE), compared to 99% (mole/mole) in the R1 reactor. The R2 reactor, with no inoculation, produced only trichloroethene (TCE), averaging 43% (mole/mole) of the effluent chloroethenes. No dechlorination of PCE was observed in an abiotic control consisting of sterile granules without inoculum. During continuous operation with stepwise-reduced hydraulic retention times (HRTs), both the test reactor and the R1 reactor showed conversion of PCE to DCE, even at HRTs much lower than the reciprocal maximum specific growth rate of D. multivorans, indicating that this bacterium was immobilized in the living and autoclaved granular sludge. In contrast, the R2 reactor, with no inoculation of D. multivorans, only converted PCE to TCE under the same conditions. Immobilization could be confirmed by using fluorescein-labeled antibody probes raised against D. multivorans. In granules obtained from the R1 reactor, D. multivorans grew mainly in microcolonies located in the centers of the granules, while in the test reactor, the bacterium mainly covered the surfaces of granules.
Tetrachloroethene, also known as perchloroethylene (PCE), has commonly been used as a degreasing agent for metals and as a solvent for dry cleaning. Due to improper storage and disposal, significant amounts of PCE are spread throughout the environment worldwide. Microbial transformation of PCE has been demonstrated exclusively under anaerobic conditions by the mechanism of reductive dechlorination (13). Reductive dechlorination of PCE has been observed in anaerobic mixed and enrichment cultures (5, 6, 8). Recently, the anaerobic bacteria Dehalospirillum multivorans, “Dehalobacter restrictus,” and Desulfitobacterium sp. strain PCE1, which perform reductive dechlorination of PCE (9, 10, 13), have been isolated.
Manipulation of the biomass can be essential in technical systems for optimal degradation of xenobiotic compounds. In earlier studies, we have shown that de novo activity can be introduced into granular sludge by use of an anaerobic bacterium actively degrading the target compound; Desulfomonile tiedjei, a 3-chlorobenzoate-dechlorinating bacterium, was inoculated into the granular sludge of an upflow anaerobic sludge blanket (UASB) reactor, resulting in 3-chlorobenzoate-degrading capability of the sludge, which previously did not have this capability (1). It was further verified that the bacterium was immobilized in the granular sludge layer, where it expressed its dechlorinating activity. By the use of immunofluorescence microscopy on slices of granules, it was shown that the bacteria made microcolonies in the granular sludge. In a further study, we introduced the pentachlorophenol-dechlorinating bacterium Desulfitobacterium hafniense into sterile granular sludge (2, 3). This bacterium was found to be immobilized in a net-like structure inside the granules, where it expressed its ortho-dechlorinating pathway.
The purpose of the present study was to investigate dechlorination of PCE in UASB reactors with and without addition of the PCE-degrading bacterium D. multivorans and further to compare the performance depending on whether the granules were active or sterile.
MATERIALS AND METHODS
Reactor design.
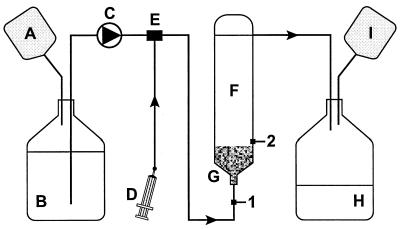
Three glass UASB reactors with an active volume of 204 ml and 31 ml of headspace were used for the experiments (Fig. 1). Viton, stainless steel, and glass tubing were used for connections to minimize chloroethene adsorption and evaporation. The reactor systems were checked for abiotic loss of chloroethenes before addition of granules to the reactors. The reactors were operated continuously, with a hydraulic retention time (HRT) decreasing stepwise from 2 days (flow rate, 100 ml/day) to 14.4, 7.2, and finally 1.9 h. PCE was supplied by continuously injecting a 780 μM aqueous PCE solution into the mixing vessel, from which it flowed into the reactor. Samples were taken from sample ports 1 and 2, representing the reactor influent and effluent, respectively. The samples were analyzed for chloroethenes, formate, and acetate. All sample ports were closed with Teflon-lined butyl septa. The reactors were operated at room temperature (22 to 25°C) and kept in the dark.
FIG. 1.
Reactor setup. A and I, gas bags; B, medium bottle; C, pump; D, PCE injection syringe; E, stirred mixing vessel; F, UASB reactor; G, granular sludge blanket; H, waste bottle; 1 and 2, sample ports 1 and 2.
Source of inoculum.
The granular sludge was taken from a lab scale UASB reactor fed PCE and ethanol for 1 year (4). Originally, the granules came from a full-scale UASB reactor treating paper mill effluent. The granules were stored at 4°C for approximately 2 months prior to use. The test and reference 2 (R2) reactors were filled with 20 ml of wet granules, gassed with N2-CO2 (4:1), and filled with anaerobic medium. The reference 1 (R1) reactor was also filled with 20 ml of wet granules but was autoclaved three times at 140°C before being gassed with sterile N2-CO2 (4:1) and filled with anaerobic medium. The test and R1 reactors, containing living and autoclaved granules, respectively, were further inoculated with 50 ml of a pure culture of D. multivorans, kindly provided by H. Scholz-Muramatsu (Institute for Sanitary Engineering, Department of Biology, University of Stuttgart, Stuttgart, Germany) and allowed to acclimate for 3 days before feeding of the reactors was initiated.
As an abiotic control, a 118-ml serum vial was filled with 5 ml of wet granules and 50 ml of anaerobic medium. The bottle was gassed (with N2-CO2 [4:1]), autoclaved three times, and supplemented with formate and acetate to final concentrations equivalent to those in the reactor system. PCE was added as an aqueous sterile solution. The batch was tested for dechlorination activity every 4 to 5 days during the experimental period.
Sterility check.
The sterility of the autoclaved reactor inoculated with D. multivorans was checked daily, and that of the sterile control bottle was checked every 4 to 5 days, by light-microscopic inspection of a liquid sample.
Medium.
A basal medium was prepared as previously described (12), except that 0.5 mg of resazurin/liter, 0.48 g of Na2S · 7H2O to 9H2O/liter, 0.5 mM acetate, and 2 mM formate were added from anaerobic stock solutions. The final pH of the medium ranged from 7.0 to 7.5.
Immunological methods.
Granules were sampled from each reactor after reactors were operated at an HRT of 1.9 h. The granules and inoculum were fixed in 4% formalin, embedded in paraffin, cut into 5-μm slices, and placed on glass slides. Paraffin was removed with xylene, and the granules were hydrated in decreasing concentrations of ethanol. Polyclonal rabbit antiserum against D. multivorans labeled with FLUOS (5-6-carboxyfluorescein-N-hydroxysuccinimide ester) was obtained from the Institute for Sanitary Engineering, Germany. The hydrated granules were incubated with normal rabbit serum, washed with bovine serum albumin in phosphate-buffered saline (pH 7.6), and incubated with the antiserum for 25 min. As a control, granules were prepared without antiserum incubation. The slides were investigated by immunofluorescence microscopy.
Analytical methods.
PCE, trichloroethene (TCE), and dichloroethene (DCE) were measured with a mass spectrometer (MS QMG 421-1; Balzers, Liechtenstein) for membrane inlet mass spectrometry (MIMS) (11). PCE, TCE, and DCE were analyzed at mass-to-charge ratios (m/z) of 165.8, 129.9, and 61.0 atomic mass units, respectively. The detection limit for the MIMS is <1 μg/liter (11a). The range of concentrations tested was 1 to 500 μM for PCE, TCE, and DCE, corresponding to the range of the standards. Standards were prepared by weighing exact amounts of chloroethenes into double-distilled water, stirring overnight for complete mixing, and diluting into serum bottles. The liquid phase was then measured. In order to detect unknowns, reactor liquid was pumped from the reactor through the MIMS and discarded, by using viton tubing. The same flow rate was used as that for measuring the standards (60 ml/h).
Formate was analyzed by high-pressure liquid chromatography (Lambda Max 410; Waters). An acidified sample (20 μl) was injected into an Aminex HPX-87-H column heated to 60°C. The mobile phase was 0.01 N sulfuric acid with a flow rate of 0.7 ml/min. Formate was detected by UV detection at 190 nm. Standards were tested in a range from 0.1 to 10 mM formate. The detection limit was 0.05 mM. Samples and standards were acidified to below pH 2 with 20 μl of sulfuric acid (10%) and were centrifuged. The supernatant was filtered (45-μm-pore-size filter; Millipore) and injected. Acetate was measured by gas chromatography as previously described (14).
The stereoisomeric composition of DCE was determined by gas chromatography. Chloroethenes were extracted by the addition of 150 μl of pentane containing 4.2 mg of bromotrichloromethane/liter as the internal standard for a 1.5-ml sample. Three microliters of the pentane phase was then injected into a gas chromatograph (HRGC 5300 Mega series; Carlo Erba Instruments, Milan, Italy) connected to an electron capture detector. The detection limit was 500 μg of cis-1,2-DCE/liter. Standards were prepared in the range from 500 to 2,540 μg/liter. Unknowns were determined by using a liquid sample from the reactor known not to contain any DCE.
RESULTS
Reactor studies.
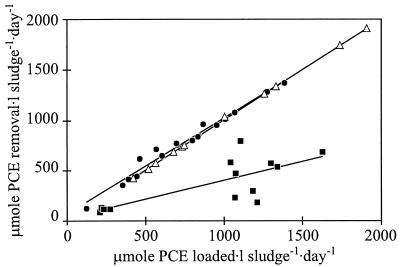
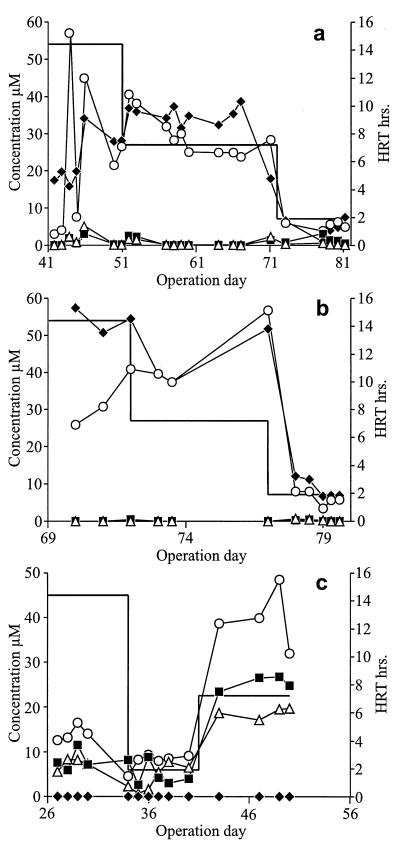
The test reactor inoculated with D. multivorans transformed an average of 96% of the incoming PCE, up to a PCE loading rate of 1.4 mmol · liter of sludge−1 · day−1 (Fig. 2). The main product of the reactor was DCE, averaging 93% (mole/mole) of effluent chloroethenes (Fig. 3a). DCE effluent concentrations sometimes exceeded the PCE influent concentration. Only small amounts of PCE and TCE (<5 μM) could occasionally be detected in the effluent. Samples taken at day 61 showed that the stereoisomeric composition was 100% cis-1,2-DCE. The conversion of PCE to DCE was almost equimolar at different HRTs. However, when the HRT was reduced from 7.2 to 1.9 h, the PCE effluent concentration increased to a maximum of 25% (mole/mole) of the sum of chloroethenes in the effluent. Nine days after the HRT was reduced to 1.9 h, PCE dropped to less than 7% (mole/mole) of the sum of chloroethenes.
FIG. 2.
PCE removal rates versus PCE loading in the UASB reactors studied. The slopes of the straight lines represent the average percentages of PCE removal. •, test reactor (slope, 0.96); ▵, R1 reactor (slope, 1.0); ▪, R2 reactor (slope, 0.4).
FIG. 3.
Dechlorination performance of the reactors at decreasing HRTs. (a) Test reactor; (b) R1 reactor; (c) R2 reactor. Open circles, influent PCE; solid squares, effluent PCE; open triangles, effluent TCE; solid diamonds, effluent DCE; solid line, HRT.
The R2 reactor, containing only granular sludge, showed PCE dechlorination activity with TCE as the only product (Fig. 2 and 3c). TCE accounted on average for 43% (mole/mole) of chloroethenes in the effluent; the rest was PCE. No DCE could be detected at any time.
The R1 reactor, containing autoclaved granules inoculated with D. multivorans, was able to transform an average of 100% of the incoming PCE, up to a PCE loading rate of 1.9 mmol · liter of sludge−1 · day−1 (Fig. 2). During continuous operation, DCE was the main chloroethene found at sample port 2 (Fig. 3b), representing an average of 99% (mole/mole) of the sum of chloroethenes in the effluent. PCE and TCE were detected only in trace amounts (<1 μM) in the effluent on the first 2 days after the HRT had been reduced from 7.2 to 1.9 h. DCE in the effluent averaged 150% of the PCE influent concentration.
Abiotic control experiments with the reactors were carried out before start-up of the experiment and showed that the PCE concentrations at sample ports 1 and 2 deviated within the accuracy of the MIMS measurement (standard deviation, 5%). This excluded a measurable abiotic loss of PCE in the two reactor systems. In the abiotic control with autoclaved granular sludge, addition of 500 μM PCE did not result in any TCE or DCE production. The PCE concentrations were fluctuating but tended to decrease with time, corresponding to the decrease of the total PCE amount through sampling. Sterility checks by light-microscopic inspection of liquid samples from the sterile inoculated reactor and the sterile control bottle did not reveal any bacteria except D. multivorans at any time.
Acetate consumption by the test and R2 reactors averaged 3.4, 6.7, and 27.0 mmol · day−1 · liter of sludge−1 at HRTs of 14.4, 7.2, and 1.9 h, respectively. No acetate consumption or production was observed with the R1 reactor. Formate was completely consumed by the test and R2 reactors, equaling an average uptake of 21.8, 43.7, and 175.5 mmol · day−1 · liter of sludge−1 at HRTs of 14.4, 7.2, and 1.9 h, whereas in the R1 reactor only 8.4, 16.8, and 67.2 mmol · day−1 · liter of sludge−1 at HRTs of 14.4, 7.2, and 1.9 h were consumed, respectively, resulting in an effluent concentration of 0.8 mM.
Immunological studies.
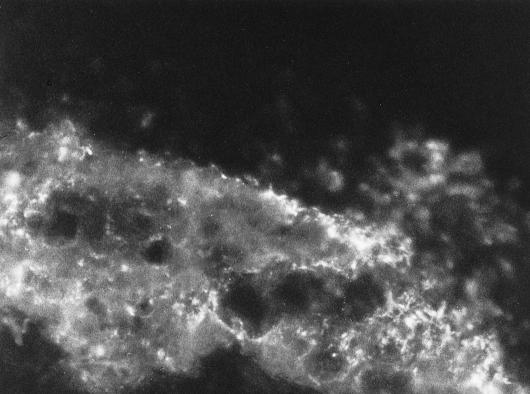
The placement of D. multivorans within the granules of the reactors was investigated by immunofluorescence microscopy. Granules derived from the test reactor were typically densely covered with D. multivorans on the surface in a net-like structure (Fig. 4). Aggregations at surfaces occurred but were scarce. At the surfaces of some granules prepared from the R2 reactor and of the original granules used for the experiment, single cells which were fluorescing brightly were seen (data not shown). However, many of the slides from both sources showed no fluorescing signals but looked like the control slide (data not shown).
FIG. 4.
Net-like growth of D. multivorans on the surface of a granule from the reactor inoculated with D. multivorans.
In granules from the R1 reactor, D. multivorans was found to grow in microcolonies mainly located in the central regions of the granules. Microcolonies also appeared at the edges of the granules (data not shown). A control slide prepared from the unsterile inoculated reactor without antiserum coupling did not give any fluorescing signal but showed a homogeneous dark color (data not shown).
DISCUSSION
In medium containing PCE, formate, and acetate, the reciprocal maximum specific growth rate of D. multivorans was approximately 10 h at 20°C (7). Operating the reactors at an HRT lower than the reciprocal maximum specific growth rate, therefore, will lead to washout of free-living cells of D. multivorans. Both the test and R1 reactors dechlorinated PCE to DCE, even at an HRT much lower than the reciprocal maximum specific growth rate of D. multivorans. The living granular sludge in the R2 reactor was also able to dechlorinate PCE; however, the PCE removal rates were more than 2 times lower than in the test reactor at identical HRTs. Furthermore, PCE was dechlorinated only to TCE in the R2 reactor, and DCE was never detected. No abiotic loss was observed in the test reactor, the R2 reactor, or the abiotic control of the granules, and there was a mass balance of the chlorinated ethenes over the reactor system that in general was within the standard deviation of the analytical apparatus. These results indicate that D. multivorans was immobilized in the test and R1 reactors and was responsible for the enhanced dechlorination activity compared to that in the R2 reactor.
The immobilization of D. multivorans in reactor R1 was verified by immunofluorescence microscopy. D. multivorans grew in dense microcolony-like structures inside the autoclaved granules derived from the reactor operating at an HRT of 1.9 h (data not shown). Microscopic investigation of the immunolabeled cuts of the granular inoculum and of granules from the R2 reactor revealed a few sporadic cells at granular surfaces detected with the antibody for D. multivorans. The cells detected are not likely to be the dechlorinating strain of D. multivorans, since this bacterium would be expected to show quick proliferation, resulting in a large population, as seen in the test and R1 reactors. This was the case in an earlier experiment where a reactor similar to the R2 reactor accidentally became contaminated by D. multivorans. The bacterium spread throughout the granular sludge within days (data not shown).
PCE removal increased linearly with increasing PCE loading of the test and R1 reactors, both inoculated with D. multivorans, indicating that no inhibitory effects were seen at any PCE loading rate tested. The slightly lower PCE removal rate of the test reactor was further seen as a slower adaptation capability when the HRT was lowered. This resulted in a temporary appearance of PCE and TCE in the effluent. In contrast, the dechlorinating performance of the R1 reactor did not change with changes in the HRT. This effect could be due to D. multivorans being present in lower cell numbers per unit of granules in the test reactor than in the R1 reactor, making the unsterile inoculated system more sensitive to changes in HRT.
A total consumption of the formate added was seen for the R2 reactor, compared to only 39% consumption of the formate added to reactor R1. This indicates that formate was used by bacteria other than D. multivorans in the unsterile granular sludge. Formate was never detected in the effluent of the test reactor, and a lack of this electron donor is possible in this reactor. PCE dechlorination is an energy-conserving process for D. multivorans, and therefore the lack of the electron donor might shorten the energy gain and result in a lower cell number than that observed when the formate supply is sufficient.
The placement of the introduced bacteria observed in this study is in contrast to that in previous studies. Immobilization of the dechlorinating bacterium D. hafniense in sterile granules resulted in a net-like uniform growth of the organism, while immobilization of D. tiedjei in unsterile sludge resulted in the formation of microcolonies (1, 3). These results show that the placement of the introduced bacteria is controlled by individual factors within the specific system. Since the conditions for D. multivorans, for instance, regarding exposure to formate, in sterile granules will be different from those in active granules, differences in placement can be expected.
In the present study, we showed for the first time that D. multivorans could be immobilized in granular sludge, where it enhanced the PCE dechlorination activity significantly. Furthermore, this incorporation of D. multivorans resulted in a change in product formation towards a compound (DCE) which is much more amenable to further conversion in aerobic aftertreatment than the original product (TCE). These results are of considerable significance to the bioremediation of PCE-contaminated aquifers.
ACKNOWLEDGMENTS
We thank Jacob Rasmussen for technical assistance.
This research was supported by grants from the Commission of the European Communities, contract EV5V-CT92-0239 (BIODEC project), and the Danish Technical Science Council (no. 9502657-28813).
REFERENCES
- 1.Ahring B K, Christiansen N, Mathrani I, Hendriksen H V, Macario A J L, Conway de Macario E. Introduction of a de novo bioremediation ability, aryl reductive dechlorination, into anaerobic granular sludge by inoculation of sludge with Desulfomonile tiedjei. Appl Environ Microbiol. 1992;58:3677–3682. doi: 10.1128/aem.58.11.3677-3682.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christiansen N, Ahring B K. Desulfitobacterium hafniense sp. nov., an anaerobic, reductively dechlorinating bacterium. Int J Syst Bacteriol. 1996;46:442–448. [Google Scholar]
- 3.Christiansen N, Ahring B K. Introduction of a de novo bioremediation activity into anaerobic granular sludge using the dechlorinating bacterium DCB-2. Antonie Leeuwenhoek. 1996;69:61–66. doi: 10.1007/BF00641612. [DOI] [PubMed] [Google Scholar]
- 4.Christiansen N, Christensen S R, Arvin E, Ahring B K. Transformation of tetrachloroethene in an upflow anaerobic sludge blanket reactor. Appl Microbiol Biotechnol. 1996;47:91–94. [Google Scholar]
- 5.de Bruin W P, Kotterman M J J, Posthumus M A, Schraa G, Zehnder A J B. Complete biological reductive transformation of tetrachloroethene to ethane. Appl Environ Microbiol. 1992;58:1996–2000. doi: 10.1128/aem.58.6.1996-2000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiStefano T D, Gossett J M, Zinder S H. Reductive dechlorination of high concentrations of tetrachloroethene to ethene by an anaerobic enrichment culture in the absence of methanogenesis. Appl Environ Microbiol. 1991;57:2287–2292. doi: 10.1128/aem.57.8.2287-2292.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenbeis, M. (Institute for Sanitary Engineering, Department of Biology, University of Stuttgart, Stuttgart, Germany). Personal communication.
- 8.Freedman D L, Gossett J M. Biological reductive dechlorination of tetrachloroethylene and trichloroethylene to ethylene under methanogenic conditions. Appl Environ Microbiol. 1989;55:2144–2151. doi: 10.1128/aem.55.9.2144-2151.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerritse J, Renard V, Pedro Gomes T M, Lawson P A, Collins M D, Gottschalk J C. Desulfitobacterium sp. strain PCE1, an anaerobic bacterium that can grow by reductive dechlorination of tetrachloroethene or ortho-chlorinated phenols. Arch Microbiol. 1996;165:132–140. doi: 10.1007/s002030050308. [DOI] [PubMed] [Google Scholar]
- 10.Holliger C, Schumacher W. Reductive dehalogenation as a respiratory process. Antonie Leeuwenhoek. 1994;66:239–246. doi: 10.1007/BF00871642. [DOI] [PubMed] [Google Scholar]
- 11.Lauritsen F R, Gylling S. On-line monitoring of biological reactions at low parts-per-trillion levels by membrane inlet mass spectrometry. Anal Chem. 1995;67:1418–1420. [Google Scholar]
- 11a.Lauritsen F R, Lloyd D. Direct detection of volatile metabolites produced by microorganisms: membrane inlet mass spectrometry. Am Chem Soc Symp Ser. 1994;541:91–106. [Google Scholar]
- 12.Neumann A, Scholz-Muramatsu H, Diekert G. Tetrachloroethene metabolism of Dehalospirillum multivorans. Arch Microbiol. 1994;162:295–301. doi: 10.1007/BF00301854. [DOI] [PubMed] [Google Scholar]
- 13.Scholz-Muramatsu H, Neumann A, Meßmer M, Moore E, Diekert G. Isolation and characterization of Dehalospirillum multivorans gen. nov., sp. nov., a tetrachloroethene-utilizing, strictly anaerobic bacterium. Arch Microbiol. 1995;163:48–56. [Google Scholar]
- 14.Sørensen A H, Winther-Nielsen M, Ahring B K. Kinetics of lactate, acetate and propionate in unadapted and lactate-adapted thermophilic, anaerobic sewage sludge: the influence of sludge adaption for start-up of thermophilic UASB-reactors. Appl Environ Biotechnol. 1991;34:823–827. [Google Scholar]