Abstract
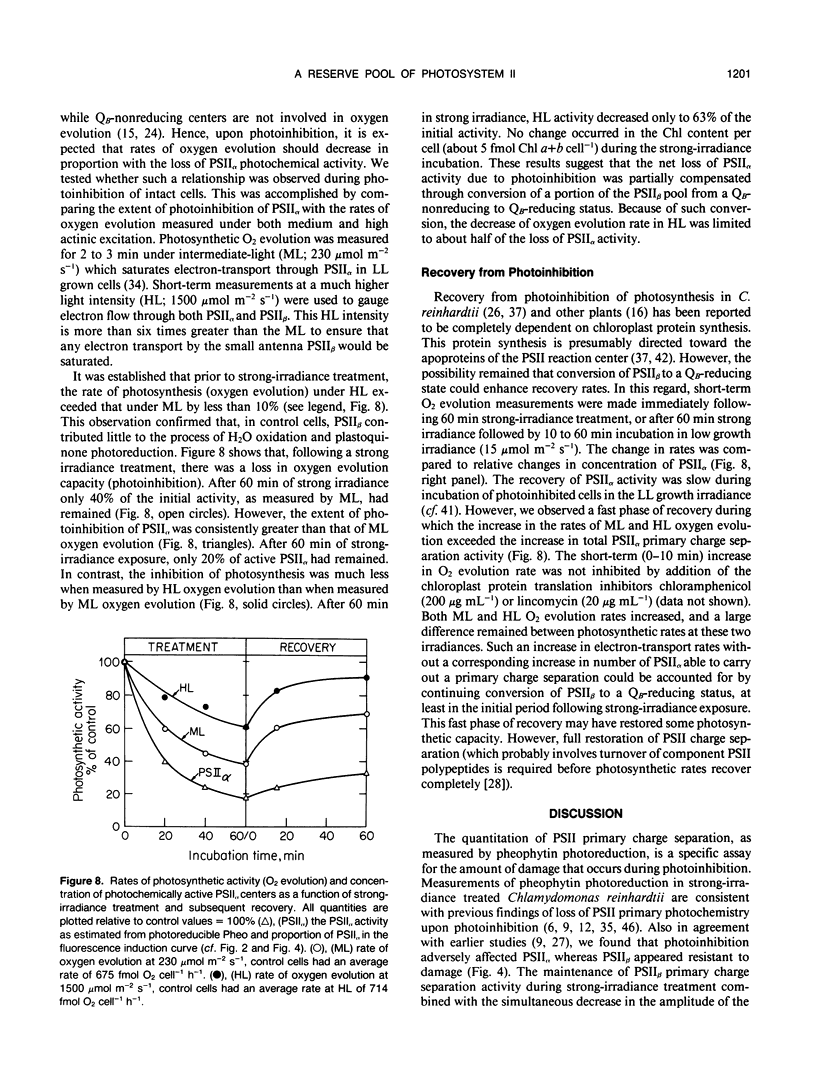
The effect of strong irradiance (2000 micromole photons per square meter per second) on PSII heterogeneity in intact cells of Chlamydomonas reinhardtii was investigated. Low light (LL, 15 micromole photons per square meter per second) grown C. reinhardtii are photoinhibited upon exposure to strong irradiance, and the loss of photosynthetic functioning is due to damage to PSII. Under physiological growth conditions, PSII is distributed into two pools. The large antenna size (PSIIα) centers account for about 70% of all PSII in the thylakoid membrane and are responsible for plastoquinone reduction (Qb-reducing centers). The smaller antenna (PSIIβ) account for the remainder of PSII and exist in a state not yet able to photoreduce plastoquinone (Qb-nonreducing centers). The exposure of C. reinhardtii cells to 60 minutes of strong irradiance disabled about half of the primary charge separation between P680 and pheophytin. The PSIIβ content remained the same or slightly increased during strong-irradiance treatment, whereas the photochemical activity of PSIIα decreased by 80%. Analysis of fluorescence induction transients displayed by intact cells indicated that strong irradiance led to a conversion of PSIIβ from a Qb-nonreducing to a Qb-reducing state. Parallel measurements of the rate of oxygen evolution revealed that photosynthetic electron transport was maintained at high rates, despite the loss of activity by a majority of PSIIα. The results suggest that PSIIβ in C. reinhardtii may serve as a reserve pool of PSII that augments photosynthetic electron-transport rates during exposure to strong irradiance and partially compensates for the adverse effect of photoinhibition on PSIIα.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Melis A. Localization of different photosystems in separate regions of chloroplast membranes. Proc Natl Acad Sci U S A. 1983 Feb;80(3):745–749. doi: 10.1073/pnas.80.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chylla R. A., Whitmarsh J. Inactive Photosystem II Complexes in Leaves : Turnover Rate and Quantitation. Plant Physiol. 1989 Jun;90(2):765–772. doi: 10.1104/pp.90.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darr S. C., Somerville S. C., Arntzen C. J. Monoclonal antibodies to the light-harvesting chlorophyll a/b protein complex of photosystem II. J Cell Biol. 1986 Sep;103(3):733–740. doi: 10.1083/jcb.103.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbush B., Kok B. Reaction between primary and secondary electron acceptors of photosystem II of photosynthesis. Biochim Biophys Acta. 1968 Aug 20;162(2):243–253. doi: 10.1016/0005-2728(68)90106-0. [DOI] [PubMed] [Google Scholar]
- Klimov V. V., Klevanik A. V., Shuvalov V. A., Kransnovsky A. A. Reduction of pheophytin in the primary light reaction of photosystem II. FEBS Lett. 1977 Oct 15;82(2):183–186. doi: 10.1016/0014-5793(77)80580-2. [DOI] [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Edelman M. Intramembrane translocation and posttranslational palmitoylation of the chloroplast 32-kDa herbicide-binding protein. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1497–1501. doi: 10.1073/pnas.84.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A., Schreiber U. The kinetic relationship between the C-550 absorbance change, the reduction of Q(delta A320) and the variable fluorescence yield change in chloroplasts at room temperature. Biochim Biophys Acta. 1979 Jul 10;547(1):47–57. doi: 10.1016/0005-2728(79)90094-x. [DOI] [PubMed] [Google Scholar]
- Nanba O., Satoh K. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc Natl Acad Sci U S A. 1987 Jan;84(1):109–112. doi: 10.1073/pnas.84.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I., Kyle D. J., Arntzen C. J. Membrane protein damage and repair: removal and replacement of inactivated 32-kilodalton polypeptides in chloroplast membranes. J Cell Biol. 1984 Aug;99(2):481–485. doi: 10.1083/jcb.99.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson G., Lönneborg A., Rosenqvist E., Gustafsson P., Oquist G. Photoinhibition and Reactivation of Photosynthesis in the Cyanobacterium Anacystis nidulans. Plant Physiol. 1985 Dec;79(4):992–995. doi: 10.1104/pp.79.4.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster G., Timberg R., Ohad I. Turnover of thylakoid photosystem II proteins during photoinhibition of Chlamydomonas reinhardtii. Eur J Biochem. 1988 Nov 1;177(2):403–410. doi: 10.1111/j.1432-1033.1988.tb14389.x. [DOI] [PubMed] [Google Scholar]
- Thompson L. K., Brudvig G. W. Cytochrome b-559 may function to protect photosystem II from photoinhibition. Biochemistry. 1988 Sep 6;27(18):6653–6658. doi: 10.1021/bi00418a002. [DOI] [PubMed] [Google Scholar]