Abstract
Background:
Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) is an inflammatory disorder of the CNS with a variety of clinical manifestations, including cerebral edema.
Case Summary:
A 7-year-old boy presented with headaches, nausea, and somnolence. He was found to have cerebral edema that progressed to brainstem herniation. Invasive multimodality neuromonitoring was initiated to guide management of intracranial hypertension and cerebral hypoxia while he received empiric therapies for neuroinflammation. Workup revealed serum myelin oligodendrocyte glycoprotein antibodies. He survived with a favorable neurologic outcome.
Conclusion:
We describe a child who presented with cerebral edema and was ultimately diagnosed with MOGAD. Much of his management was guided using data from invasive multimodality neuromonitoring. Invasive multimodality neuromonitoring may have utility in managing life-threatening cerebral edema due to neuroinflammation.
Keywords: brain tissue oxygenation, cerebral, edema, intracranial hypertension, myelin oligodendrocyte glycoprotein antibody-associated disease, neuroinflammation, neuromonitoring
KEYPOINTS
Question: Does invasive multimodality neuromonitoring have utility in the management of myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease?
Findings: We describe a child with MOG antibody-associated disease who presented with life-threatening cerebral edema and received invasive multimodality neuromonitoring. The physiologic data provided by the monitor allowed episodes of increased intracranial pressure and/or cerebral tissue hypoxia to be promptly and aggressively treated to prevent progression to irreversible brain injury. The child additionally received immunomodulatory therapies and had a favorable neurologic outcome.
Meaning: This management reflects adaptation of invasive multimodality neuromonitoring to treat an entity distinct from pediatric traumatic brain injury that causes severe cerebral edema.
BACKGROUND
Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) is a neuroinflammatory disorder with a range of clinical phenotypes, including optic neuritis, acute disseminated encephalomyelitis (ADEM), transverse myelitis, and cerebral encephalitis. Cerebral inflammation due to MOGAD puts patients at risk for cerebral edema, intracranial hypertension, and cerebral ischemia. MOGAD typically responds to corticosteroids. IV immunoglobulin (IVIG) or plasmapheresis can be beneficial in severe disease.
Invasive multimodality neuromonitoring provides continuous measurements of intracranial pressure (ICP) and brain tissue oxygenation (Pbto2), identifying perturbations in cerebral physiology that, when addressed, can prevent secondary brain injury. Although invasive multimodality neuromonitoring is most commonly used in patients after traumatic brain injury (TBI), it has been successfully employed in acute nontraumatic neurologic conditions causing cerebral edema in adults and children (1–3). Importantly, elevated ICP and low Pbto2 have been shown to occur exclusive of one another and are independently associated with poor outcomes (1, 4–6). To our knowledge, there have been no prior reports of invasive multimodality neuromonitoring used to guide management of elevated ICP and brain tissue hypoxia in MOGAD.
We describe a previously healthy child who developed diffuse cerebral edema and symptoms of uncal and brainstem herniation. Invasive multimodality neuromonitoring was employed to guide clinical care, allowing for episodes of increased ICP and/or low Pbto2 to be treated to prevent progression to irreversible neurologic injury. Diagnostic workup revealed a diagnosis of MOGAD, and he was treated with immunomodulatory therapy. The patient made a remarkable recovery.
CASE SUMMARY
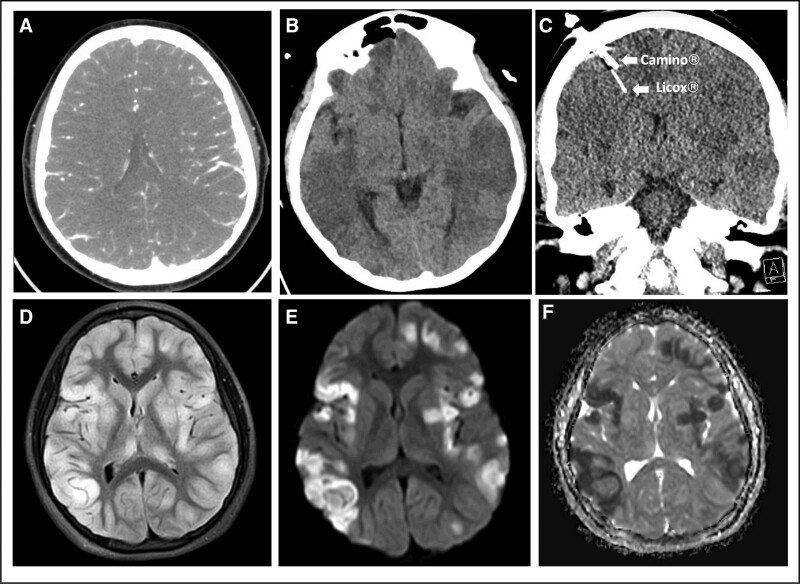
A 7-year-old previously healthy boy presented with 6 days of headache, somnolence, and fever. On the day of presentation to an outside institution, he became unarousable and was intubated in the emergency department. His initial head CT scan was normal. A lumbar puncture had an opening pressure exceeding 39 cm H2O and cerebral spinal fluid studies were notable for a lymphocytic pleocytosis (WBCs 28 cells/μL, 68% lymphocytes). He was initially admitted to the PICU at the outside institution and received antimicrobials and methylprednisolone for presumed meningitis and vasculitis, respectively. Four days following his presentation, he developed anisocoria with an unreactive left pupil, decerebrate posturing, and loss of cough and gag reflexes. CT angiography revealed concern for diffuse cerebral edema (Fig. 1A), at which time he was transferred to our institution. On arrival, his neurologic examination was unchanged. He was administered hypertonic (3%) saline and sedative medications, was hyperventilated, and had the head of bed raised to 30 degrees, which restored left pupillary reactivity and cough and gag reflexes. He was also given dexamethasone for cerebral edema. Invasive ICP (Camino; Natus Global, Middleton, WI) and Pbto2 (Licox; Integra LifeSciences, Princeton, NJ) intraparenchymal neuromonitors were placed the day of admission. High-resolution continuous monitor data was synchronized and recorded using a Moberg Component Neuromonitoring System (Moberg Research, Inc., Ambler, PA). Figure 1, B and C, demonstrates diffuse cerebral edema on the day of admission to our institution and confirms intraparenchymal monitor placement on CT scan.
Figure 1.
Brain imaging. A, Preadmission CT angiography demonstrating narrowing of the lateral ventricles and sulcal effacement. B, CT head obtained the day of admission to our institution demonstrating patchy areas of hypoattenuation involving the white and gray matter of the entire brain parenchyma and sulcal effacement. C, CT head showing intraparenchymal monitor placement. D, T2/fluid-attenuated inversion recovery (FLAIR) image showing extensive confluent areas of hyperintensity with diffuse cerebral edema, affecting the bilateral cortex and deep gray matter, including the bilateral thalami. E, Diffusion weighted imaging and (F) apparent diffusion coefficient demonstrating multifocal areas of restricted diffusion corresponding with areas of T2/FLAIR hyperintensity primarily in the bilateral cortex and left lentiform nucleus.
We adapted our ICP and Pbto2 management strategy from our institution’s guidelines on pediatric TBI (7–9). For example, we chose a higher cerebral perfusion pressure (CPP) goal (60 mm Hg) than suggested in the clinical pathway due to his size (55 kg). Upon placing the monitors, ICP was low (< 10 mm Hg), presumably due in part to hyperventilation with subsequent cerebral vasoconstriction. We titrated ventilator settings to normocarbia without causing elevated ICP. After the Pbto2 monitor had calibrated, we confirmed adequate Pbto2. Within the first several hours of neuromonitoring, the patient developed elevations in ICP greater than or equal to 20 mm Hg (> 5 min) and/or decreases in CPP less than 60 mm Hg. These perturbations were treated successfully with boluses of hypertonic (3%) saline and/or analgesic or sedative medications. The patient also experienced episodes of Pbto2 less than 15 mm Hg (> 5 min), which were treated with increased supplemental oxygen. Within 24 hours of therapies titrated to the above targets, the patient’s anisocoria resolved and he regained cough and gag reflexes. Due to concern for a neuroinflammatory process upon review of the outside hospital MRI, high-dose methylprednisolone was initiated 24 hours and plasma exchange 48 hours after admission.
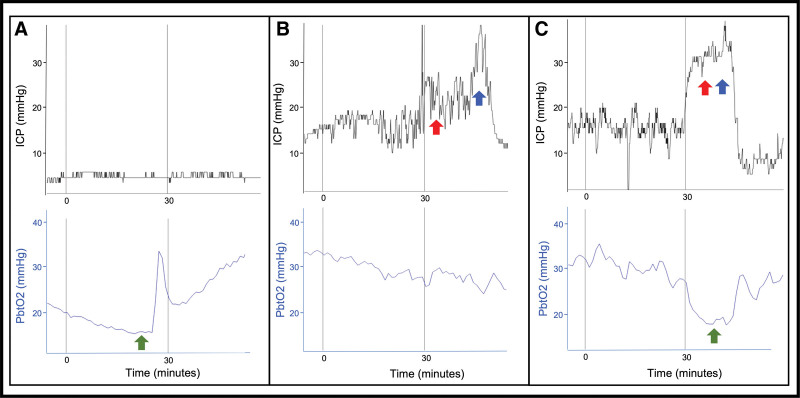
Over the subsequent week, multimodality neuromonitoring demonstrated episodes of cerebral tissue hypoxia when the ICP was within goal range (Fig. 2A). At other times, there were episodes of elevated ICP while the Pbto2 was within goal range (Fig. 2B). There were further episodes in which elevations in ICP and cerebral tissue hypoxia were simultaneously observed (Fig. 2C). These data allowed us to optimize one or both variables while monitoring for and minimizing risk of harm. For instance, during episodes of cerebral tissue hypoxia with normal ICP, adjustments to the ventilator were made to achieve mild hypercarbia, promoting increased cerebral blood flow and oxygen delivery without causing a rise in ICP. In one instance of cerebral tissue hypoxia persisting despite increasing the Fio2 up to 60%, and in the setting of atelectasis on chest radiograph, positive end-expiratory pressure was increased while ensuring ICP did not concomitantly rise due to decreased cerebral venous drainage. We continued to employ analgesia and sedation, including bolus ketamine, and were able to avoid neuromuscular blockade almost entirely. Induced hypernatremia was continued only long as physiology responded to and required it. Importantly, perturbations of ICP and/or Pbto2 were not due to seizures, as confirmed via continuous electroencephalography (discontinued hospital day 9 with no seizures captured). Based on improvements in the child’s examination and trends in ICP and Pbto2, the neuromonitors were removed after 1 week.
Figure 2.
Intracranial pressure (ICP) and brain tissue oxygenation (Pbto2) monitoring. Selected invasive multimodality neuromonitoring data. Interventions are marked by colored arrows (green represents increase in Fio2; red, hypertonic 3% saline; blue, pentobarbital). A, On hospital day 5, the patient had a decrease in Pbto2 less than 15 mm Hg without a concurrent increase in ICP. Fio2 was increased resulting in improved Pbto2. B, On hospital day 4, the patient had an increase in ICP greater than or equal to 20 mm Hg without a decrease in Pbto2 below our threshold for intervention. Hypertonic 3% saline followed by pentobarbital were administered resulting in improved ICP. C, On hospital day 3, the patient had a simultaneous increase in ICP greater than or equal to 20 mm Hg and decrease in Pbto2 less than 15 mm Hg. Hypertonic 3% saline followed by pentobarbital were administered in response to increased ICP, resulting in improvement. Simultaneously, Fio2 was increased in response to decreased Pbto2, resulting in improvement.
With regard to diagnosis, infectious and metabolic studies were unrevealing. Brain MRI was repeated, revealing extensive confluent T2/fluid-attenuated inversion recovery hyperintense lesions with multifocal areas of restricted diffusion (Fig. 1D–F). One week following admission, serum myelin oligodendrocyte glycoprotein (MOG) antibodies were detected at a high titer, confirming a diagnosis of MOGAD. He received IVIG and tocilizumab (an interleukin-6 receptor antagonist) with further improvements in his neurologic examination, including purposeful movements and response to commands. He was extubated on hospital day 17. He continued to improve during his 2-month inpatient hospital and rehabilitation admission and was able to return to school following discharge. One year after his MOGAD attack, he had fully recovered his swallowing ability and gross and fine motor skills and could independently perform activities of daily living, with some residual deficits in speech and language.
The patient’s parents, who are his legal guardians, provided written informed consent for the publication of this case. A complementary case series discussing immunomodulatory therapies in pediatric MOGAD was recently published (10).
DISCUSSION
We report the first case to our knowledge of pediatric MOGAD managed with both invasive ICP and Pbto2 monitoring. While severe cerebral edema is not a common feature of MOGAD, it has previously been reported in both children and adults. Table 1 describes published cases, including medical and surgical management and patient outcomes (11–14). There are also reports of severe cerebral edema in cases of ADEM without MOG antibodies or for which MOG status was not tested or reported. Among reported cases of ADEM with significant cerebral edema, some patients received standard medical therapy alone, whereas others received invasive monitoring for directed ICP management (15, 16). Severe cases requiring decompressive hemicraniectomy have also been reported (17–20). In these cases, it is unclear whether injury to the brain derives primarily from the burden of inflammatory disease or secondary injury due to elevated ICP and resultant ischemia. This patient presented with cerebral edema and clinical herniation syndromes that were initially of unclear etiology. The decision to place invasive neuromonitors was collaborative between pediatric critical care and pediatric neurosurgery based on disease severity, potential for reversibility (regaining of brainstem reflexes with initial ICP lowering therapies), and absence of contraindications (thrombocytopenia, coagulopathy, local infection). Although data are sparse for use of this technology outside of TBI, we anticipated that a tailored approach to treating increased ICP and cerebral hypoxia could minimize secondary neurologic injury, as well as iatrogenic effects of therapies used to treat increased ICP (e.g., hyperventilation, neuromuscular blockade [21, 22], and hypernatremia/hyperchloremia [23]). Furthermore, growing appreciation for the potential role for invasive neuromonitoring in neuroinflammatory disease is reflected in the proposed best practice recommendations by the Autoimmune Encephalitis Clinicians Network, which advises consideration in cases with severe edema (24).
TABLE 1.
Summary of Published Cases of Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease With Cerebral Edema
| Publication | Age (Sex) | Immunomodulatory Therapies | Surgical Therapies | Outcome |
|---|---|---|---|---|
| Narayan et al (11) | 6 yo (male) | Steroids, plasmapheresis, cyclophosphamide, rituximab | None | Blinked to answer questions, spasticity, nonambulatory |
| 3 yo (female) | Steroids, plasmapheresis, IVIG, rituximab | EVD | Left hemiparesis, left hemianopia, low-average intellectual ability | |
| 4 yo (female) | Steroids, plasmapheresis, IVIG, cyclophosphamide | EVD | Spasticity, gait instability, language impairments, visual and auditory deficits | |
| Hochmeister et al (13) | 52 yo (female) | Steroids | None | Death during hospital admission |
| Sinha et al (12) | 10 yo (female) | Steroids, plasmapheresis, IVIG, cyclophosphamide | Hemicraniectomy, EVD | Spoke in full sentences, left greater than right hemiparesis but could ambulate independently |
| Kannan et al (14) | 2 yo (female) | Steroids, plasmapheresis, IVIG | None | PCPC score of 2 and FSS score of 6 at hospital follow-up, indicating mild disability |
| 2 yo (male) | Steroids, IVIG | None | PCPC score of 3 and FSS score of 8 at hospital follow-up, indicating mild to moderate disability, and epilepsy | |
| 3 yo (male) | Steroids, plasmapheresis, IVIG, tocilizumab | EVD | PCPC score of 4 and FSS score of 15 at hospital follow-up, indicating moderate to severe disability, and epilepsy | |
| 5 yo (male) | Steroids, plasmapheresis | None | PCPC score of 2 and FSS score of 6 at hospital follow-up, indicating mild disability | |
| 7 yo (male) | Steroids, plasmapheresis, IVIG | None | PCPC score of 1 and FSS score of 6 at hospital follow-up, indicating no residual disability | |
| 7 yo (male) | Steroids, plasmapheresis, IVIG | EVD | PCPC score of 2 and FSS score of 7 at hospital follow-up, indicating mild disability |
EVD = extraventricular drain, FSS = Functional Status Score, IVIG = IV immunoglobulin, PCPC = Pediatric Cerebral Performance Category, yo = yr old.
CONCLUSION
We report a pediatric MOGAD patient with severe cerebral edema and herniation syndromes who had a favorable neurologic outcome following invasive multimodality neuromonitoring and immunomodulatory therapies. While further studies are needed, clinicians may consider invasive multimodality neuromonitoring to guide medical management in cases of MOGAD with significant cerebral edema.
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Yang E, Sekhon M, Griesdale D: Transtentorial herniation syndrome from meningococcal meningitis in a young woman: The case for neurocritical care. BMJ Case Rep 2022; 15:e253191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirschen MP, LaRovere K, Balakrishnan B, et al. ; Pediatric Neurocritical Care Research Group (PNCRG): A survey of neuromonitoring practices in North American pediatric intensive care units. Pediatr Neurol 2022; 126:125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang SS, Paden W, Steenhoff AP, et al. : Intracranial pressure and brain tissue oxygen neuromonitoring in pediatric cerebral malaria. World Neurosurg 2020; 141:115–118 [DOI] [PubMed] [Google Scholar]
- 4.Le Roux P, Menon DK, Citerio G, et al. : Consensus summary statement of the international multidisciplinary consensus conference on multimodality monitoring in neurocritical care: A statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit Care 2014; 21(Suppl 2): S1–S26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helbok R, Olson DM, Le Roux PD, et al. ; Participants in the International Multidisciplinary Consensus Conference on Multimodality Monitoring: Intracranial pressure and cerebral perfusion pressure monitoring in non-TBI patients: Special considerations. Neurocrit Care 2014; 21(Suppl 2):S85–S94 [DOI] [PubMed] [Google Scholar]
- 6.Lang SS, Rahman R, Kumar N, et al. : Invasive neuromonitoring modalities in the pediatric population. Neurocrit Care 2023; 38:470–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Children’s Hospital of Philadelphia: Traumatic Brain Injury (TBI) Clinical Pathway — Emergency Department and ICU. 2015. Available at: https://www.chop.edu/clinical-pathway/brain-injury-severe-traumatic-picu-clinical-pathway. Accessed August 8, 2023
- 8.Kochanek PM, Tasker RC, Carney N, et al. : Guidelines for the management of pediatric severe traumatic brain injury, third edition: Update of the brain trauma foundation guidelines, executive summary. Neurosurgery 2019; 84:1169–1178 [DOI] [PubMed] [Google Scholar]
- 9.Lang SS, Kumar NK, Zhao C, et al. : Invasive brain tissue oxygen and intracranial pressure (ICP) monitoring versus ICP-only monitoring in pediatric severe traumatic brain injury. J Neurosurg Pediatr 2022; 17:1–11 [DOI] [PubMed] [Google Scholar]
- 10.McLendon LA, Gambrah-Lyles C, Viaene A, et al. : Dramatic response to anti-IL-6 receptor therapy in children with life-threatening myelin oligodendrocyte glycoprotein-associated disease. Neurol Neuroimmunol Neuroinflamm 2023; 10:e200150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narayan RN, Wang C, Greenberg BM: Acute disseminated encephalomyelitis (ADEM) and increased intracranial pressure associated with anti-myelin oligodendrocyte glycoprotein antibodies. Pediatr Neurol 2019; 99:64–68 [DOI] [PubMed] [Google Scholar]
- 12.Sinha S, Banwell B, Tucker A, et al. : Hemicraniectomy and externalized ventricular drain placement in a pediatric patient with myelin oligodendrocyte glycoprotein-associated tumefactive demyelinating disease. Childs Nerv Syst 2022; 38:185–189 [DOI] [PubMed] [Google Scholar]
- 13.Hochmeister S, Gattringer T, Asslaber M, et al. : A fulminant case of demyelinating encephalitis with extensive cortical involvement associated with anti-MOG antibodies. Front Neurol 2020; 11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kannan V, Sandweiss AJ, Erickson TA, et al. : Fulminant anti-myelin oligodendrocyte glycoprotein-associated cerebral cortical encephalitis: Case series of a severe pediatric myelin oligodendrocyte glycoprotein antibody-associated disease phenotype. Pediatr Neurol 2023; 147:36–43 [DOI] [PubMed] [Google Scholar]
- 15.Yae Y, Kawano G, Yokochi T, et al. : Fulminant acute disseminated encephalomyelitis in children. Brain Dev 2019; 41:373–377 [DOI] [PubMed] [Google Scholar]
- 16.VanLandingham M, Hanigan W, Vedanarayanan V, et al. : An uncommon illness with a rare presentation: Neurosurgical management of ADEM with tumefactive demyelination in children. Childs Nerv Syst 2010; 26:655–661 [DOI] [PubMed] [Google Scholar]
- 17.Granget E, Milh M, Pech-Gourg G, et al. : Life-saving decompressive craniectomy for acute disseminated encephalomyelitis in a child: A case report. Childs Nerv Syst 2012; 28:1121–1124 [DOI] [PubMed] [Google Scholar]
- 18.Dombrowski KE, Mehta AI, Turner DA, et al. : Life-saving hemicraniectomy for fulminant acute disseminated encephalomyelitis. Br J Neurosurg 2011; 25:249–252 [DOI] [PubMed] [Google Scholar]
- 19.Refai D, Lee MC, Goldenberg FD, et al. : Decompressive hemicraniectomy for acute disseminated encephalomyelitis: Case report. Neurosurgery 2005; 56:E872; discussion E871 [DOI] [PubMed] [Google Scholar]
- 20.Sekula RF, Marchan EM, Baghai P, et al. : Central brain herniation secondary to fulminant acute disseminated encephalomyelitis: Implications for neurosurgical management. Case report. J Neurosurg 2006; 105:472–474 [DOI] [PubMed] [Google Scholar]
- 21.Smith HAB, Besunder JB, Betters KA, et al. : 2022 Society of Critical Care Medicine clinical practice guidelines on prevention and management of pain, agitation, neuromuscular blockade, and delirium in critically ill pediatric patients with consideration of the ICU environment and early mobility. Pediatr Crit Care Med 2022; 23:e74–e110 [DOI] [PubMed] [Google Scholar]
- 22.Renew JR, Ratzlaff R, Hernandez-Torres V, et al. : Neuromuscular blockade management in the critically ill patient. J Intensive Care 2020; 8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dabrowski W, Siwicka-Gieroba D, Robba C, et al. : Potentially detrimental effects of hyperosmolality in patients treated for traumatic brain injury. J Clin Med 2021; 10:4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abboud H, Probasco JC, Irani S, et al. ; Autoimmune Encephalitis Alliance Clinicians Network: Autoimmune encephalitis: Proposed best practice recommendations for diagnosis and acute management. J Neurol Neurosurg Psychiatry 2021; 92:757–768 [DOI] [PMC free article] [PubMed] [Google Scholar]