Abstract
Background and Objectives
The association of motor function with cognitive health remains controversial, and the mechanisms underlying this relationship are unclear. We aimed to examine the association between motor function and long-term cognitive trajectories and further explore the underlying mechanisms using brain MRI.
Methods
In the Rush Memory and Aging Project, a prospective cohort study, a total of 2,192 volunteers were recruited from the communities in northeastern Illinois and followed up for up to 22 years (from 1997 to 2020). Individuals with dementia, disability, missing data on motor function at baseline, and missing follow-up data on cognitive function were excluded. At baseline, global motor function was evaluated using the averaged z scores of 10 motor tests covering dexterity, gait, and hand strength; the composite score was tertiled as low, moderate, or high. Global and domain-specific cognitive functions—including episodic memory, semantic memory, working memory, visuospatial ability, and perceptual speed—were measured annually through 19 cognitive tests. A subsample (n = 401) underwent brain MRI scans and regional brain volumes were measured. Data were analyzed using linear mixed-effects models and linear regression.
Results
Among the 1,618 participants (mean age 79.45 ± 7.32 years) included in this study, baseline global motor function score ranged from 0.36 to 1.82 (mean 1.03 ± 0.22). Over the follow-up (median 6.03 years, interquartile range 3.00–10.01 years), low global motor function and its subcomponents were related to significantly faster declines in global cognitive function (β = −0.005, 95% CI −0.006 to −0.005) and each of the 5 cognitive domains. Of the 344 participants with available MRI data, low motor function was also associated with smaller total brain (β = −25.848, 95% CI −44.902 to −6.795), total white matter (β = −18.252, 95% CI −33.277 to −3.226), and cortical white matter (β = −17.503, 95% CI −32.215 to −2.792) volumes, but a larger volume of white matter hyperintensities (β = 0.257, 95% CI 0.118–0.397).
Discussion
Low motor function is associated with an accelerated decline in global and domain-specific cognitive functions. Both neurodegenerative and cerebrovascular pathologies might contribute to this association.
Introduction
Motor function is an important indicator of an individual's functional ability.1 Age-related motor impairments—including diminished dexterity, slower gait speed, poor coordination, and decreased muscle strength—are common and associated with poorer quality of life and a higher risk of all-cause mortality.2-4 Given that motor function can be heterogeneous, the integration of multiple motor measures may help to more comprehensively examine their association with cognitive function changes among older adults.5
Growing evidence points to an association between motor impairment and cognitive decline among older adults.6-13 However, the relationship between different components of motor function and cognition, including specific cognitive domains, needs to be clarified. Previous studies have related impairments in individual motor indicators (including gait speed and hand strength) to a faster decline in memory function, visuospatial ability, and processing speed.9-11 However, other studies found no association between the loss of hand strength and memory, attention, or processing speed.12,13 In light of these mixed findings, the association between motor function and long-term cognitive trajectories remains unclear. In addition, previous studies on this topic were limited by relatively short follow-up time and the use of basic cognitive assessments or only a single indicator of motor function.
The mechanisms underlying the association between motor function and changes in cognitive function warrant further investigation. MRI has been widely applied to examine structural brain changes and can provide insight into the pathophysiology underlying changes in cognition.14 Previous neuroimaging studies reported an association between motor function and reduced gray matter, white matter, and hippocampal volumes,15-19 though other studies produced conflicting results.15,16 So far, few studies have systematically addressed the association between motor function and domain-specific cognitive trajectories and brain structural measures assessed by MRI scans.
Within the Rush Memory and Aging Project (MAP), we have previously reported that poor motor function is associated with incident cognitive impairment.5,8,17 In this study, we aimed to (1) assess the longitudinal associations between motor function (including dexterity, gait, and hand strength) and trajectories of global and domain-specific cognitive functions and (2) explore the association between poor motor function and structural brain differences. We hypothesize that poor motor function is related to accelerated declines in cognitive function and that neurodegeneration and cerebrovascular pathologies might underlie this association. In this study, we tested these hypotheses using 22-year follow-up data from MAP, a prospective cohort study including brain MRI and comprehensive assessments of motor function and cognitive function over time.
Methods
Study Population
MAP is an ongoing prospective cohort study focused on cognitive decline and the development of common chronic diseases among older adults. Study design and assessment protocols have been previously described in detail.18 In brief, participants were recruited from continuous care retirement communities, senior and subsidized housing, social service agencies, church groups, and individual homes in northeastern Illinois and the Chicago area.18 All participants were native English speakers. Between 1997 and 2020, the study included 2,192 participants who were followed up annually for up to 22 years, and 401 of them underwent brain MRI scans between 2009 and 2011. Because MAP is an ongoing open cohort study, MRI scans could occur at baseline for newly recruited participants or during a follow-up examination for already enrolled participants. Thus, the motor function-MRI association is considered cross-sectional. We excluded 574 individuals from the total MAP population, including 117 with no motor function data, 217 with impairment in the activities of daily living (ADL), 117 with prevalent dementia at baseline, and 123 with missing follow-up data on cognitive function. This left a study population of 1,618 participants for this investigation, 344 of whom had MRI data available (Figure 1).
Figure 1. Flowchart of the Study Population.
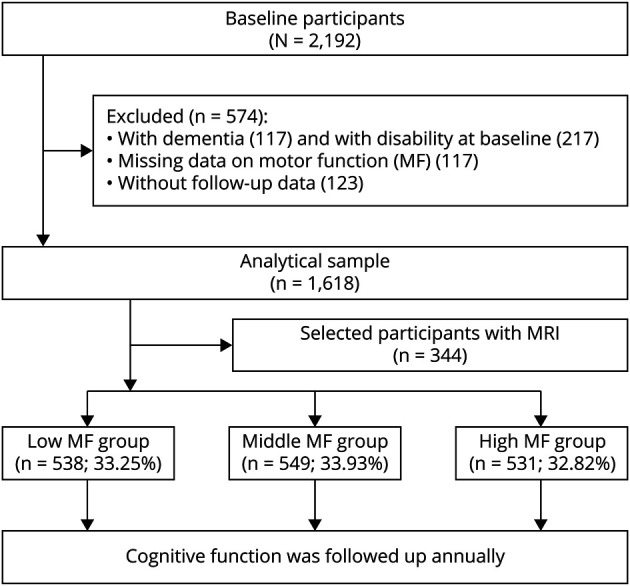
MF = motor function.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Institutional Review Board of Rush University Medical Center and was conducted in accordance with the ethical standards set forth in the 1964 Declaration of Helsinki and its subsequent amendments. All participants provided written informed consent and repository consent to allow sharing of their data.19
Assessment of Motor Function and ADL
A comprehensive measure of global motor function reflecting dexterity, gait, and hand strength was assessed at baseline using 10 individual performance tests of common motor behaviors.20
Motor dexterity was assessed based on 2 tasks: the Purdue pegboard task and the finger-tapping task. In the Purdue pegboard task, participants were required to insert as many nails as possible into the holes of a pegboard within a 30-second period. In the finger-tapping task, participants were required to strike an electric tapper (Western Psychological Services, Los Angeles, CA) with their forefinger as fast as they could for 10 seconds. Each task was repeated 4 times (twice for each hand). The number of nails placed on the pegboard and the number of taps performed in each of the 4 sessions were averaged to obtain an overall Purdue pegboard score and tapping score, respectively, for each participant.
Motor gait was assessed using 2 lower extremity function tasks. Participants were required to walk forward 8 feet and make two 360-degree turns. The time (in seconds) and the number of steps taken for each task were recorded separately and the averages were obtained to yield scores for walking time, walking steps, turning time, and turning steps.
Motor hand strength consisted of 2 dimensions: grip strength and pinch strength. Using the Jamar hydraulic hand and pinch dynamometers (Lafayette Instruments, Lafayette, IN), participants performed a grip and a pinch twice with each hand. Overall grip/pinch strength scores were calculated as the mean grip/pinch strength (in pounds) from these 4 trials.
Balance was assessed by recording the amount of time (up to 10 seconds) participants were able to stand on each leg and their toes. Because the balance tasks were sometimes not attempted, we did not aggregate balance scores into a single measure of overall balance performance.
Given differences in how performance was measured in each of the 10 motor function tests, performance scores for each test were converted to z scores and averaged together to obtain a composite measure of global motor function, with higher scores indicating better motor performance.5 Global motor function score was tertiled to yield 3 equally sized groups reflecting different levels of motor function (low, moderate, and high).
Disability was assessed using the self-reported Katz ADL scale, which covers 6 basic physical abilities including eating, toileting, bathing, dressing, transferring from bed to chair, and walking across a small room.21 Participants who reported difficulty in 1 or more items were considered to have a disability and were excluded from the study population.
Assessment of Cognitive Function, Mild Cognitive Impairment, and Dementia
Cognitive function was assessed using a battery of 19 cognitive performance tests administered at baseline and annual follow-up visits.22 The 19 tests captured 5 cognitive domains, including episodic memory (Word List Memory, Word List Recognition, Word List Recall, Immediate and Delayed Recall of Story A from Logical Memory and East Boston Story), semantic memory (Verbal Fluency, Boston Naming Test, National Adult Reading Test), working memory (Digit Ordering, Digit Span Forward and Backward), perceptual speed (Symbol Digit Modalities Test, Number Comparison, Stroop Word Reading, and Stroop Color Naming Test), and visuospatial ability (Judgment of Line Orientation, Standard Progressive Matrices). Raw scores from all tests were converted to z scores and averaged to generate a composite measure of global cognitive function, with higher scores reflecting better cognitive function.23
Dementia and mild cognitive impairment (MCI) were diagnosed based on a standard procedure that included computerized scoring of the 19 aforementioned cognitive tests, clinical evaluation by a neuropsychologist, and diagnostic classification by a clinician.24,25 Dementia was diagnosed according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS/ADRDA) joint working group criteria.24 Participants were diagnosed with MCI if they showed evidence of cognitive impairment in the neuropsychologist's examination (i.e., impairment in at least 1 cognitive domain based on normative cognitive data considering age, sex, and education), but did not meet the NINCDS/ADRDA criteria for dementia in the clinician's examination.25
Assessment of Structural Brain Volumes
The MAP MRI protocol is described in eMethods 1 (links.lww.com/WNL/D98). In this study, we obtained the volumes (in cubic centimeters) of the whole brain, total gray matter (cerebellar gray matter, cortical gray matter, and subcortical gray matter), total white matter (cerebellar white matter, cortical white matter), the hippocampus, and white matter hyperintensities (WMH).
Assessment of Other Variables
During enrollment, participants underwent a comprehensive clinical assessment and physical assessment performed by trained staff and provided information on demographic characteristics, socioeconomic status, daily lifestyle, and medical history. Information on the assessment of education level, body mass index (BMI), alcohol consumption, smoking, physical activity, social activity, cardiometabolic diseases (CMDs; i.e., diabetes, heart disease, or stroke), hypertension, depression, and APOE ε4 carrier status are described in detail in eMethods 2 (links.lww.com/WNL/D98). More detailed information about the data collection can be found at the Rush Alzheimer's Disease Center Resource Sharing Hub.
Statistical Analysis
Baseline characteristics of the study population were compared according to tertiles of motor function. One-way analysis of variance or Kruskal-Wallis rank sum tests were used for continuous variables, and Chi-square tests were used for categorical variables.
Linear mixed-effects models were used to estimate β coefficients and 95% CIs for the association between motor function (both as a continuous and a categorical variable) and annual changes in global cognitive function and 5 cognitive domains. The fixed effect included motor function, quadratic follow-up time (year2), and their interaction based on the distribution of the observed association. The random effect included random intercepts and slopes, allowing for the individual differences at baseline and during follow-up. We additionally assessed the joint effect of motor function and other factors of interest on changes in global cognition over follow-up time by creating dummy variables based on the joint exposure of global motor function (low vs high) and CMDs (absence vs presence), APOE genotype (ε4 vs no ε4), or physical activity (low vs high). Next, we examined statistical interactions by creating indicator variables containing global motor function, CMDs/APOE genotype/physical activity, and their cross-product term in the same model. Finally, linear regression models were used to estimate the β coefficients and 95% CIs for the association between global motor function and structural brain volumes.
All models were adjusted for age, sex, education, BMI, alcohol consumption, smoking, physical activity, social activity, hypertension, diabetes, heart diseases, stroke, depression, and APOE ε4 carrier status.
In the sensitivity analysis, we repeated the original analyses after (1) excluding individuals with MCI at baseline, (2) excluding measures of cognitive function assessed during the first 5 years of follow-up to minimize reverse causality, (3) imputing missing values for the covariates using multiple imputation by chained equation, (4) additionally adjusting for global cognition at baseline, and (5) stratifying by sex. A 2-tailed p value <0.05 was considered to be statistically significant for all tests. All statistical analyses were performed using Stata SE 15.0 for Windows (StataCorp, College Station, TX).
Data Availability
Requests for access to MAP data can be made at radc.rush.edu.
Results
Characteristics of the Study Population
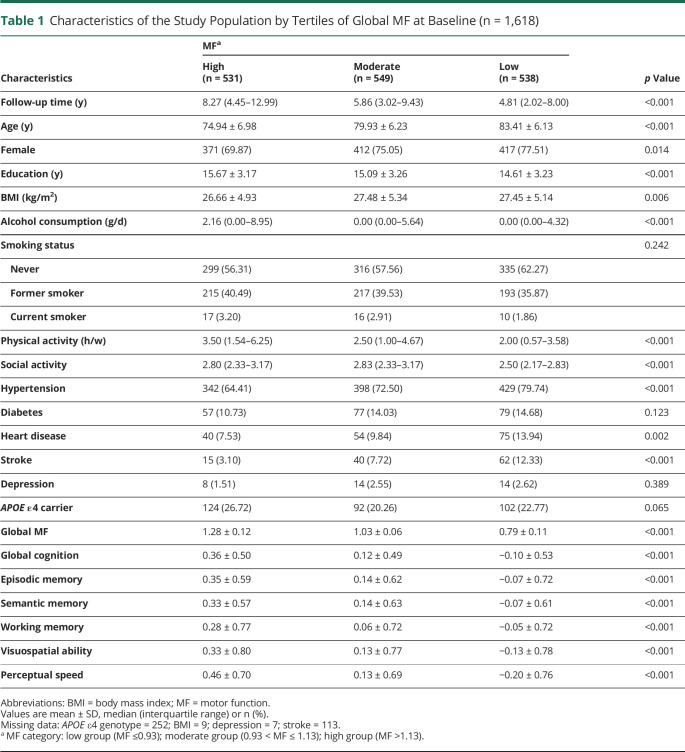
Of the 1,618 participants included in the study (mean age 79.45 ± 7.32 years, 74.17% female), the global motor function score ranged from 0.36 to 1.82 (mean 1.03 ± 0.22) at baseline. There were 538 (33.25%), 549 (33.93%), and 531 (32.82%) participants in the low, moderate, and high motor function groups, respectively.
Participants with moderate/low motor function were more likely to be older, be female, have less formal education, and have higher BMI compared with those with high global motor function. In addition, participants with moderate/low motor function tended to drink less alcohol, tended to have less physical and social activities, tended to have poorer cognitive performance, and were more likely to have hypertension, heart disease, and stroke. No significant differences among the motor function groups were found in terms of APOE ε4 carrier status, smoking, diabetes, or depression (Table 1).
Table 1.
Characteristics of the Study Population by Tertiles of Global MF at Baseline (n = 1,618)
| Characteristics | MFa | p Value | ||
| High (n = 531) | Moderate (n = 549) | Low (n = 538) | ||
| Follow-up time (y) | 8.27 (4.45–12.99) | 5.86 (3.02–9.43) | 4.81 (2.02–8.00) | <0.001 |
| Age (y) | 74.94 ± 6.98 | 79.93 ± 6.23 | 83.41 ± 6.13 | <0.001 |
| Female | 371 (69.87) | 412 (75.05) | 417 (77.51) | 0.014 |
| Education (y) | 15.67 ± 3.17 | 15.09 ± 3.26 | 14.61 ± 3.23 | <0.001 |
| BMI (kg/m2) | 26.66 ± 4.93 | 27.48 ± 5.34 | 27.45 ± 5.14 | 0.006 |
| Alcohol consumption (g/d) | 2.16 (0.00–8.95) | 0.00 (0.00–5.64) | 0.00 (0.00–4.32) | <0.001 |
| Smoking status | 0.242 | |||
| Never | 299 (56.31) | 316 (57.56) | 335 (62.27) | |
| Former smoker | 215 (40.49) | 217 (39.53) | 193 (35.87) | |
| Current smoker | 17 (3.20) | 16 (2.91) | 10 (1.86) | |
| Physical activity (h/w) | 3.50 (1.54–6.25) | 2.50 (1.00–4.67) | 2.00 (0.57–3.58) | <0.001 |
| Social activity | 2.80 (2.33–3.17) | 2.83 (2.33–3.17) | 2.50 (2.17–2.83) | <0.001 |
| Hypertension | 342 (64.41) | 398 (72.50) | 429 (79.74) | <0.001 |
| Diabetes | 57 (10.73) | 77 (14.03) | 79 (14.68) | 0.123 |
| Heart disease | 40 (7.53) | 54 (9.84) | 75 (13.94) | 0.002 |
| Stroke | 15 (3.10) | 40 (7.72) | 62 (12.33) | <0.001 |
| Depression | 8 (1.51) | 14 (2.55) | 14 (2.62) | 0.389 |
| APOE ε4 carrier | 124 (26.72) | 92 (20.26) | 102 (22.77) | 0.065 |
| Global MF | 1.28 ± 0.12 | 1.03 ± 0.06 | 0.79 ± 0.11 | <0.001 |
| Global cognition | 0.36 ± 0.50 | 0.12 ± 0.49 | −0.10 ± 0.53 | <0.001 |
| Episodic memory | 0.35 ± 0.59 | 0.14 ± 0.62 | −0.07 ± 0.72 | <0.001 |
| Semantic memory | 0.33 ± 0.57 | 0.14 ± 0.63 | −0.07 ± 0.61 | <0.001 |
| Working memory | 0.28 ± 0.77 | 0.06 ± 0.72 | −0.05 ± 0.72 | <0.001 |
| Visuospatial ability | 0.33 ± 0.80 | 0.13 ± 0.77 | −0.13 ± 0.78 | <0.001 |
| Perceptual speed | 0.46 ± 0.70 | 0.13 ± 0.69 | −0.20 ± 0.76 | <0.001 |
Abbreviations: BMI = body mass index; MF = motor function.
Values are mean ± SD, median (interquartile range) or n (%).
Missing data: APOE ε4 genotype = 252; BMI = 9; depression = 7; stroke = 113.
MF category: low group (MF ≤0.93); moderate group (0.93 < MF ≤ 1.13); high group (MF >1.13).
Moreover, we further compared the baseline characteristics of participants with (n = 344) and without (n = 1,274) MRI, and the analysis showed no significant differences between the 2 groups in terms of sex, education, BMI, alcohol consumption, smoking, hypertension, diabetes, heart disease, stroke, depression, or APOE ε4 carrier status (all p > 0.05) (eTable 1, links.lww.com/WNL/D98).
Association of Motor Function With Cognitive Decline
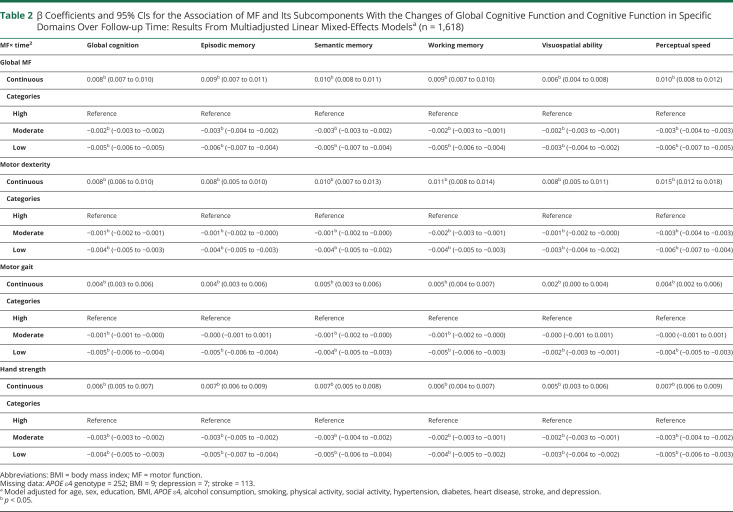
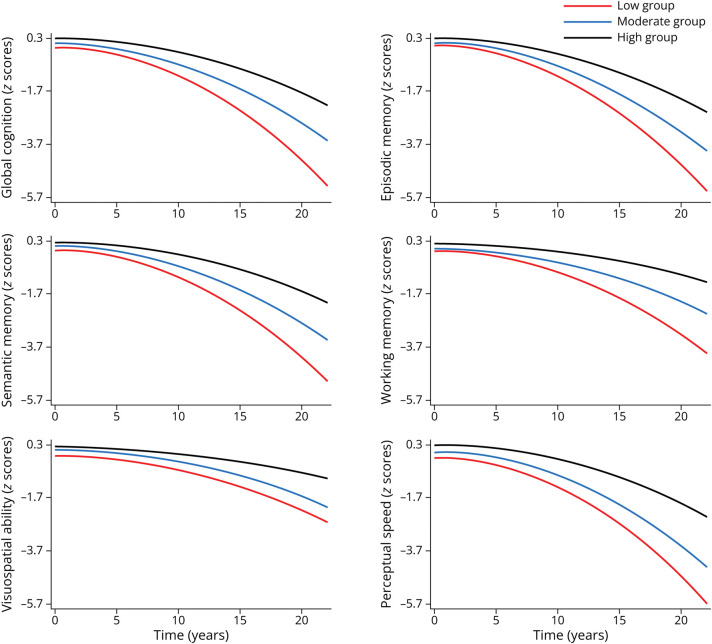
During the follow-up (median 6.03 years, interquartile range 3.00–10.01 years), better global motor function and its subcomponents including dexterity, gait, and hand strength (as continuous variables) were associated with slower declines in global cognition, episodic memory, semantic memory, working memory, visuospatial ability, and perceptual speed over time. When motor function and its subcomponents were analyzed as categorical variables, participants with moderate/low motor function showed significantly faster declines in global cognitive function and each of the 5 domain-specific cognitive functions over time, compared with those with high motor function (Table 2 and Figure 2).
Table 2.
β Coefficients and 95% CIs for the Association of MF and Its Subcomponents With the Changes of Global Cognitive Function and Cognitive Function in Specific Domains Over Follow-up Time: Results From Multiadjusted Linear Mixed-Effects Modelsa (n = 1,618)
| MF× time2 | Global cognition | Episodic memory | Semantic memory | Working memory | Visuospatial ability | Perceptual speed |
| Global MF | ||||||
| Continuous | 0.008b (0.007 to 0.010) | 0.009b (0.007 to 0.011) | 0.010b (0.008 to 0.011) | 0.009b (0.007 to 0.010) | 0.006b (0.004 to 0.008) | 0.010b (0.008 to 0.012) |
| Categories | ||||||
| High | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderate | −0.002b (−0.003 to −0.002) | −0.003b (−0.004 to −0.002) | −0.003b (−0.003 to −0.002) | −0.002b (−0.003 to −0.001) | −0.002b (−0.003 to −0.001) | −0.003b (−0.004 to −0.003) |
| Low | −0.005b (−0.006 to −0.005) | −0.006b (−0.007 to −0.004) | −0.005b (−0.007 to −0.004) | −0.005b (−0.006 to −0.004) | −0.003b (−0.004 to −0.002) | −0.006b (−0.007 to −0.005) |
| Motor dexterity | ||||||
| Continuous | 0.008b (0.006 to 0.010) | 0.008b (0.005 to 0.010) | 0.010b (0.007 to 0.013) | 0.011b (0.008 to 0.014) | 0.008b (0.005 to 0.011) | 0.015b (0.012 to 0.018) |
| Categories | ||||||
| High | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderate | −0.001b (−0.002 to −0.001) | −0.001b (−0.002 to −0.000) | −0.001b (−0.002 to −0.000) | −0.002b (−0.003 to −0.001) | −0.001b (−0.002 to −0.000) | −0.003b (−0.004 to −0.003) |
| Low | −0.004b (−0.005 to −0.003) | −0.004b (−0.005 to −0.003) | −0.004b (−0.005 to −0.002) | −0.004b (−0.005 to −0.003) | −0.003b (−0.004 to −0.002) | −0.006b (−0.007 to −0.004) |
| Motor gait | ||||||
| Continuous | 0.004b (0.003 to 0.006) | 0.004b (0.003 to 0.006) | 0.005b (0.003 to 0.006) | 0.005b (0.004 to 0.007) | 0.002b (0.000 to 0.004) | 0.004b (0.002 to 0.006) |
| Categories | ||||||
| High | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderate | −0.001b (−0.001 to −0.000) | −0.000 (−0.001 to 0.001) | −0.001b (−0.002 to −0.000) | −0.001b (−0.002 to −0.000) | −0.000 (−0.001 to 0.001) | −0.000 (−0.001 to 0.001) |
| Low | −0.005b (−0.006 to −0.004) | −0.005b (−0.006 to −0.004) | −0.004b (−0.005 to −0.003) | −0.005b (−0.006 to −0.003) | −0.002b (−0.003 to −0.001) | −0.004b (−0.005 to −0.003) |
| Hand strength | ||||||
| Continuous | 0.006b (0.005 to 0.007) | 0.007b (0.006 to 0.009) | 0.007b (0.005 to 0.008) | 0.006b (0.004 to 0.007) | 0.005b (0.003 to 0.006) | 0.007b (0.006 to 0.009) |
| Categories | ||||||
| High | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderate | −0.003b (−0.003 to −0.002) | −0.003b (−0.005 to −0.002) | −0.003b (−0.004 to −0.002) | −0.002b (−0.003 to −0.001) | −0.002b (−0.003 to −0.001) | −0.003b (−0.004 to −0.002) |
| Low | −0.004b (−0.005 to −0.003) | −0.005b (−0.007 to −0.004) | −0.005b (−0.006 to −0.004) | −0.004b (−0.005 to −0.002) | −0.003b (−0.004 to −0.002) | −0.005b (−0.006 to −0.003) |
Abbreviations: BMI = body mass index; MF = motor function.
Missing data: APOE ε4 genotype = 252; BMI = 9; depression = 7; stroke = 113.
Model adjusted for age, sex, education, BMI, APOE ε4, alcohol consumption, smoking, physical activity, social activity, hypertension, diabetes, heart disease, stroke, and depression.
p < 0.05.
Figure 2. Cognitive Trajectories in Global Cognition and Specific Domains by MF in Tertiles.
Trajectories represent β coefficients from linear mixed-effect models adjusted for age, sex, education, body mass index, apolipoprotein E epsilon 4, alcohol consumption, smoking, physical activity, social activity, hypertension, diabetes, heart disease, stroke, and depression with the highest MF group as reference group. MF = motor function.
In the joint effect analyses (eTable 2, links.lww.com/WNL/D98), we combined the moderate and low global motor function groups because both were significantly related to cognitive decline. The association between poor global motor function and changes in global cognition over time was stronger among people with CMDs (β = −0.004, 95% CI −0.005 to −0.003). However, after excluding participants with CMDs, the relationship between poor motor function and faster decline in cognitive function still remained significant in people without CMDs (eTable 3). There was a remarkable multiplicative interaction between global motor function and CMDs on cognitive decline (p < 0.001). We failed to find the interactions between global motor function and APOE ε4 carrier status or physical activity (all p > 0.05) (eTables 4 and 5).
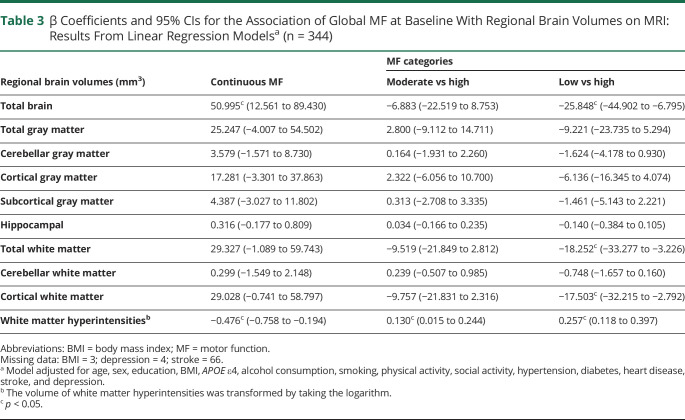
Association of Motor Function With Structural Brain Differences
In the low, moderate, and high motor function groups, 74, 107, and 163 participants, respectively, underwent an MRI scan. The median and interquartile range of the intervals between the baseline examination and the MRI scan was 2.96 (0.00–5.58), 3.87 (0.00–6.02), 3.79 (0.00–6.04) years in each group, and the difference in interval time was not statistically significant (p = 0.468). In multiadjusted linear regression models, greater global motor function (as a continuous variable) was related to larger total brain volume (β = 50.995, 95% CI 12.561–89.430) and smaller WMH volume (β = −0.476, 95% CI −0.758 to −0.194). When global motor function was treated as a categorical variable, compared with high motor function, low motor function was related to smaller total brain volume (β = −25.848, 95% CI −44.902 to −6.795), total white matter volume (β = −18.252, 95% CI −33.277 to −3.226), and cortical white matter volume (β = −17.503, 95% CI −32.215 to −2.792), but a larger volume of WMH (β = 0.257, 95% CI 0.118–0.397) (Table 3). The associations between global motor function and gray matter or hippocampal volume were not significant.
Table 3.
β Coefficients and 95% CIs for the Association of Global MF at Baseline With Regional Brain Volumes on MRI: Results From Linear Regression Modelsa (n = 344)
| Regional brain volumes (mm3) | Continuous MF | MF categories | |
| Moderate vs high | Low vs high | ||
| Total brain | 50.995c (12.561 to 89.430) | −6.883 (−22.519 to 8.753) | −25.848c (−44.902 to −6.795) |
| Total gray matter | 25.247 (−4.007 to 54.502) | 2.800 (−9.112 to 14.711) | −9.221 (−23.735 to 5.294) |
| Cerebellar gray matter | 3.579 (−1.571 to 8.730) | 0.164 (−1.931 to 2.260) | −1.624 (−4.178 to 0.930) |
| Cortical gray matter | 17.281 (−3.301 to 37.863) | 2.322 (−6.056 to 10.700) | −6.136 (−16.345 to 4.074) |
| Subcortical gray matter | 4.387 (−3.027 to 11.802) | 0.313 (−2.708 to 3.335) | −1.461 (−5.143 to 2.221) |
| Hippocampal | 0.316 (−0.177 to 0.809) | 0.034 (−0.166 to 0.235) | −0.140 (−0.384 to 0.105) |
| Total white matter | 29.327 (−1.089 to 59.743) | −9.519 (−21.849 to 2.812) | −18.252c (−33.277 to −3.226) |
| Cerebellar white matter | 0.299 (−1.549 to 2.148) | 0.239 (−0.507 to 0.985) | −0.748 (−1.657 to 0.160) |
| Cortical white matter | 29.028 (−0.741 to 58.797) | −9.757 (−21.831 to 2.316) | −17.503c (−32.215 to −2.792) |
| White matter hyperintensitiesb | −0.476c (−0.758 to −0.194) | 0.130c (0.015 to 0.244) | 0.257c (0.118 to 0.397) |
Abbreviations: BMI = body mass index; MF = motor function.
Missing data: BMI = 3; depression = 4; stroke = 66.
Model adjusted for age, sex, education, BMI, APOE ε4, alcohol consumption, smoking, physical activity, social activity, hypertension, diabetes, heart disease, stroke, and depression.
The volume of white matter hyperintensities was transformed by taking the logarithm.
p < 0.05.
Sensitivity Analysis
We obtained similar results after (1) excluding 402 participants with baseline MCI (eTables 6 and 7, links.lww.com/WNL/D98), (2) excluding measurements of cognitive function collected during the first 5 years of follow-up (n = 878) (eTable 8), (3) imputing missing data for covariates (n = 374) (eTable 9), and (4) further adjusting for global cognition at baseline (eTables 10 and 11). After stratifying by sex, the association between motor function and cognitive decline was still significant in both male and female participants (eTables 12 and 13), but the association between poor global motor function and smaller white matter volume and larger WMH volume was significant only in female participants, possibly owing to the smaller sample size (n = 87) of male participants (eTables 14 and 15).
Discussion
In this community-based cohort study of older adults, we found that (1) low motor function (including dexterity, gait, and hand strength) was associated with accelerated declines in global cognition, episodic memory, semantic memory, working memory, visuospatial ability, and perceptual speed and (2) low global motor function was further related to smaller total brain, total white matter, and cortical white matter volume, but larger WMH volume.
Longitudinal studies have shown that the deterioration of motor dexterity, gait, and hand strength is related to global cognitive decline among older adults.6-8 However, previous studies on the association between motor function and domain-specific cognitive function have shown mixed results.9-13 Two previous studies have reported significant relationships between slower gait speed and reduced memory performance and visuospatial ability.9,10 A systematic review indicated that weakened hand strength was related not only to impaired memory but also a poorer performance on tests of language and processing speed.11 However, another study reported no association between motor function and cognitive decline in any domain.12 Moreover, one previous investigation even reported that stronger hand strength was related to less decline in memory, spatial ability, and processing speed in people older than 65 years, though this association was not significant among individuals younger than 65 years.13 In this study, we combined dexterity, gait, and hand strength as a comprehensive assessment of global motor function and evaluated global cognitive function in addition to 5 cognitive domains. We found that low motor function was related to more rapid decreases in global cognitive function and all 5 domain-specific cognitive functions.
Some studies have linked poor motor function to severe brain atrophy or lesions in people with specific neurologic disorders, such as Alzheimer disease, Parkinson disease, and multiple sclerosis.26-28 However, the evidence on the relationship between specific motor functions and structural brain difference has been limited.15,16,29-31 Poorer motor dexterity has been associated with larger white matter lesions.29 Moreover, gait disorders have been related to smaller white matter, gray matter, and hippocampal volumes.30,31 A British birth cohort suggested that reduced hand strength was associated with smaller total brain volume,15 while another study on people with dementia showed that hand strength was associated only with smaller left hippocampal volume.16 In this study, we demonstrated that poor global motor function was related to smaller total brain, total white matter, and cortical white matter volumes and larger WMH volume. Reduction in total brain volume is a typical marker of neurodegeneration,32 whereas reduction in white matter volume can reflect vascular damage to the brain.33 In addition, WMH are considered to be a typical MRI expression of microvascular lesions in cerebral white matter.33 Our findings suggest that both neurodegeneration and vascular lesions in the brain may underlie the motor function-cognitive decline association.
It is well known that impairment in certain cognitive domains can reflect specific changes in the brain structure. Poor performance in semantic memory, working memory, visuospatial ability, and processing speed is associated with reduced total brain atrophy and an increase in the volume of white matter lesions, such as WMH.34-37 Decline in working memory and processing speed might be related to white matter atrophy.38,39 Furthermore, poorer performance in perceptual speed and global cognition have been associated with smaller total brain volume.40 Thus, the 2 sections of this study—on cognitive domains and brain structural changes—complement each other and strengthen the evidence of a connection between motor function and cognition.
Higher prevalence of diabetes or cardiovascular diseases among people with poor motor function could be one possible explanation for the association between poor motor function and cognitive decline. Type 2 diabetes, heart disease, and stroke have been related to cognitive impairment and irreversible brain pathologies and are prevalent in people with poor motor function.41-43 Indeed, we found a significant joint effect of poor global motor function and CMDs on cognitive decline, whereby the poor motor function-cognitive decline association was stronger among people with CMDs. Our finding suggests that CMDs could be a potential factor of risk for faster cognitive decline among older adults, particularly those with diminished motor function. Motor function is an important predictor of vascular risk, which could cause cognitive decline through increased pulsatile cerebral blood flow velocity and decreased cerebral perfusion.44,45 Moreover, poor motor function could lead to reduced glucose metabolism and the development of insulin resistance. Insulin resistance in the CNS could trigger cognitive impairment by promoting the phosphorylation of tau proteins.46,47 Further long-term studies are warranted to confirm the interaction between poor motor function and CMDs on cognitive decline, given the small sample size in our joint exposure analysis. Notably, in our study, the relationship between poor motor function and faster decline of cognitive functions was still significant among CMD-free participants, so the motor function-cognition association cannot be completely explained by the higher prevalence of CMDs among people with poor motor function. Indeed, poor motor function could cause chronic low-grade local or systemic inflammation by affecting the secretion of cytokines and other peptides, thus resulting in cognitive decline.48-50
As a longitudinal community-based cohort study, our study has the advantage of a long follow-up period and a relatively large sample size. In addition, we used a composite measure of motor function and assessed global and domain-specific cognitive functions through a comprehensive battery of 19 tests. Although many studies have reported an association between motor function and cognitive function, no study so far has incorporated brain MRI data, which could provide evidence regarding the mechanisms underlying the motor-cognition association. In addition to the poor motor function-cognitive decline association, we found that poor motor function was associated with brain MRI markers indicative of neurodegeneration and vascular lesions, suggesting the involvement of both neurodegenerative and vascular pathways in the motor-cognition association. Nevertheless, some limitations of our study should be pointed out. First, the study population was older (mean age 79.45 years), was approximately 70% female, and composed of predominantly highly educated people living in urban communities who thus performed better than the general population on cognitive tests. Thus, caution is required when generalizing our findings to other (especially younger) populations. Second, because the exclusion of people with missing data on motor function and cognition may have resulted in a comparatively healthier study population, the observed association between motor function and cognitive function might have been underestimated. However, we repeated the analysis after multiple imputations, and the results were consistent with those in from the initial analysis. Third, composite motor function score was based on z scores of 10 individual motor tests and thus may have limited clinical application. Future studies are warranted to develop a standard cutoff in motor function that can be more clinically relevant. Fourth, the relationship between motor function and regional brain volumes could not be examined longitudinally; thus, the temporality of this association is unclear. Moreover, we considered only MRI-derived structural brain volumes, and future imaging studies using modalities such as functional MRI and diffusion tensor imaging are warranted to further explore the mechanisms underlying the association between motor function and cognition. Fifth, the diagnoses of some clinical disorders were based on participants' retrospective self-reports; thus, information bias could not be ruled out. Finally, although we considered a wide range of potential confounders, detailed information on other factors of interest (e.g., hearing, duration and amount of smoking, and the diagnosis of movement disorders such as ataxia and dystonia) was unavailable in the study.
In summary, our study provides evidence that poor motor function is associated with accelerated decline in global cognition, episodic memory, semantic memory, working memory, visuospatial ability, and perceptual speed over time. Neurodegeneration and vascular lesions in the brain might underlie the motor function-cognitive decline association. Future large population-based cohort studies are warranted to corroborate our findings.
Acknowledgment
The authors thank the participants and staff involved in data collection and management in the Rush Memory and Aging Project.
Glossary
- ADL
activities of daily living
- ADRDA
Alzheimer's Disease and Related Disorders Association
- BMI
body mass index
- CMD
cardiometabolic disease
- MAP
Rush Memory and Aging Project
- MCI
mild cognitive impairment
- NINCDS
National Institute of Neurological and Communicative Disorders and Stroke
- WMH
white matter hyperintensities
Appendix. Authors
| Name | Location | Contribution |
| Zhangyu Wang, MSc | Tianjin Medical University, China | Analyzed and interpreted the data; drafted the article; performed statistical analysis; revised the article for intellectual content |
| Jiao Wang, PhD | Tianjin Medical University, China | Analyzed and interpreted the data; performed statistical analysis; revised the article for intellectual content |
| Jie Guo, PhD | Karolinska Institutet, Stockholm, Sweden | Analyzed and interpreted the data; performed statistical analysis; revised the article for intellectual content |
| Abigail Dove, MSc | Karolinska Institutet, Stockholm, Sweden | Interpreted the data; revised the article for intellectual content |
| Konstantinos Arfanakis, PhD | Rush University Medical Center, Chicago, IL | Interpreted the data; revised the article for intellectual content |
| Xiuying Qi, PhD | Tianjin Medical University, China | Revised the article for intellectual content |
| David A. Bennett, MD | Rush University Medical Center, Chicago, IL | Design and conceptualized study; revised the article for intellectual content; major role in the acquisition of data |
| Weili Xu, PhD | Tianjin Medical University, China; Karolinska Institutet, Stockholm, Sweden | Design and conceptualized study; interpreted the data; revised the article for intellectual content; major role in the acquisition of data |
Study Funding
No targeted funding reported.
Disclosure
Z. Wang, J. Wang, J. Guo, A. Dove, K. Arfanakis, and X. Qi report no disclosures relevant to the manuscript. D.A. Bennett received grants from the NIH (R01AG17917). W. Xu received grants from the Swedish Research Council (No. 2021-01647), the Swedish Council for Health Working Life and Welfare (2021-01826), Lindhés Advokatbyrå AB (2021-0134), and Stiftelsen För Gamla Tjänarinnor (2021–2022). Go to Neurology.org/N for full disclosures.
References
- 1.Strand BH, Cooper R. Monitoring changes in motor function in the years before death. BMJ. 2021;374:n1882. doi: 10.1136/bmj.n1882 [DOI] [PubMed] [Google Scholar]
- 2.Buchman AS, Bennett DA. Loss of motor function in preclinical Alzheimer's disease. Expert Rev Neurother. 2011;11(5):665-676. doi: 10.1586/ern.11.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchman AS, Wilson RS, Leurgans SE, Bennett DA, Barnes LL. Change in motor function and adverse health outcomes in older African-Americans. Exp Gerontol. 2015;70:71-77. doi: 10.1016/j.exger.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Change in motor function and risk of mortality in older persons. J Am Geriatr Soc. 2007;55(1):11-19. doi: 10.1111/j.1532-5415.2006.01032.x [DOI] [PubMed] [Google Scholar]
- 5.Buchman AS, Leurgans SE, Boyle PA, Schneider JA, Arnold SE, Bennett DA. Combinations of motor measures more strongly predict adverse health outcomes in old age: the rush memory and aging project, a community-based cohort study. BMC Med. 2011;9:42. doi: 10.1186/1741-7015-9-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abe T, Soma Y, Kitano N, et al. Change in hand dexterity and habitual gait speed reflects cognitive decline over time in healthy older adults: a longitudinal study. J Phys Ther Sci. 2017;29(10):1737-1741. doi: 10.1589/jpts.29.1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou MY, Nishita Y, Nakagawa T, et al. Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatr. 2019;19(1):186. doi: 10.1186/s12877-019-1199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol. 2009;66(11):1339-1344. doi: 10.1001/archneurol.2009.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kikkert LHJ, Vuillerme N, van Campen JP, Hortobagyi T, Lamoth CJ. Walking ability to predict future cognitive decline in old adults: a scoping review. Ageing Res Rev. 2016;27:1-14. doi: 10.1016/j.arr.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 10.Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68(8):929-937. doi: 10.1093/gerona/gls256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi-Cuya KE, Sakurai R, Suzuki H, Ogawa S, Takebayashi T, Fujiwara Y. Observational evidence of the association between handgrip strength, hand dexterity, and cognitive performance in community-dwelling older adults: a systematic review. J Epidemiol. 2018;28(9):373-381. doi: 10.2188/jea.JE20170041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooghiemstra AM, Ramakers I, Sistermans N, et al. Gait speed and grip strength reflect cognitive impairment and are modestly related to incident cognitive decline in memory clinic patients with subjective cognitive decline and mild cognitive impairment: findings from the 4C study. J Gerontol A Biol Sci Med Sci. 2017;72(6):846-854. doi: 10.1093/gerona/glx003 [DOI] [PubMed] [Google Scholar]
- 13.Sternäng O, Reynolds CA, Finkel D, Ernsth-Bravell M, Pedersen NL, Dahl Aslan AK. Grip strength and cognitive abilities: associations in old age. J Gerontol B Psychol Sci Soc Sci. 2016;71(5):841-848. doi: 10.1093/geronb/gbv017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braskie MN, Thompson PM. A focus on structural brain imaging in the Alzheimer's disease neuroimaging initiative. Biol Psychiatry. 2014;75(7):527-533. doi: 10.1016/j.biopsych.2013.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dercon Q, Nicholas JM, James SN, Schott JM, Richards M. Grip strength from midlife as an indicator of later-life brain health and cognition: evidence from a British birth cohort. BMC Geriatr. 2021;21(1):475. doi: 10.1186/s12877-021-02411-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon Y, Moon WJ, Kim JO, Kwon KJ, Han SH. Muscle strength is independently related to brain atrophy in patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 2019;47(4-6):306-314. doi: 10.1159/000500718 [DOI] [PubMed] [Google Scholar]
- 17.Beeri MS, Leurgans SE, Bennett DA, Barnes LL, Buchman AS. Diverse motor performances are related to incident cognitive impairment in community-dwelling older adults. Front Aging Neurosci. 2021;13:717139. doi: 10.3389/fnagi.2021.717139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2018;64(suppl 1):S161-S189. doi: 10.3233/JAD-179939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Yang W, Wang J, et al. High lifelong cognitive reserve prolongs disability-free survival: the role of cognitive function. Alzheimers Dement. 2023;19(1):208-216. doi: 10.1002/alz.12670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson RS, Segawa E, Buchman AS, Boyle PA, Hizel LP, Bennett DA. Terminal decline in motor function. Psychol Aging. 2012;27(4):998-1007. doi: 10.1037/a0028182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6(3):493-508. doi: 10.2190/uurl-2ryu-wryd-ey3k [DOI] [PubMed] [Google Scholar]
- 22.Wilson RS, Boyle PA, Yu L, et al. Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology. 2015;85(11):984-991. doi: 10.1212/WNL.0000000000001935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson R, Barnes L, Bennett D. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol. 2003;25(5):634-642. doi: 10.1076/jcen.25.5.634.14572 [DOI] [PubMed] [Google Scholar]
- 24.Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27(3):169-176. doi: 10.1159/000096129 [DOI] [PubMed] [Google Scholar]
- 25.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59(2):198-205. doi: 10.1212/wnl.59.2.198 [DOI] [PubMed] [Google Scholar]
- 26.Nadkarni NK, McIlroy WE, Mawji E, Black SE. Gait and subcortical hyperintensities in mild Alzheimer's disease and aging. Dement Geriatr Cogn Disord. 2009;28(4):295-301. doi: 10.1159/000245158 [DOI] [PubMed] [Google Scholar]
- 27.Lee Y, Ko J, Choi YE, et al. Areas of white matter hyperintensities and motor symptoms of Parkinson disease. Neurology. 2020;95(3):e291-e298. doi: 10.1212/WNL.0000000000009890 [DOI] [PubMed] [Google Scholar]
- 28.Grothe M, Lotze M, Langner S, Dressel A. Impairments in walking ability, dexterity, and cognitive function in multiple sclerosis are associated with different regional cerebellar gray matter loss. Cerebellum. 2017;16(5-6):945-950. doi: 10.1007/s12311-017-0871-8 [DOI] [PubMed] [Google Scholar]
- 29.Nyquist PA, Yanek LR, Bilgel M, et al. Effect of white matter lesions on manual dexterity in healthy middle-aged persons. Neurology. 2015;84(19):1920-1926. doi: 10.1212/WNL.0000000000001557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Annweiler C, Beauchet O, Celle S, et al. Contribution of brain imaging to the understanding of gait disorders in Alzheimer's disease: a systematic review. Am J Alzheimers Dis Other Demen. 2012;27(6):371-380. doi: 10.1177/1533317512454710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wennberg AM, Savica R, Mielke MM. Association between various brain pathologies and gait disturbance. Dement Geriatr Cogn Disord. 2017;43(3-4):128-143. doi: 10.1159/000456541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smolinski L, Litwin T, Redzia-Ogrodnik B, Dziezyc K, Kurkowska-Jastrzebska I, Czlonkowska A. Brain volume is related to neurological impairment and to copper overload in Wilson's disease. Neurol Sci. 2019;40(10):2089-2095. doi: 10.1007/s10072-019-03942-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822-838. doi: 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oosterman JM, Van Harten B, Weinstein HC, Scheltens P, Sergeant JA, Scherder EJ. White matter hyperintensities and working memory: an explorative study. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2008;15(3):384-399. doi: 10.1080/13825580701879998 [DOI] [PubMed] [Google Scholar]
- 35.Schmidt R, Ropele S, Enzinger C, et al. White matter lesion progression, brain atrophy, and cognitive decline: the Austrian stroke prevention study. Ann Neurol. 2005;58(4):610-616. doi: 10.1002/ana.20630 [DOI] [PubMed] [Google Scholar]
- 36.Brugulat-Serrat A, Salvado G, Operto G, et al. White matter hyperintensities mediate gray matter volume and processing speed relationship in cognitively unimpaired participants. Hum Brain Mapp. 2020;41(5):1309-1322. doi: 10.1002/hbm.24877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyle PA, Yu L, Fleischman DA, et al. White matter hyperintensities, incident mild cognitive impairment, and cognitive decline in old age. Ann Clin Transl Neurol. 2016;3(10):791-800. doi: 10.1002/acn3.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charlton RA, Barrick TR, Lawes IN, Markus HS, Morris RG. White matter pathways associated with working memory in normal aging. Cortex. 2010;46(4):474-489. doi: 10.1016/j.cortex.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 39.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vibha D, Tiemeier H, Mirza SS, et al. Brain volumes and longitudinal cognitive change: a population-based study. Alzheimer Dis Assoc Disord. 2018;32(1):43-49. doi: 10.1097/WAD.0000000000000235 [DOI] [PubMed] [Google Scholar]
- 41.Horton WB, Barrett EJ. Microvascular dysfunction in diabetes mellitus and cardiometabolic disease. Endocr Rev. 2021;42(1):29-55. doi: 10.1210/endrev/bnaa025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dove A, Marseglia A, Shang Y, et al. Cardiometabolic multimorbidity accelerates cognitive decline and dementia progression. Alzheimers Dement. 2022. doi: 10.1002/alz.12708 [DOI] [PubMed] [Google Scholar]
- 43.Sinclair AJ, Abdelhafiz AH. Cardiometabolic disease in the older person: prediction and prevention for the generalist physician. Cardiovasc Endocrinol Metab. 2020;9(3):90-95. doi: 10.1097/XCE.0000000000000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fonseca Alves DJ, Bartholomeu-Neto J, Júnior ER, Ribeiro Zarricueta BS, Nóbrega OT, Córdova C. Walking speed, risk factors, and cardiovascular events in older adults-systematic review. J Strength Cond Res. 2017;31(11):3235-3244. doi: 10.1519/jsc.0000000000002182 [DOI] [PubMed] [Google Scholar]
- 45.Pase MP, Grima NA, Stough CK, Scholey A, Pipingas A. Cardiovascular disease risk and cerebral blood flow velocity. Stroke. 2012;43(10):2803-2805. doi: 10.1161/STROKEAHA.112.666727 [DOI] [PubMed] [Google Scholar]
- 46.Seko T, Akasaka H, Koyama M, et al. Lower limb muscle mass is associated with insulin resistance more than lower limb muscle strength in non-diabetic older adults. Geriatr Gerontol Int. 2019;19(12):1254-1259. doi: 10.1111/ggi.13805 [DOI] [PubMed] [Google Scholar]
- 47.Ma L, Wang J, Li Y. Insulin resistance and cognitive dysfunction. Clin Chim Acta. 2015;444:18-23. doi: 10.1016/j.cca.2015.01.027 [DOI] [PubMed] [Google Scholar]
- 48.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457-465. doi: 10.1038/nrendo.2012.49 [DOI] [PubMed] [Google Scholar]
- 49.Looijaard S, Te Lintel Hekkert ML, Wust RCI, Otten RHJ, Meskers CGM, Maier AB. Pathophysiological mechanisms explaining poor clinical outcome of older cancer patients with low skeletal muscle mass. Acta Physiol (Oxf). 2021;231(1):e13516. doi: 10.1111/apha.13516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ashraf-Ganjouei A, Moradi K, Bagheri S, Aarabi MH. The association between systemic inflammation and cognitive performance in healthy adults. J Neuroimmunol. 2020;345:577272. doi: 10.1016/j.jneuroim.2020.577272 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Requests for access to MAP data can be made at radc.rush.edu.