Abstract
A 74-year-old woman presented with acute-onset right ptosis and binocular diplopia. CT scan showed low-density lesions in the bilateral basal ganglia and adjacent to lateral ventricles. Intracranial aneurysm was not detected. This case highlights the importance of neurologic localization of ophthalmoplegia based on physical examination and the microanatomy of the oculomotor nerve.
Section 1
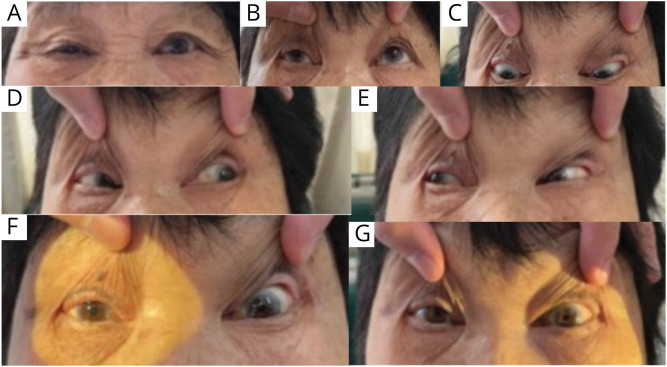
A 74-year-old woman with a history of hypertension presented with right ptosis and binocular diplopia when she woke up in the morning. She denied prodromal fever or headache. She also denied history of diabetes or recent head trauma. She was admitted to the emergency department of local hospital. On physical examination, her body mass index was 26.8 kg/m2. The visual acuity was 20/30 for both eyes. Upward movement of the right eye was limited (Figure 1). Eyeball movement toward other directions and pupillary reactivity were normal. Her vertical diplopia also worsened on upward gaze. No tremor, ataxia, choreiform movement, or limb weakness was found. Other neurologic examinations were unremarkable. CT scan showed low-density lesions in bilateral basal ganglia adjacent to lateral ventricles. Cranial CT angiography (CTA) did not detect intracranial aneurysm or stenosis.
Figure 1. Extraocular Movement Examination.
(A) Right ptosis. (B–E) Extraocular movements: (B) Impaired elevation of right eye on upgaze. (C–E) Normal bilateral depression, abduction, and adduction. (F–G) Normal bilateral pupillary light reflex.
The patient was referred to our hospital for further evaluation 6 days later. Laboratory investigations including full blood count, electrolytes, blood glucose, renal and liver function, erythrocyte sedimentation rate, and antinuclear antibodies were unrevealing. The level of triglycerides was 2.14 mmol/L (normal range 0.45–1.69). Repeated electrical stimulation was negative.
Questions for Consideration:
Where is the lesion responsible for the monocular ptosis and binocular diplopia?
What is the differential diagnosis for a monocular ptosis along with diplopia?
What further testing would you perform?
Section 2
The distribution and temporal progression of the paralytic extraocular muscles hold clues to the anatomical location of the lesion. Critical characteristics of ophthalmoplegia include unilateral or bilateral, persistent or fluctuant, and the distribution of paralytic muscles. Monocular, nonfluctuating, and isolated extraocular muscle paralysis suggests lesions in a peripheral nerve instead of muscle or neuromuscular junction, which would paralyze muscles that do not follow nerve innervation pattern.1
Coexistence of right ptosis and vertical binocular diplopia suggests levator palpebrae and superior rectus palsy on the right side.
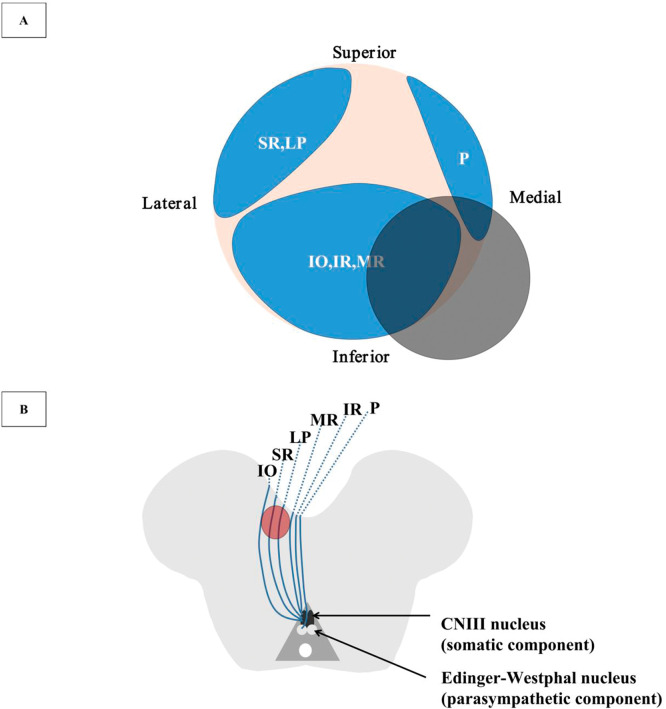
The central caudal subnucleus of the third cranial nerve (CNIII) nucleus gives rise to crossed and uncrossed fibers that innervate bilateral levator palpebrae superioris,2 that is, supranuclear and nuclear palsy of the CNIII results in bilateral ptosis. The lesion for unilateral ptosis and upward palsy is therefore located to structures peripheral to oculomotor nucleus. Before entering the superior orbital fissure, the oculomotor nerve separates as superior and inferior branches. Levator palpebrae and superior rectus are innervated by the superior branch. Ksiazek proposed a 3-dimensional anatomic model for oculomotor nerve.3 The topographic arrangement of oculomotor fascicles is arranged as shown by Figure 2. Such model supports the notion that a divisional oculomotor nerve paresis may occur from damage at any location along the course of the oculomotor nerve once it leaves the nuclei, instead of just cavernous sinus or orbit, where superior and inferior divisional palsy have classically been located.4 In this sense, the presence of monocular ptosis and vertical binocular diplopia localizes the lesion to the any course of the oculomotor nerve from the fascicle to the orbit part.
Figure 2. Illustration of the CNIII Microanatomy in the Cistern Space and the Diagram of the CNIII Nerve Fascicular Topography in the Midbrain (Not to Scale).
(A) Illustration of the CNIII microanatomy. The gray shaded area demonstrates the region of the neurovascular conflict in our patient showing the possibly involved CNIII fascicles, which is not in accordance with the symptomatology. (B) Diagram of the topographic fascicular arrangement of the CNIII nerve. The gray shaded area shows the midbrain lesion in our patient. CNIII = third cranial nerve; IO = inferior oblique; IR = inferior rectus; LP = levator palpebrae; MR = medial rectus; P = pupillary fibers; SR = superior rectus.
Differential diagnoses of persistent unilateral oculomotor nerve palsy include intrinsic (such as diabetes, infarction, and CNS infection) and extrinsic causes (such as aneurysm, trauma, cavernous sinus lesion, subarachnoid hemorrhage, and neurovascular conflict).5 Neurovascular contact is a scenario in which a vessel contacts a nerve with the disappearance of CSF signal in between. It is most often seen in asymptomatic individuals. In comparison, neurovascular conflict is defined as a direct perpendicular compression between an artery and the root entry/exit zone of a cranial nerve. The diagnosis can only be made when there is nerve deviation or indentation.6 Based on different etiologies, painful ophthalmoplegia can be caused by intrinsic or extrinsic factors. CTA of this patient did not reveal any intracranial vascular abnormalities. Neither was there laboratory evidence for hyperglycemia or CNS infection. Oculomotor damage due to cavernous sinus lesion and painful ophthalmoplegia is often accompanied by paralysis of trigeminal, trochlear, and abducens nerves. Isolated partial oculomotor nerve paresis is extremely rare in these 2 conditions. The nonfluctuating nature of ophthalmoplegia in this patient and negative repeated electrical stimulation test make neuromuscular junction disorders unlikely.
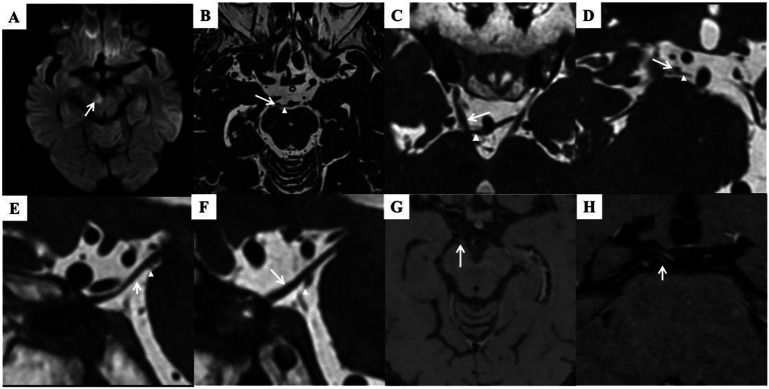
Further investigatory tests were performed. Synoptophore test revealed right superior rectus palsy, confirming physical examination findings. Brain MRI revealed hyperintensity of periventricular and deep white matter matching the results of CT scan, suggesting previous lacunar stroke. Diffusion-weighted image (DWI, slice thickness 5 mm) showed a diffusion restricted lesion of 8 mm diameter involving the medial part of the right cerebral peduncle (Figure 3). Three-dimensional constructive interference in steady-state (3D-CISS) MRI (slice thickness 0.4 mm) revealed subtle swelling and deviation of right oculomotor nerve in interpeduncular cistern. The right superior cerebellar artery (SCA) compresses from behind the oculomotor nerve, resulting in absence of CSF signal in between (Figure 3). High-resolution magnetic resonance vessel wall imaging (HRMR-VWI, slice thickness 0.6 mm) showed stenosis and a plaque in the origin of right posterior cerebral artery (PCA, Figure 3).
Figure 3. Neuroimaging.
(A) Restricted diffusion involving the medial aspect of the right cerebral peduncle. (B–F) Axial (B, C), coronal (D, showing right CNIII nerve), and sagittal (E, right CNIII nerve; F, left CNIII nerve) 3D-CISS MRI showing the right SCA (arrowhead) indenting the inferior and medial part of the CNIII nerve (arrow) in the cistern space, resulting in nerve extension and deviation. HRMR-VWI showed stenosis and a plaque in the origin of right PCA (G–H, arrow). 3D-CISS = 3-dimensional constructive interference in steady state; CNIII = third cranial nerve; HRMR-VWI = high-resolution magnetic resonance vessel wall imaging; PCA = posterior cerebral artery.
Question for Consideration:
Which is the culprit lesion for the partial oculomotor palsy?
Section 3
Physical examination and synoptophore test of this patient suggest lesions in fibers innervating the right superior rectus and levator palpebrae and sparing of the pupillomotor fibers. Based on the imaging results, midbrain lacunar ischemic stroke and neurovascular conflict are candidate reasons for the isolated oculomotor nerve palsy. The right SCA compresses the inferior and medial portion part of the nerve (Figure 3). Topography of the CNIII nerve makes it unlikely that the SCA compression could spare the medial and dorsal pupillary fibers (Figure 2).7,8 Moreover, the presence of multiple stroke risk factors (older age, sex, hypertension, dyslipidemia, obesity) supports a cerebrovascular cause in this patient. We therefore deem that the compression of the CNIII nerve by SCA is not responsible for this partial oculomotor palsy. The acute midbrain infarction accorded with the third nerve fascicular topography (Figure 2).3 The stenotic PCA blocked the medial artery group that supplies the brainstem section of the oculomotor fascicles (Figure 3).2 We conclude that the midbrain infarction between the red nucleus and the cerebral peduncle accounts for the monocular ptosis and diplopia. The American Academy of Neurology's guideline for symptomatic intracranial atherosclerotic arterial stenosis recommends 3 months of dual antiplatelet therapy (clopidogrel and aspirin) for severe stenosis (70%–99%) to reduce further stroke risk.9 Given she had excessive gingival bleeding when on aspirin, only clopidogrel was given as monoantiplatelet therapy. Lipid-lowering treatment was also initiated. Follow-up 1 month later indicates that the ptosis was significantly improved and the diplopia disappeared.
Discussion
A combination of monocular ptosis and binocular diplopia is characteristic of superior CNIII nerve palsy. Fascicular oculomotor nerve palsy secondary to midbrain lacunar stroke is rare in clinical practice. In his first report of such case, Hriso proposed that the fibers destined to the elevators of the eye and eyelid coursed laterally in nerve fascicle.10 Choi reviewed the literature of similar cases and located the lesion in overlapping regions between the red nucleus and cerebral peduncle in the midbrain.11
Lacunar stroke (LS) is a type of ischemic stroke with lesion diameter ranging from 3 to 20 mm. It is attributed to occlusion of small deep penetrating branches of cerebral arteries. Etiology of LS includes lipohyalinosis, atherosclerosis, branch atheromatous disease (BAD), and embolism. Fisher first coined the term ‘lipohyalinosis’ as fibrinoid material accumulation and collagenous sclerosis of the wall of small vessels. Lipohyalinosis is considered secondary to uncontrolled hypertension.12 For an alternative pathomechanism of LS, BAD was first described by Caplan13 as a pathologic finding of occlusion at the origin of a deep penetrating artery due to a microatheroma or a parent vessel atheromatous plaque. Lesions associated with BAD are located proximally along the perforator artery, usually larger than cerebral small vascular disease-related LS.14 Diagnosis of BAD was previously based on the vascular territory, size, and/or shape of the infarcts as identified by conventional imaging methods. More advanced HR-MRI can show artery wall changes and aid BAD diagnosis.15 LS can also be attributed to embolism arising from the carotid, aortic arch or heart, or watershed infarction between adjacent vascular territories. The lesion of our patient meets the size and the noncortical and nonwatershed location of LS. The atheromatous plaque in the right PCA most likely resulted in occlusion of a perforating artery from PCA and the infarction which compromises the fibers that innervate superior rectus and levator palpebrae (Figure 2).
As discussed above, our patient fulfilled the criteria of neurovascular conflict. Nevertheless, this conflict does not accord with the symptomatology of this patient according to the topography of oculomotor nerve. So the final diagnosis of this patient is fascicular oculomotor nerve palsy caused by midbrain lacunar ischemic stroke secondary to BAD of the ipsilateral PCA.
Monocular partial oculomotor palsy bears many differential diagnoses. Meticulous history questioning and physical examination pave way for correct localization of the lesion. Careful selection of pertinent investigation would limit the economic cost and ensure identification of the underlying cause. More advanced imaging such as 3D-CISS and HRMR-VWI enables illustration of fine changes of intracranial structures that are otherwise undetected by conventional imaging tools.
Appendix. Authors

| Name | Location | Contribution |
| Zhenghui Liu, MMed | Department of Neurology, Xiangya Hospital, Central South University, Changsha, China | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Wei Wei Zhang, MD | Department of Radiology, Xiangya Hospital, Central South University, Changsha, China | Major role in the acquisition of data; analysis or interpretation of data |
| Piaoyu Dai, MMed | Department of Nephrology, Xiangya Hospital, Central South University, Changsha, China | Major role in the acquisition of data; analysis or interpretation of data |
| Xiaoyan Long, MD | Department of Neurology, Xiangya Hospital, Central South University, Changsha, China | Major role in the acquisition of data; analysis or interpretation of data |
| Lu Shen, MD | Department of Neurology, Xiangya Hospital, National Clinical Research Center for Geriatric Disorders, Engineering Research Center of Hunan Province in Cognitive Impairment Disorders; Hunan International Scientific and Technological Cooperation Base of Neurodegenerative and Neurogenetic Diseases; Key Laboratory of Hunan Province in Neurodegenerative Disorders, Central South University; Key Laboratory of Organ Injury, Aging and Regenerative Medicine of Hunan Province, Changsha, China | Major role in the acquisition of data; analysis or interpretation of data |
| Yue-Bei Luo, PhD, MBBS | Department of Neurology, Xiangya Hospital, Central South University, Changsha, China | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
No targeted funding reported.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Keene KR, Kan HE, van der Meeren S, et al. Clinical and imaging clues to the diagnosis and follow-up of ptosis and ophthalmoparesis. J Cachexia Sarcopenia Muscle. 2022;13(6):2820-2834. doi: 10.1002/jcsm.13089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park HK, Rha HK, Lee KJ, Chough CK, Joo W. Microsurgical anatomy of the oculomotor nerve. Clin Anat. 2017;30(1):21-31. doi: 10.1002/ca.22811 [DOI] [PubMed] [Google Scholar]
- 3.Ksiazek SM, Slamovits TL, Rosen CE, Burde RM, Parisi F. Fascicular arrangement in partial oculomotor paresis. Am J Ophthalmol. 1994;118(1):97-103. doi: 10.1016/s0002-9394(14)72848-x [DOI] [PubMed] [Google Scholar]
- 4.Ksiazek SM, Repka MX, Maguire A, et al. Divisional oculomotor nerve paresis caused by intrinsic brainstem disease. Ann Neurol. 1989;26(6):714-718. doi: 10.1002/ana.410260605 [DOI] [PubMed] [Google Scholar]
- 5.Sivasubramaniyan KM, Nagarajan K, Rajeswari A, Sathiaprabhu A. A case of oculomotor nerve palsy caused by neurovascular compression by the fetal posterior communicating artery with a review of literature. Neurol India. 2019;67(5):1390-1392. doi: 10.4103/0028-3886.271270 [DOI] [PubMed] [Google Scholar]
- 6.Borges A, Casselman J. Imaging the cranial nerves: part II: primary and secondary neoplastic conditions and neurovascular conflicts. Eur Radiol. 2007;17(9):2332-2344. doi: 10.1007/s00330-006-0572-z [DOI] [PubMed] [Google Scholar]
- 7.Miyazaki S. Location of motoneurons in the oculomotor nucleus and the course of their axons in the oculomotor nerve. Brain Res. 1985;348(1):57-63. doi: 10.1016/0006-8993(85)90359-2 [DOI] [PubMed] [Google Scholar]
- 8.Atasever A, Durgun B, Celik HH, Yilmaz E. Somatotopic organization of the axons innervating the superior rectus muscle in the oculomotor nerve of the rat. Acta Anat (Basel). 1993;146(4):251-254. doi: 10.1159/000147464 [DOI] [PubMed] [Google Scholar]
- 9.Turan TN, Zaidat OO, Gronseth GS, et al. Stroke prevention in symptomatic large artery intracranial atherosclerosis practice advisory: report of the AAN Guideline Subcommittee. Neurology. 2022;98(12):486-498. doi: 10.1212/wnl.0000000000200030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hriso E, Masdeu JC, Miller A. Monocular elevation weakness and ptosis: an oculomotor fascicular syndrome? J Clin Neuroophthalmol. 1991;11(2):111-113. doi: 10.3109/01658109108997304 [DOI] [PubMed] [Google Scholar]
- 11.Choi YJ, Lee SH, Park MS, Kim BC, Kim MK. Midbrain infarction presenting with monocular elevation palsy and ptosis: topographic lesion analysis. J Neuroophthalmol. 2015;35(2):175-178. doi: 10.1097/wno.0000000000000208 [DOI] [PubMed] [Google Scholar]
- 12.Fisher CM. Lacunar strokes and infarcts: a review. Neurology. 1982;32(8):871-876. doi: 10.1212/wnl.32.8.871 [DOI] [PubMed] [Google Scholar]
- 13.Caplan LR. Intracranial branch atheromatous disease: a neglected, understudied, and underused concept. Neurology. 1989;39(9):1246-1250. doi: 10.1212/wnl.39.9.1246 [DOI] [PubMed] [Google Scholar]
- 14.Nah HW, Kang DW, Kwon SU, Kim JS. Diversity of single small subcortical infarctions according to infarct location and parent artery disease: analysis of indicators for small vessel disease and atherosclerosis. Stroke. 2010;41(12):2822-2827. doi: 10.1161/strokeaha.110.599464 [DOI] [PubMed] [Google Scholar]
- 15.Petrone L, Nannoni S, Del Bene A, Palumbo V, Inzitari D. Branch atheromatous disease: a clinically meaningful, yet unproven concept. Cerebrovasc Dis. 2016;41(1-2):87-95. doi: 10.1159/000442577 [DOI] [PubMed] [Google Scholar]