Abstract
Background/Objective
Guidelines on obesity management reinforce regular exercise to reduce body fat. Exercise modalities, including high-intensity interval training (HIIT), appear to produce a similar effect to continuous aerobic training (CAT) on body fat. However, they have not addressed the chronic effect of HIIT vs. CAT on body fat assessed by dual energy X-ray absorptiometry (DEXA). Thus, we compared the effectiveness of CAT vs. HIIT protocols on body fat (absolute or relative) (%BF) and abdominal visceral fat reduction, assessed by DEXA, in adults with overweight and obesity.
Methods
We conducted a systematic review and meta-analysis of randomized clinical trials (RCTs) including both female or male adults with excess body weight. We performed searches in the databases MEDLINE (PubMed), EMBASE, Scopus, LILACS, Web of Science and Cochrane.
Results
In our analysis (11 RCTs), we found no greater benefit on %BF of HIIT vs. CAT (MD –0.55%, 95% CI –1.42 to 0.31; p = 0.209). As for abdominal visceral fat, no training modality was superior (SMD: −0.05, 95% CI –0.29 to 0.19; p = 0.997). Regarding secondary outcomes (body weight, BMI, VO2 max, glycemic and lipid profiles), HIIT shows greater benefit than CAT in increasing VO2 max and fasting blood glucose and reducing total cholesterol.
Conclusion
HIIT is not superior to CAT in reducing %BF or abdominal visceral fat in individuals characterized by excess weight. However, HIIT showed beneficial effects on cardiorespiratory fitness, total cholesterol and fasting blood glucose when compared to CAT.
Keywords: Exercise training, Excess weight, DEXA, Body composition
1. Introduction
Obesity is defined as excessive accumulation of body fat1 that is strongly associated with several harmful health conditions2 such as insulin resistance, type 2 diabetes mellitus, dyslipidemia, atherosclerosis, systemic arterial hypertension, acute myocardial infarction, stroke, among others.3,4 In Brazil, it has been estimated in 2021 that excess weight affected 57.2% of the population and the obesity prevalence was 22.4%.5
Excess body fat—body and/or abdominal visceral fat assessed as total mass or percentage—is a major health concern as people engage in sedentary lifestyles. There is no consensus on the gold standard for body composition. However, dual energy X-ray absorptiometry (DEXA) provides accurate measures of bone mineral content, body fat mass, lean mass and visceral fat in limb or whole-body assessments.6 It is thus currently the reference method for body composition assessment and is used to validate predictive equations for doubly indirect methods.7, 8, 9, 10
Several research studies have investigated the relationship between exercise training and weight reduction in individuals with obesity.11,12 Traditional aerobic exercises involving continuous aerobic training (CAT, moderate-intensity exercise ranges between 40% and 60–65% of maximal VO2 max) and/or resistance exercise can have beneficial effects and improve body composition by reducing body fat mass and increasing lean mass.13,14 However, other exercise modalities, including high-intensity interval training (HIIT), appear to produce at least similar beneficial effects on body composition as traditional aerobic exercises.15 There is no universal definition of HIIT. Typically, it is characterized by short bursts of high-intensity activity interspersed by periods of (active or passive) rest with the advantage of a shorter time frame and total session duration over CAT. The American College of Sports Medicine states that for high-intensity exercise is above 65% of maximal VO2 max.14 However, intensity below 80% of maximal VO2 max, we should not consider generally this as high-intensity training.16
HIIT mostly involves greater energy expenditure than CAT (of moderate intensity and volume). It is thus logical to believe that greater energy expenditure would result in greater reduction of body fat. This is further supported by a greater effect of HIIT on VO2 max17 as well as the effect of excess post-exercise oxygen consumption (EPOC)18 compared to CAT. These adaptations would be associated with fatty acid oxidation, thereby contributing to body fat reduction.
Some evidence suggests that HIIT offers significant benefits in reducing body fat,19, 20, 21 but other studies have not shown these same benefits.22, 23, 24, 25 A number of meta-analyses have aimed to further examine these effects, but they involved different populations26,27 or different body composition assessment methods27,28 as well as other factors that may affect the results.
Given that DEXA is currently the reference method of body composition assessment7, 8, 9, 10 and that there are only two studies relevant to our research question, the present systematic review aimed to compare the effectiveness of HIIT and moderate-intensity CAT in improving body fat (BF) and abdominal visceral fat measurements in adults with excess weight (overweight and obesity) assessed by DEXA. As secondary outcomes, we compared biochemical markers of the two interventions. The hypothesis of this study was that HIIT is superior to CAT in improving body fat (absolute and relative) and abdominal visceral fat).
2. Methods
2.1. Study protocol and registration
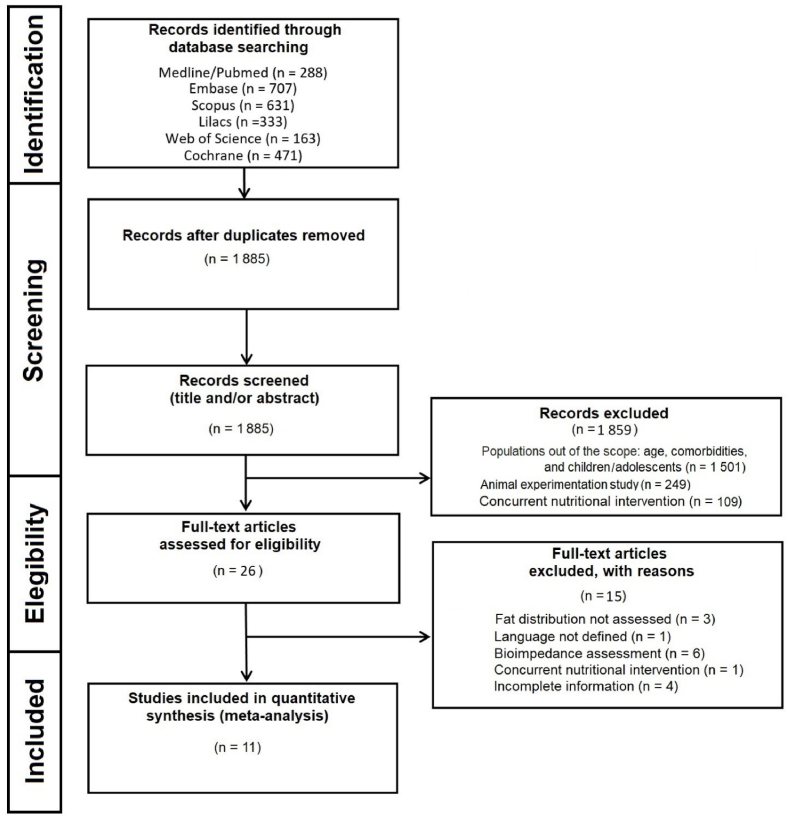
We conducted this systematic review following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)29 and Cochrane Handbook for Systematic Reviews of Interventions.30 This review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (“https://www.crd.york.ac.uk/prospero/#searchadvanced”, ID “CRD42022357195”; Date of first submission: August 31, 2022; Date of registration in PROSPERO: 11 September 2022). Fig. 1 shows the flowchart of the study. We performed database searches until October 12, 2022. The database used in this systematic review is available on Mendeley Data repository (“https://data.mendeley.com/datasets/67mwfxtmzv”, https://doi.org/10.17632/67mwfxtmzv.3).
Fig. 1.
Flowchart of the study.
2.2. Eligibility criteria
For consistency in the terminology and considering that reduction of both subcutaneous and visceral fat induces weight loss,31,32 we chose to use “abdominal visceral fat” as a general term to refer to fat in the abdominal cavity.
We used PICOS framework to develop our search strategies, as follows: Population – adults ≥18 years of age with excess weight (BMI ≥25.0 kg/m2);5 Intervention – HIIT and CAT; Comparison – HIIT at 85–120% of HRmax, HRR or VO2 max14 compared to moderate-intensity CAT (60–75% HRmax or 50–65% HRR or VO2 max); Outcome – % BF and abdominal fat (abdominal visceral fat) assessed by DEXA; Study – randomized controlled trial (RCT).
2.3. Search strategy
Two reviewers (AMK and JBM) conducted all searches and the selection of studies eligible for this systematic review as described in the Cochrane Handbook for Systematic Reviews of Interventions.30 They independently read titles and abstracts for the selection and review of studies. Searches were conducted in the electronic databases MEDLINE (PubMed), EMBASE, Scopus, LILACS (Latin America and the Caribbean Health Sciences Literature/VHL – Virtual Health Library), Web of Science and Cochrane. When the abstract did not contain enough information, full-text articles were examined. Additional searches were undertaken in the Brazilian Clinical Trials Registry (ReBEC), Clinical Trial.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) for unpublished studies in order to minimize potential publication bias or distortions in the results.
Any disagreements were resolved through a consensus discussion and, if necessary, by a third reviewer (AML). When reviewers encountered restrictions on access to full-text articles, the authors were contacted by e-mail. Articles in Portuguese, English and Spanish with no date of publication limits were eligible for inclusion in our review.
The main key terms used in our search strategy were “obesity,” “overweight,” “exercise,” “high-intensity interval training” and “body fat”. To increase the accuracy and sensitivity of our search, the terms for RCTs in the MEDLINE33 and EMBASE34 databases were added to the search terms. The complete search strategy is detailed in Chart 1S.
2.4. Inclusion and exclusion criteria
Our searches were exported into EndNote X9 and screened against the inclusion and exclusion criteria. All studies were independently rated by two reviewers (AMK and JBM). Those that met the following criteria were included in the review: sample of female or male adults (≥18 years old); study population with excess body weight (overweight or obesity; BMI ≥25 kg/m2). It should be stressed that the studies were labeled based on BMI classifications of overweight or obesity provided by the authors,5 and randomized controlled design comparing HIIT with CAT. Studies evaluating other exercise interventions with well-defined independent HIIT or CAT groups were fully reviewed to ascertain their eligibility. Furthermore, there were excluded studies involving animals and children/adolescents, dietary interventions, and duplicates.
2.5. Data extraction and management
The primary outcome was changes in body fat (absolute and relative) and abdominal visceral fat. We assessed the following parameters as secondary outcomes due to their association with cardiometabolic risk: body weight (kg); BMI (kg/m2); VO2 max (mL/kg/min); fasting blood glucose (mg/dL or mmol/L); total cholesterol (mg/dL or mmol/L); and triglycerides (mg/dL or mmol/L). Fasting blood glucose, lipid profile and VO2 max are associated with adiposity and may change in response to exercise training.
Three blinded reviewers (AMK, GW, and AML) independently extracted and compiled data into a spreadsheet in Office Excel 2010. The three data spreadsheets were compared and any disagreements were resolved through consensus. We extracted data from pre- and post-intervention means in each group (HIIT or CAT), and estimated measures of dispersion (standard error, standard deviation [SD] and 95% confidence interval) as well as mean differences (Δ = post- – pre-training). We also extracted training-related data including weekly exercise frequency, exercise intensity, session duration and sample characterization.
2.6. Risk of bias and quality of evidence
Two reviewers (AMK and PCO) independently assessed the risk of bias of individual studies using Cochrane Risk of Bias 2 (RoB 2) tool (www.riskofbias.info/) according to guidance provided in the Cochrane Handbook.30 The risk of bias assessment is based on a set of six domains of bias and judgement: randomization process, deviations from the intervention (allocation sequence concealment), outcome data, outcome assessment, selection of the reported result, and selective outcome reporting.
Based on that, studies were classified as low risk of bias (in all domains for this result); some concerns of bias (in at least one domain for this outcome, but not at high risk of bias in any domain); or high risk of bias (in at least one domain for this outcome, or the study was judged at some concerns for several domains in a way that significantly reduces confidence in the outcome). Since it is not feasible to blind participants to exercise interventions, all studies were classified as high risk of bias in the domain “blinding of participants and personnel.” No study was excluded based on the risk of bias assessment. It should be pointed out that the risk of bias was assessed for the primary outcomes of interest.
We evaluated the quality of evidence (strength of recommendations) using GRADE tool (“www.gradeworkinggroup.org/”). This tool classifies the quality of evidence into four levels (high, moderate, low and very low) based on the assessment of confidence in specific estimates in five domains: methodological limitations (risk of bias); inconsistency (heterogeneity); indirectness of evidence; imprecision; and publication bias.30,35
2.7. Statistical analysis
We performed analyses to compare the effects of HIIT and CAT on % BF and abdominal visceral fat. Effect measures of % BF were presented as mean differences (MDs) between HIIT vs. CAT groups (Δ % BF to HIIT vs. Δ % BF to CAT) and their related 95% confidence intervals (95% CIs) using the inverse variance method. Since abdominal visceral fat measurements were reported in different units (cm3, % or kg), standardized mean differences (SMDs) and related 95% CIs were used as they express the size of the intervention effect in each study relative to the variability observed. The results (MDs or SMDs) were pooled using a random-effects model. Taking into consideration that the confidence interval from a random-effects analysis describes uncertainty in the location of the mean of systematically different effects in the different studies and to ensure that the results are interpreted correctly, we considered the 95% prediction interval (95% PI) because it expresses the interval of uncertainty of the possible effect in a new RCT.36
For interpreting secondary data expressed as SMD we used Cohen's (1988) effect size classified as small (0.2), moderate (0.5) and large effect size (0.8).37 To assess heterogeneity, we used Higgins inconsistency test (I2) (0%–40%, might not be important; 30%–60%, may represent moderate heterogeneity; 50%–90%, may represent substantial heterogeneity; and 75%–100%, considerable heterogeneity).30,38 We tested heterogeneity (p < 0.05) using subgroup analyses or meta-regression for effect modifiers with normal distribution in a quartile-quartile plot (qq-plot) confirmed by the Shapiro-Wilk test.39 In addition, to detect and remove discrepant data from the meta-analysis, forest plots were constructed to visualize the effect estimate of individual studies and allow to detect outliers based on non-CI overlapping that is due to heterogeneity.39 We performed sensitivity analysis by including or excluding one study each time.39 Potential effect modifiers such as age, baseline BMI and total exercise sessions (weekly frequency x total duration of the intervention) were analyzed separately. If applicable (≥10 studies), we performed the Egger's test using a funnel plot to assess publication bias. To avoid a unit-of-analysis error for RCTs with multiple treatment arms and a single control group, the sample size for the control group was weighed by the number of groups and participants treated.40 When SD of differences (post-minus pre-intervention) was not available in an eligible study, SDs were calculated at each time point (pre- and post-intervention) using an imputed correlation coefficient of 0.5:30,41 ΔSD = √ SD2 baseline + SD2 final – (2 * 0,5 * SD baseline * SD final). The measures of dispersion expressed as CIs or standard errors were converted into standard deviations (SDs = EP * √n) prior to the analysis.
All statistical tests were two-tailed and the significance level was set at p < 0.05. All measures of dispersion presented as CIs or standard errors were converted into standard deviations (SDs) before the analysis. We used RStudio for statistical analyses. Chart 2S (Supplementary Material) shows the Main RStudio script.
3. Results
3.1. Selection of articles
A total of 2593 studies were identified in our initial searches. Of these, 708 were excluded due to duplicates. Of the remaining 1885 studies, 1859 were screened out after title and abstract reading (non-RCTs; children/adolescents or animal experimentation; and concomitant use of other interventions, including diets, food supplements, medications, among others). Of 26 full-text articles reviewed, 14 were excluded because they did not have a control group or information provided was uncertain. As a result, 11 studies42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52 were included in the quantitative analysis (Fig. 1). It is noteworthy that the study by Cooper (2016) had two groups of interest (HIIT) and Zhang (2021) evaluated in their study three groups of interest (HIIT) that followed PICOS criteria. These groups were individually analyzed in the meta-analysis making a total of 14 arms for the primary outcome (% BF) – Table 1. Because not all studies evaluated abdominal visceral fat, there were 11 data entries for abdominal visceral fat in the meta-analysis.
Table 1.
Exercise protocols of the studies included in the analysis.
| Sijie (2012)50 |
|
| Gripp (2020)43 |
|
| Keating (2014)46 |
|
| Zhang (2017)51 |
|
| Cooper (2016)42 |
|
| Hornbuckle (2018)45 |
|
| Ram (2020)48 |
|
| Higgins (2016)44 |
|
| Kong (2016)47 |
|
| Zhang (2021)52 |
|
| Rowley (2017)49 |
|
HR, heart rate; VO2, oxygen consumption volume; HIIT, high-intensity interval training; CAT, continuous aerobic training.
3.2. Characteristics of the studies
Table 2 shows the main characteristics of the studies. A total of 379 participants were included in the primary outcome analysis (% BF). Of these, 194 were in the intervention group (HIIT) and 185 in the comparator group (CAT). As for clinical characteristics of the participants, 11 studies involved adults with BMI of 25.0–29.9 kg/m2 and four studies involved adults with BMI ≥30.0 kg/m2.42,44,45,49 Of 14 groups included in the meta-analysis, six consisted of women only,44,45,49, 50, 51, 52 three of men only,42,43,48 one included both men and women,46 and one study did not report this information.47 All studies reported the participants’ ages that ranged from 19 to 51 years, mean age of 29.5 ± 10.4 years. HIIT intervention involved 3–5 sessions a week, average duration of 26.6 min for a total of 5–16 weeks. CAT intervention involved 3–5 sessions a week, average duration of 44 min for 5–16 weeks. Only one study49 evaluated data at different time points (baseline, 6 and 12 weeks), and we chose to prioritize data more frequently assessed in the selected studies (12 weeks). Of the 11 studies included, six involved an intervention duration of 12 weeks,42,46,49, 50, 51, 52 four <12 weeks43,44,47,48 and only one >12 weeks.45 Also, of the eligible studies, only one study51 compared data from three different HIIT arms against one CAT arm.
Table 2.
Changes in body weight, BMI, and maximal oxygen uptake in included studies.
| Group |
n |
Sex |
Body weight (kg) |
BMI (kg/m2) |
VO2 max (mL/kg/min) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post | Δ | Baseline | Post | Δ | Baseline | Post | Δ | ||||
| Sijie (2012)50 | HIIT | 17 | F | 74 ± 8 | 68 ± 7 | −6 | 27.7 ± 1.8 | 26.3 ± 1.9 | −1.4 | 33 ± 4 | 36 ± 3 | 3 |
| CAT | 16 | F | 74 ± 9 | 70 ± 9 | −4 | 28.3 ± 2.0 | 26.6 ± 2.2 | −1.7 | 33 ± 5 | 35 ± 4 | 2 | |
| Gripp (2020)43 | HIIT | 11 | M | 81 ± 7 | – | – | 27.2 ± 13.0 | 26.8 ± 1.3 | −0.4 | 46 ± 10 | 53 ± 10 | 7 |
| CAT | 11 | M | 84 ± 15 | – | – | 28.7 ± 3.1 | 28.2 ± 3.5 | −0.5 | 45 ± 4 | 49 ± 15 | 4 | |
| Keating (2014)46 | HIIT | 13 | 10 F/3 M | – | 76 ± 11 | – | 28.2 ± 1.8 | – | – | 25 ± 37 | 30 ± 1 | 5 |
| CAT | 11 | 9 F/2 M | – | 80 ± 6 | – | 28.5 ± 1.9 | – | – | 24 ± 4 | 28 ± 2 | 4 | |
| Zhang (2017)51 | HIIT | 15 | F | – | 64 ± 6 | – | – | – | – | 32 ± 2 | – | – |
| CAT | 15 | F | – | 65 ± 8 | – | – | – | – | 31 ± 4 | – | – | |
| Cooper (2016)42 | HIIT/active rest | 15 | M | 94 ± 19 | 94 ± 19 | 0 | 29.7 ± 4.0 | 30.1 ± 1.2 | 0.4 | 28 ± 4 | 31 ± 4 | 3 |
| HIIT/passive rest | 15 | M | 95 ± 20 | 96 ± 21 | 1 | 29.7 ± 4.1 | 30.3 ± 1.0 | 0.6 | 25 ± 5 | 30 ± 5 | 5 | |
| CAT | 15 | M | 96 ± 11 | 96 ± 12 | 0 | 30.6 ± 3.4 | 30.6 ± 1.5 | 0.0 | 24 ± 4 | 27 ± 5 | 3 | |
| Hornbuckle (2018)45 | HIIT | 11 | F | 100 ± 12 | 100 ± 11 | 0 | 35.7 ± 3.3 | 35.7 ± 2.9 | 0.0 | 24 ± 3 | 26 ± 2 | 2 |
| CAT | 3 | F | 94 ± 5 | 94 ± 5 | 0 | 33.5 ± 1.0 | 33.6 ± 1.2 | 0.1 | 24 ± 3 | 24 ± 4 | 0 | |
| Ram (2020)48 | HIIT | 16 | M | 86 ± 19 | 85 ± 17 | −1 | 28.1 ± 4.1 | 28.4 ± 4.1 | 0.3 | 34 ± 7 | 36 ± 6 | 2 |
| CAT | 12 | M | 87 ± 17 | 89 ± 16 | 2 | 27.4 ± 4.0 | 27.8 ± 3.8 | 0.4 | 33 ± 7 | 35 ± 6 | 2 | |
| Higgins (2016)44 | HIIT | 23 | F | 82 ± 12 | 81 ± 12 | −1 | 30.3 ± 4.5 | – | – | 29 ± 5 | 33 ± 4 | 4 |
| CAT | 29 | F | 83 ± 14 | 83 ± 15 | 0 | 30.3 ± 4.6 | – | – | 27 ± 5 | 29 ± 4 | 2 | |
| Kong (2016)47 | HIIT | 10 | – | 68 ± 9 | 69 ± 9 | 1 | 25.5 ± 2.1 | 25.7 ± 2.2 | 0.2 | 34 ± 6 | 37 ± 7 | 3 |
| CAT | 8 | – | 69 ± 9 | 68 ± 8 | −1 | 26.2 ± 2.4 | 25.9 ± 2.2 | −0.3 | 34 ± 4 | 38 ± 7 | 4 | |
| Zhang (2021)52 | HIIT 90 | 12 | F | 66 ± 7 | 63 ± 7 | −3 | 26.0 ± 2.9 | 24.9 ± 1.1 | −1.1 | 29 ± 2 | 38 ± 3 | 9 |
| HIIT 120 | 12 | F | 70 ± 10 | 68 ± 9 | −2 | 26.1 ± 3.2 | 25.5 ± 1.5 | −0.6 | 26 ± 4 | 35 ± 5 | 9 | |
| All-HIIT | 11 | F | 67 ± 7 | 64 ± 6 | −3 | 25.6 ± 2.4 | 24.6 ± 1.6 | −1.0 | 27 ± 3 | 33 ± 3 | 6 | |
| CAT | 11 | F | 65 ± 9 | 64 ± 8 | −1 | 25.1 ± 3.0 | 25.2 ± 1.3 | 0.1 | 29 ± 3 | 34 ± 4 | 5 | |
| Rowley (2017)49 | HIIT | 8 | F | 87 ± 15 | 87 ± 15 | 0 | 32.3 ± 4.7 | 32.0 ± 4.6 | −0.3 | 30 ± 3 | – | – |
| CAT | 7 | F | 83 ± 4 | 82 ± 4 | −1 | 29.5 ± 1.8 | 28.9 ± 1.6 | −0.6 | 27 ± 2 | – | – | |
Values are presented as mean ± standard deviation; BMI, body mass index; HIIT, high-intensity interval training; CAT, continuous aerobic training. VO2 max, maximal oxygen uptake. F, female; M, male.
Table 3 shows biochemical profiles of the study participants. Of 14 studies, five43,45, 46, 47,52 reported complete information for fasting blood glucose (seven arms of HIIT); five43,45,46,49,52 for total cholesterol and triglycerides (seven arms of HIIT); and four studies43,45,46,49 for HDL (four arms of HIIT).
Table 3.
Biochemical profile.
| Fasting Blood Glucose (mg/dL) |
Total Cholesterol (mg/dL) |
High Density Lipoprotein (mg/dL) |
Triglycerides (mg/dL) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | Baseline | Post | Δ | Baseline | Post | Δ | Baseline | Post | Δ | Baseline | Post | Δ | |
| Sijie (2012)50 | HIIT | – | – | – | – | – | – | – | – | – | – | – | – |
| CAT | – | – | – | – | – | – | – | – | – | – | – | – | |
| Gripp (2020)43 | HIIT | 94 ± 8 | 94 ± 8 | 0 | 216 ± 33 | 186 ± 28 | −30 | 50 ± 9 | 46 ± 7 | −4 | 189 ± 79 | 118 ± 50 | −71 |
| CAT | 94 ± 9 | 92 ± 9 | −2 | 224 ± 43 | 213 ± 42 | −11 | 50 ± 9 | 50 ± 8 | 0 | 198 ± 80 | 161 ± 54 | −37 | |
| Keating (2014)46 | HIIT | 79 ± 13 | 77 ± 6 | −2 | 208 ± 42 | 209 ± 28 | 1 | 66 ± 42 | 66 ± 28 | 0 | 115 ± 32 | 115 ± 64 | 0 |
| CAT | 77 ± 6 | 79 ± 6 | 2 | 205 ± 38 | 213 ± 25 | 8 | 54 ± 13 | 54 ± 13 | 0 | 106 ± 58 | 97 ± 29 | −9 | |
| Zhang (2017)51 | HIIT | – | – | – | – | – | – | – | – | – | – | – | – |
| CAT | – | – | – | – | – | – | – | – | – | – | – | – | |
| Cooper (2016)42 | HIIT/active rest | 105 ± 11 | – | – | 201 ± 46 | – | – | 42 ± 12 | – | – | 106 ± 62 | – | – |
| HIIT/passive rest | 103 ± 9 | – | – | 217 ± 31 | – | – | 43 ± 15 | – | – | 159 ± 71 | – | – | |
| CAT | 105 ± 7 | – | – | 216 ± 50 | – | – | 43 ± 8 | – | – | 168 ± 97 | – | – | |
| Hornbuckle (2018)45 | HIIT | 88 ± 7 | 87 ± 11 | −1 | 161 ± 27 | 161 ± 25 | 0 | 48 ± 7 | 48 ± 0 | 0 | 71 ± 18 | 78 ± 24 | 7 |
| CAT | 87 ± 3 | 86 ± 3 | −1 | 158 ± 40 | 156 ± 59 | −2 | 56 ± 10 | 59 ± 0 | 3 | 61 ± 6 | 52 ± 6 | −9 | |
| Ram (2020)48 | HIIT | – | – | – | – | – | – | – | – | – | – | – | – |
| CAT | – | – | – | – | – | – | – | – | – | – | – | – | |
| Higgins (2016)44 | HIIT | – | – | – | – | – | – | – | – | – | – | – | – |
| CAT | – | – | – | – | – | – | – | – | – | – | – | – | |
| Kong (2016)47 | HIIT | 81 ± 4 | 79 ± 11 | −2 | – | – | – | – | – | – | – | – | – |
| CAT | 83 ± 9 | 79 ± 7 | −4 | – | – | – | – | – | – | – | – | – | |
| Zhang (2021)52 | HIIT 90 | 70 ± 7 | 59 ± 11 | −11 | 213 ± 43 | 190 ± 43 | −23 | – | – | – | 150 ± 27 | 133 ± 27 | −17 |
| HIIT 120 | 72 ± 11 | 61 ± 7 | −11 | 193 ± 54 | 174 ± 43 | −112 | – | – | – | 186 ± 142 | 133 ± 27 | −53 | |
| HIIT all | 70 ± 5 | 59 ± 11 | −11 | 197 ± 58 | 158 ± 39 | −123 | – | – | – | 168 ± 71 | 133 ± 18 | −35 | |
| CAT | 72 ± 18 | 70 ± 13 | −2 | 182 ± 46 | 174 ± 35 | −8 | – | – | – | 168 ± 35 | 168 ± 35 | 0 | |
| Rowley (2017)49 | HIIT | – | – | – | 203 ± 10 | 190 ± 12 | −13 | 52 ± 22 | 54 ± 23 | 2 | 167 ± 65 | 106 ± 27 | −61 |
| CAT | – | – | – | 228 ± 46 | 240 ± 46 | 12 | 59 ± 7 | 62 ± 15 | 3 | 180 ± 111 | 133 ± 53 | −47 | |
Values are presented as mean ± standard deviation; HIIT, high-intensity interval training; CAT, continuous aerobic training; Post, post-training.
3.3. Risk of bias and quality of studies
Of 11 studies selected for this review, only two reported the randomization sequence46,48 and none reported blinding participants and/or evaluators. Thus, all studies were classified as “some concerns” of risk of bias (Fig. 1S of supplementary material). All studies performed intention-to-treat analyses and reported point estimates of effect size.
Regarding the strength of evidence assessed by GRADE,30,35 the outcomes of interest (% BF and abdominal visceral fat) were rated as “low quality of evidence”. Although all studies included in the meta-analysis were considered of high quality (RCT design), we removed one point in the “risk of bias” domain due to no blinding of volunteers and evaluators for the intervention (exercise), and one point in the “inconsistency (heterogeneity)” domain due to substantial heterogeneity (>75%) because of different characteristics of the samples examined and the small number of studies (Fig. 2S of supplementary material).
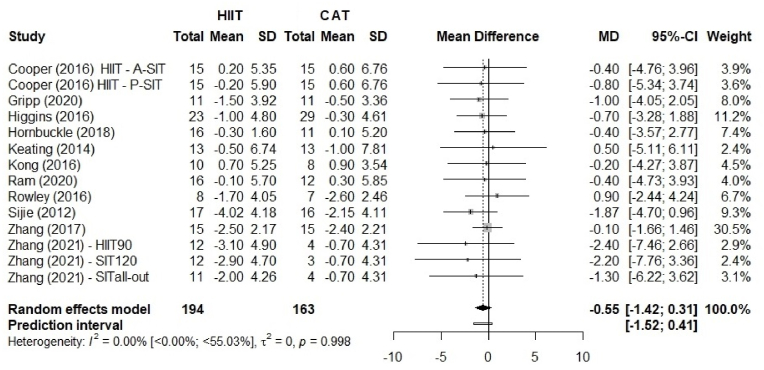
Fig. 2.
Comparison of body fat percentage (%BF) between HIIT and CAT. HIIT, high-intensity interval training; continuous aerobic training (CAT); SD, standard deviation; MD, mean difference; CI, confidence interval.
3.4. Publication bias
The risk of publication bias assessment did not show substantial concerns of bias for both % BF and abdominal visceral fat. The funnel plot of % BF and abdominal visceral fat data is presented in the supplementary material (Fig. 3S of supplementary material).
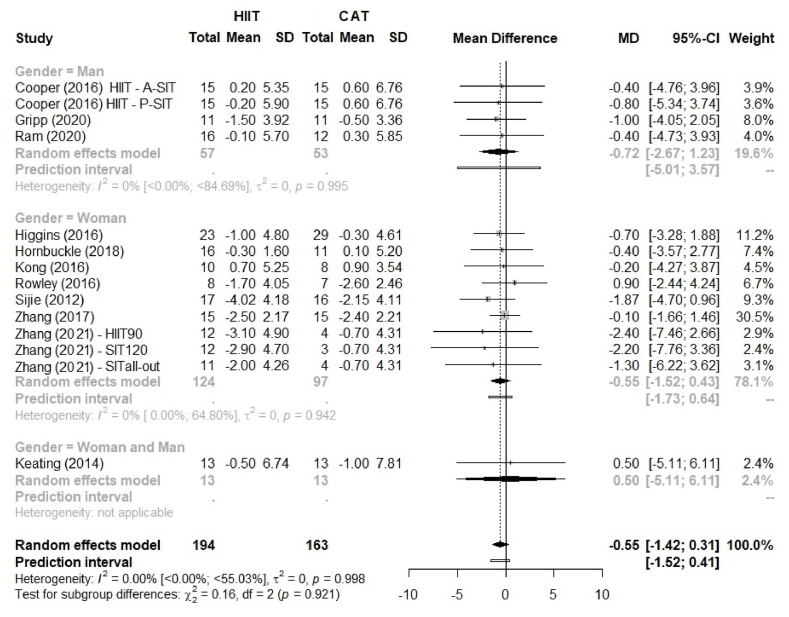
Fig. 3.
Comparison of body fat percentage (%BF) between HIIT and CAT by sex as a potential moderating effect. HIIT, high-intensity interval training; continuous aerobic training (CAT); SD, standard deviation; MD, mean difference; CI, confidence interval.
3.5. Effect size of HIIT vs. CAT
Fig. 2 shows the effect size for % BF with no advantage of HIIT over CAT, MD 0.55% (95% CI –1.42 to 0.31; p = 0.209), and 95% PI ranged from −1.52 to 0.41. The sex-stratified analysis (Fig. 3) showed similar results: HIIT was not superior to CAT for both men and women (p = 0.921).
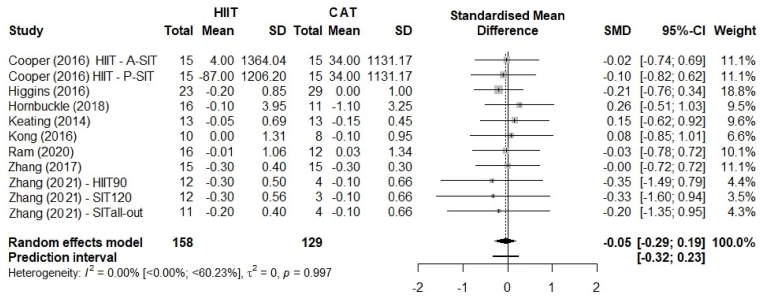
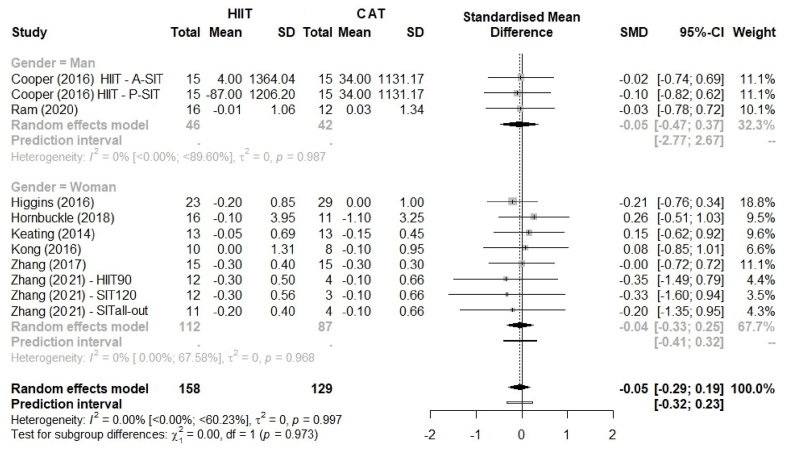
Fig. 4 shows the analysis of abdominal visceral fat. Because abdominal visceral fat was reported in different units [kg in eight studies,44,46, 47, 48,51,52% in two studies42,45 and cm3 in one study42], we used SMDs to present the size of the intervention effect. We found a SMD of −0.05 with no superiority of HIIT over CAT (95% CI –0.29 to 0.19; p = 0.997). A comparison of HIIT vs. CAT did not allow to make any conclusions based on the 95% CI, and on 95% PI (−0.32 to 0.23). The sex-stratified analysis (Fig. 5) showed there is no significant sex difference (p = 0.966).
Fig. 4.
Comparison of abdominal visceral fat between HIIT and CAT. HIIT, high-intensity interval training; CAT, continuous aerobic training; SD, standard deviation; SMD, standardized mean deviation; CI, confidence interval.
Fig. 5.
Comparison of abdominal visceral fat between HIIT and CAT by sex as a potential moderating effect. HIIT, high-intensity interval training; CAT, continuous aerobic training; SD, standard deviation; SMD, standardized mean deviation; CI, confidence interval.
Table 4 shows mean differences of secondary outcomes—body weight,42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52 BMI,42,43,45,47, 48, 49, 50,52 VO2 max,42, 43, 44, 45, 46, 47, 48, 49, 50,52 glycemic42,43,45, 46, 47 and lipid profiles.42,43,45,46,49,52 Interestingly, HIIT was superior to CAT in increasing VO2 max (SMD: 0.30, 95% CI 0.09 to 0.52, p = 0.005, small effect size), improving fasting blood glucose (SMD: −0.67, 95% CI –1.07 to −0.27, p = 0.001, moderate effect), and reducing total cholesterol (SMD: −0.42, 95% CI –0.78 to −0.05, p = 0.005, small effect).
Table 4.
Pooled effect size estimates.
| Studies | Sample size** | SMD | 95% CI | p-value | I2 (%) | 95% PI | |
|---|---|---|---|---|---|---|---|
| Body weight (kg) | 13 | 183 vs. 152 | −0.02 | −0.20; 0.24 | 0.988 | 0.0 | −0.22; 0.27 |
| BMI (kg/m2) | 6 | 78 vs. 65 | 0.02 | −0.31; 0.36 | 0.883 | 0.0 | −0.44; 0.49 |
| VO2max (mL/kg/min) | 10 | 194 vs. 163 | 0.30 | 0.09; 0.52 | 0.005* | 0.0 | 0.07; 0.55 |
| Fasting blood glucose (mg/dL OR mmol/L) | 6 | 75 vs. 46 | −0.67 | −1.07; −0.27 | 0.001* | 2.1 | −1.28; −0.07 |
| Total cholesterol (mg/dL OR mmol/L) | 6 | 80 vs. 54 | −0.42 | −0.78; −0.05 | 0.025* | 0.0 | −0.90; 0.06 |
| Triglycerides (mg/dL OR mmol/L) | 6 | 83 vs. 53 | −0.08 | −0.44; 0.28 | 0.661 | 0.0 | −0.55; 0.39 |
BMI, body mass index; VO2 max, maximum oxygen consumption; SMD, standard mean difference (effect size). ** HIIT vs. CONT; *p < 0.05.
3.6. Potential moderating effects
We tested potential moderating effects on % BF and abdominal visceral fat in a meta-regression analysis. For % BF in the HIIT group: total exercise sessions, p = 0.414; participants' age, p = 0.633; baseline BMI, p = 0.457. For the CAT group: total exercise sessions, p = 0.600; participants' age, p = 0.810; baseline BMI, p = 0.578. For testing the same potential moderating effects on abdominal visceral fat in the HIIT group: total exercise sessions, p = 0.696; participants' age, p = 0.615; baseline BMI, p = 0.533; and in the CAT group: total exercise sessions, p = 0.831; participants’ age, p = 0.741; baseline BMI, p = 0.564. We found no moderating effects on % BF and abdominal visceral fat. In addition, no publication bias was found for both outcomes assessed.
4. Discussion
This systematic review and meta-analysis examined the chronic effect of HIIT compared to CAT on body fat as assessed by DEXA in adults with overweight and obesity. Our analysis including 11 RCTs does not show advantage of HIIT over CAT regarding % BF, irrespective of sex. As for abdominal visceral fat, no training modality proved superior in reducing body fat refuting our hypothesis that HIIT is superior to CAT in improving different aspects of %BF and abdominal visceral fat.
Su (2019) evaluated the effect of HIIT vs. CAT and differences for 4–12 weeks on body composition in individuals with overweight and obesity. The methods of body composition assessment included skinfold measurements, plethysmography, absorptiometry and bioimpedance. A comparison of pre- and post-training showed a reduction in body weight, BMI and % BF with no differences between these two training modalities.53 In another meta-analysis, Keating (2017) evaluated the impact of HIIT vs. CAT on body fat assessed by absorptiometry. The authors also showed a beneficial effect of these exercise modalities compared to baseline, but not between these two modalities. Likewise, our analysis suggested that HIIT was not superior to CAT despite different sample characteristics and BF assessment methods reported in the studies by Su et al. (2019)53 and Keating et al. (2017).23
In addition, we included studies with volunteers aged 18 to 55 (mean age 29.5 ± 10.4 years) while Keating (2017) sample was characterized by a broader age range (11–65 years) that included young and elderly adults, as well as children and adolescents aged 8–16 years (three articles; total of 93 participants).23 Moreover, the studies selected for our analysis used only one method of body fat assessment (DEXA) while Su (2019) included in their review studies using several different methods (skinfold measurements, bioimpedance, and plethysmography).53
Interestingly, Andreato et al. (2019) recommended that the findings of meta-analyses with no equalization of data especially regarding energy expenditure in HIIT and CAT should be treated with caution because of a relevant risk of bias.22 In our meta-analysis, we performed a meta-regression to evaluate potential effect modifiers, including exercise session duration, weekly frequency and total duration of the intervention. We found that these variables did not affect the results. Likewise, Andreato et al. (2019) reported that HIIT and CAT have similar effects on body fat, even when the data is equalized.22 Corroborating the findings of other meta-analyses,22,23,53 our results show that HIIT is not superior to CAT in reducing body fat.
Abdominal visceral fat is more closely associated with health risks than subcutaneous fat.54 People with more fat accumulated in the android region are twice as likely to develop cardiovascular disease, type 2 diabetes mellitus and hypertension.55 In their meta-analysis, Wewege et al. (2017) reported abdominal visceral fat reduction following both HIIT and CAT with similar SMDs (HIIT, −3.1 and CAT, −3.0) when compared to baseline, and no superiority between these modalities.24 Some differences between our study and Wewege et al. study (2017) should be pointed out. In contrast to ours, Wewege study had an age limit (18–45 years) excluding older persons and menopausal women. Another point is that they included articles assessing body fat with applied hydrostatic weighing, ultrasound, dual-energy X-ray absorptiometry, bio-electrical impedance analysis, computed tomography or magnetic resonance imaging while we included in our analysis studies reporting a single assessment method—DEXA. In addition, the analyses were based on different PICOS frameworks. Yet, despite these differences, the findings are comparable, i.e., no training modality was superior in reducing body fat.
Higgins et al. (2016)44 evaluated HIIT vs. CAT as exercise interventions over 6 weeks and found that HIIT was superior to CAT in reducing % BF (CAT, Δ −0.3% and HIIT, Δ −1.0%) and android fat mass (CAT, Δ 0.0% and HIIT, Δ −0.2%). On the other hand, Hornbuckle et al. (2018)45 evaluated a 16-week intervention and found no superiority in % BF (CAT, Δ 0.1% and HIIT, Δ −0.3%) and android fat (CAT, Δ −1.1% and HIIT, Δ −0.1%). These contrasting results may be explained by the different duration of these interventions (6 vs. 16 weeks). Another explanation could be the use of different exercise machines targeting different sets of muscles. Hornbuckle et al. (2018)45 study involved treadmill training whereas Higgins et al. (2016)44 used a cycle ergometer and a smaller exercising muscle mass.
Most studies found non-superiority of HIIT vs. CAT on body fat. We can speculate that different stimuli may have similar effects on body fat reduction. Lipolysis occurring in CAT is largely due to increased levels of lipolytic hormones, such as catecholamines, cortisol, glucagon and growth hormone, dependent of exercise duration56 as exercise intensity is moderate and constant. Briefly, these hormones act on adipose tissue inducing triglyceride hydrolysis followed by free fatty acid (FFA) transport to active myocytes and to the mitochondrial matrix leading to β-oxidation.57 On the other hand, HIIT involves intense bouts of exercise that more acutely increases the levels of lipolytic hormones (catecholamines, cortisol, glucagon and GH).56 However, the fast energy requirement for the body in HIIT leads to ATP resynthesis to sustain exercise performance largely from glycolytic metabolism.57 Acetyl-CoA carboxylase catalyzes the formation of malonyl-CoA which inhibits the activity of carnitine palmitoyl transferase (CPT-I), and seems to reduce β-oxidation involved in ATP resynthesis.57 CAT has a relatively high contribution (%) to lipolysis, but at low total energy expenditure due to moderate intensity exercise. On the other hand, HIIT has a relatively low contribution (%) to lipolysis, but at high total energy expenditure due to intense workout (maximum or sub-maximum intensity). HIIT is more effective on lipolysis activation because it produces greater excess post-exercise oxygen consumption (EPOC).18 EPOC in response to CAT appears to be very low or negligible whereas it appears to be more efficient and prolonged in response to HIIT.18 Yet, this effect does not seem enough to make HIIT superior to CAT in reducing body fat; i.e., the stimuli may be different but these two modalities of exercise apparently result in similar fatty acid oxidation rates.
This rationale would explain the absence of differences in body fat reduction between HIIT vs. CAT demonstrated in our meta-analysis and in other studies as well. Interestingly, the key element of HIIT is not the shorter duration of workout, but rather greater total energy expenditure through intense bouts of exercise—the intensity of the workout is more relevant than its duration.
Evidence shows that, compared to body composition parameters (BMI or % BF), low VO2 max is more strongly associated with cardiovascular risk.3,58 Individuals with excess body weight with good cardiorespiratory endurance have lower risk of cardiovascular disease.58,59 Considering its relevance, we evaluated whether there was superiority of HIIT vs. CAT on VO2 max as a secondary outcome. CAT is a commonly recommended strategy to improve VO2 max.60 However, interval exercise protocols such as HIIT exert greater physiological stress on the cardiovascular system and appear to produce a VO2 max response that is similar or even superior to that of CAT.17 In our study, the selected set of RCTs analyzed demonstrated that HIIT is slightly superior to CAT in increasing VO2 max (SMD 0.30; 95% CI 0.09 to 0.52; p = 0.005). In their meta-analysis, Batacan (2017) evaluated short-term (<12 weeks) and long-term (>12 weeks) effects of HIIT in two populations.61 Both HIIT protocols increased VO2 max in the two groups of participants.61 Although this study did not compare HIIT with CAT, it demonstrates that, regardless of body adiposity, an increase in VO2 max induced by HIIT is time dependent. Wisloff (2009) in their review reported that the extent of exercise-induced adaptation of VO2 max and cardiomyocyte function/structure depends on exercise intensity.17 Greater exercise intensity of HIIT increases cardiomyocyte contractile function and, through the Frank-Starling mechanism, results in increased cardiomyocyte contraction force. These adaptations are associated with physiological cardiomyocyte hypertrophy, increased stroke volume as well as increased endothelium-dependent vasodilation62 and oxygen utilization in skeletal muscle17 over time.
Some evidence shows that one-third of heart diseases may be associated with high levels of total cholesterol and/or LDL-cholesterol.63 A meta-analysis with 170,000 individuals revealed that reductions in LDL levels are associated with lower cardiovascular risks.64 The authors also reported that individuals with elevated levels of total cholesterol are twice as likely to have major cardiovascular events55 and reducing LDL-cholesterol by 0.6 mmol/L (∼23 mg/dL) would reduce the risk of cardiovascular disease by 54% at age 40, and by 19% at age 80.65,66 performed a meta-analysis to evaluate the effects of HIIT and CAT, as well as strength training, stretching, and combined exercise on the levels of triglycerides and total cholesterol. Although they reported an effect size of −0.603 and −0.721 mg/dL on triglycerides and total cholesterol, respectively, their analysis included studies of exercise training together with dietary intervention which may have led to a potential confounding effect on the results. In our meta-analysis, we found consistently improved total cholesterol in the HIIT group compared to the CAT.
The present study has some limitations. The main limitation lies on the different methodological approaches and HIIT protocols used in the RCTs selected for this review, though this is a relatively common limitation for research investigating aspects related to exercise or training. Likewise, we did not include any comparisons of isocaloric interventions which may have improved our results and discussion. Another important point inherent to RCTs involving exercise and training is the impossibility to conceal group allocation. Regarding secondary outcomes—fasting glucose and lipid profile, many of the studies in our analysis reported baseline values (for sample characterization) but not post-training values. So, these articles were not included in the meta-analysis of secondary outcomes. Secondary outcomes were obtained from studies eligible for the primary outcome, so they should be interpreted with caution.
5. Conclusion
We conclude that HIIT does not show an advantage over CAT regarding %BF, irrespective of sex, in individuals with excess weight. In particular, no training modality was superior on android visceral fat. Although other studies have reported a similar finding, our analysis included studies evaluating body fat by DEXA only (a reference method of body composition assessment) revalidating the results in the literature. Given the similar effect of HIIT and CAT on reducing adiposity, exercise selection should rely on individual preferences, including exercise duration, tolerance to physical effort, motivation to exercising, and goals. We also found that HIIT has beneficial effects on VO2 max, fasting blood glucose and total cholesterol when compared to CAT.
Declarations
This study was registered in the International Prospective Register of Systematic Reviews: “https://www.crd.york.ac.uk/prospero/#searchadvanced”, ID “CRD42022357195”; Date of first submission: August 31, 2022; Date of registration in PROSPERO: 11 Sep 2022.
The database used in this systematic review is available on Mendeley Data repositor: “https://data.mendeley.com/datasets/67mwfxtmzv”, https://doi.org/10.17632/67mwfxtmzv.3.
Financial support
The authors declare AMK received support from the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES) as part of a graduate research support program.
Authors' contribution
AMK worked in the study design, data collection, data extraction and analysis, as well as in the writing of the final manuscript. JBM contributed to the collection, analysis and interpretation of data. PCO helped in the study preparation and analysis and interpretation of the quality of the studies (RoB 2 tool and GRADE). GW contributed to the extraction, analysis and interpretation of data, and critically reviewed the final manuscript. AML worked in the conception and design of the study and in the extraction, analysis and interpretation of results, and reviewed the final manuscript.
Declaration of competing interest
The authors declare no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jesf.2023.09.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Gonzalez-Muniesa P., Martinez-Gonzalez M.A., Hu F.B., et al. Obesity. Nat Rev Dis Primers. 2017;3 doi: 10.1038/nrdp.2017.34. [DOI] [PubMed] [Google Scholar]
- 2.Zalesin K., Franklin B., Miller W., Peterson E., McCullough P. Impact of obesity on cardiovascular disease. Med Clin. 2011;95(5):919–937. doi: 10.1016/j.mcna.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Kodama S., Saito K., Tanaka S., et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Jaramillo P., Joseph P., Lopez-Lopez J.P., et al. Risk factors, cardiovascular disease, and mortality in South America: a PURE substudy. Eur Heart J. 2022;43(30):2841–2851. doi: 10.1093/eurheartj/ehac113. [DOI] [PubMed] [Google Scholar]
- 5.BRAZIL. Vigitel Brazil 2021: Surveillance of Risk and Protective Factors for Chronic Diseases by Telephone Survey: Estimates of Frequency and Sociodemographic Distribution of Risk and Protective Factors for Chronic Diseases in the Capitals of the 26 Brazilian States and the Federal District in 2021. Ministério da Saúde; Brasília: 2022. p. 131. [Google Scholar]
- 6.Kasper A.M., Langan-Evans C., Hudson J.F., et al. Come back skinfolds, all is forgiven: a narrative review of the efficacy of common body composition methods in applied Sports practice. Nutrients. 2021;13(4) doi: 10.3390/nu13041075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shepherd J.A., Ng B.K., Sommer M.J., Heymsfield S.B. Body composition by DXA. Bone. 2017;104:101–105. doi: 10.1016/j.bone.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavedon V., Sandri M., Venturelli M., Zancanaro C., Milanese C. Anthropometric prediction of DXA-measured percentage of fat mass in athletes with unilateral lower limb amputation. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.620040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazess R.B., Barden H.S., Bisek J.P., Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51(6):1106–1112. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 10.Kaul S., Rothney M.P., Peters D.M., et al. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity. 2012;20(6):1313–1318. doi: 10.1038/oby.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong A., Jungbluth Rodriguez K., Sabag A., et al. Effect of aerobic exercise on waist circumference in adults with overweight or obesity: a systematic review and meta-analysis. Obes Rev. 2022;23(8) doi: 10.1111/obr.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S., Zhou H., Zhao C., He H. Effect of exercise training on body composition and inflammatory cytokine levels in overweight and obese individuals: a systematic review and network meta-analysis. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.921085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willis L.H., Slentz C.A., Bateman L.A., et al. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J Appl Physiol. 1985;113(12):1831–1837. doi: 10.1152/japplphysiol.01370.2011. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ACSM’s A.C.S.M. Guidelines for exercise testing and prescription. J Can Chiropr Assoc. 2014;58(3):328. [Google Scholar]
- 15.Gibala M.J., Jones A.M. Physiological and performance adaptations to high-intensity interval training. Nestle Nutr Inst Workshop Ser. 2013;76:51–60. doi: 10.1159/000350256. [DOI] [PubMed] [Google Scholar]
- 16.De Feo P. Is high-intensity exercise better than moderate-intensity exercise for weight loss? Nutr Metabol Cardiovasc Dis. 2013;23(11):1037–1042. doi: 10.1016/j.numecd.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Wisloff U., Ellingsen O., Kemi O.J. High-intensity interval training to maximize cardiac benefits of exercise training? Exerc Sport Sci Rev. 2009;37(3):139–146. doi: 10.1097/JES.0b013e3181aa65fc. [DOI] [PubMed] [Google Scholar]
- 18.Boutcher S.H. High-intensity intermittent exercise and fat loss. J Obes. 2011;2011 doi: 10.1155/2011/868305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irving B.A., Davis C.K., Brock D.W., et al. Effect of exercise training intensity on abdominal visceral fat and body composition. Med Sci Sports Exerc. 2008;40(11):1863–1872. doi: 10.1249/MSS.0b013e3181801d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khodadadi F., Bagheri R., Negaresh R., et al. The effect of high-intensity interval training type on body fat percentage, fat and fat-free mass: a systematic review and meta-analysis of randomized clinical trials. J Clin Med. 2023;12(6) doi: 10.3390/jcm12062291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viana R.B., Naves J.P.A., Coswig V.S., et al. Is interval training the magic bullet for fat loss? A systematic review and meta-analysis comparing moderate-intensity continuous training with high-intensity interval training (HIIT) Br J Sports Med. 2019;53(10):655–664. doi: 10.1136/bjsports-2018-099928. [DOI] [PubMed] [Google Scholar]
- 22.Andreato L.V., Esteves J.V., Coimbra D.R., Moraes A.J.P., de Carvalho T. The influence of high-intensity interval training on anthropometric variables of adults with overweight or obesity: a systematic review and network meta-analysis. Obes Rev. 2019;20(1):142–155. doi: 10.1111/obr.12766. [DOI] [PubMed] [Google Scholar]
- 23.Keating S.E., Johnson N.A., Mielke G.I., Coombes J.S. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes Rev. 2017;18(8):943–964. doi: 10.1111/obr.12536. [DOI] [PubMed] [Google Scholar]
- 24.Wewege M., van den Berg R., Ward R.E., Keech A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: a systematic review and meta-analysis. Obes Rev. 2017;18(6):635–646. doi: 10.1111/obr.12532. [DOI] [PubMed] [Google Scholar]
- 25.Leggate M., Carter W.G., Evans M.J., et al. Determination of inflammatory and prominent proteomic changes in plasma and adipose tissue after high-intensity intermittent training in overweight and obese males. J Appl Physiol. 1985;112(8):1353–1360. doi: 10.1152/japplphysiol.01080.2011. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Z., Li M., Cai J., et al. Effect of high-intensity interval training vs. Moderate-intensity continuous training on fat loss and cardiorespiratory fitness in the young and middle-aged a systematic review and meta-analysis. Int J Environ Res Publ Health. 2023;20(6) doi: 10.3390/ijerph20064741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maillard F., Pereira B., Boisseau N. Effect of high-intensity interval training on total, abdominal and visceral fat mass: a meta-analysis. Sports Med. 2018;48(2):269–288. doi: 10.1007/s40279-017-0807-y. [DOI] [PubMed] [Google Scholar]
- 28.Sultana R.N., Sabag A., Keating S.E., Johnson N.A. The effect of low-volume high-intensity interval training on body composition and cardiorespiratory fitness: a systematic review and meta-analysis. Sports Med. 2019;49(11):1687–1721. doi: 10.1007/s40279-019-01167-w. [DOI] [PubMed] [Google Scholar]
- 29.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins J., Thomas J., Chandler J., et al. 2022. Cochrane Handbook for Systematic Reviews of Interventions. (updated February 2022) [Google Scholar]
- 31.Leenen R., van der Kooy K., Deurenberg P., et al. Visceral fat accumulation in obese subjects: relation to energy expenditure and response to weight loss. Am J Physiol. 1992;263(5 Pt 1):E913–E919. doi: 10.1152/ajpendo.1992.263.5.E913. [DOI] [PubMed] [Google Scholar]
- 32.van der Kooy K., Leenen R., Seidell J.C., et al. Waist-hip ratio is a poor predictor of changes in visceral fat. Am J Clin Nutr. 1993;57(3):327–333. doi: 10.1093/ajcn/57.3.327. [DOI] [PubMed] [Google Scholar]
- 33.Robinson K.A., Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. Int J Epidemiol. 2002;31(1):150–153. doi: 10.1093/ije/31.1.150. [DOI] [PubMed] [Google Scholar]
- 34.Glanville J., Foxlee R., Wisniewski S., et al. Translating the Cochrane EMBASE RCT filter from the Ovid interface to Embase.com: a case study. Health Inf Libr J. 2019;36(3):264–277. doi: 10.1111/hir.12269. [DOI] [PubMed] [Google Scholar]
- 35.Atkins D., Best D., Briss P.A., et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.IntHout J., Ioannidis J.P., Rovers M.M., Goeman J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7) doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen J. second ed. Lawrence Erlbaum; Hillsdale: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 38.Dias S., Sutton A.J., Welton N.J., Ades A.E. Evidence synthesis for decision making 3: heterogeneity--subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making. 2013;33(5):618–640. doi: 10.1177/0272989X13485157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pedralli M.L., Eibel B., Waclawovsky G., et al. Effects of exercise training on endothelial function in individuals with hypertension: a systematic review with meta-analysis. J Am Soc Hypertens. 2018;12(12):e65–e75. doi: 10.1016/j.jash.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Balk E.M., Earley A., Patel K., Trikalinos T.A., Dahabreh I.J. Agency for Healthcare Research and Quality (US); Rockville (MD): 2012. Empirical Assessment of Within-Arm Correlation Imputation in Trials of Continuous Outcomes. Report No.: 12(13)-EHC141-EF. [PubMed] [Google Scholar]
- 42.Cooper J., Collins B., Adams D., Robergs R., Donges C. Limited effects of endurance or interval training on visceral adipose tissue and systemic inflammation in sedentary middle-aged men. J Obes. 2016;2016 doi: 10.1155/2016/2479597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gripp F., Nava R., Cassilhas R., et al. HIIT is superior than MICT on cardiometabolic health during training and detraining. Eur J Appl Physiol. 2021;121:159–172. doi: 10.1007/s00421-020-04502-6. [DOI] [PubMed] [Google Scholar]
- 44.Higgins S., Fedewa M.V., Hathaway E.D., Schmidt M.D., Evans E.M. Sprint interval and moderate-intensity cycling training differentially affect adiposity and aerobic capacity in overweight young-adult women. Appl Physiol Nutr Metabol. 2016;41(11):1177–1183. doi: 10.1139/apnm-2016-0240. [DOI] [PubMed] [Google Scholar]
- 45.Hornbuckle L.M., McKenzie M.J., Whitt-Glover M.C. Effects of high-intensity interval training on cardiometabolic risk in overweight and obese African-American women: a pilot study. Ethn Health. 2018;23(7) doi: 10.1080/13557858.2017.1294661. 752-66. [DOI] [PubMed] [Google Scholar]
- 46.Keating S.E., Machan E.A., Connor H.T., et al. Continuous exercise but not high intensity interval training improves fat distribution in overweight adults. J Obes. 2014;2014 doi: 10.1155/2014/834865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kong Z., Sun S., Liu M., Shi Q. Short-term high-intensity interval training on body composition and blood glucose in overweight and obese young women. J Diabetes Res. 2016;2016 doi: 10.1155/2016/4073618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ram A., Marcos L., Jones M.D., et al. The effect of high-intensity interval training and moderate-intensity continuous training on aerobic fitness and body composition in males with overweight or obesity: a randomized trial. Obesity Med. 2020;17 doi: 10.1038/s41440-019-0392-6. [DOI] [PubMed] [Google Scholar]
- 49.Rowley T., Espinoza J., Akers J., Wenos D., Edwards E. Effects of run sprint interval training on healthy, inactive, overweight/obese women: a pilot study. Facets. 2017;2(1):53–67. [Google Scholar]
- 50.Sijie T., Hainai Y., Fengying Y., Jianxiong W. High intensity interval exercise training in overweight young women. J Sports Med Phys Fit. 2012;52(3) 255-62. [PubMed] [Google Scholar]
- 51.Zhang H., Tong T., Qiu W., et al. Comparable effects of high-intensity interval training and prolonged continuous exercise training on abdominal visceral fat reduction in obese young women. J Diabetes Res. 2017;2017 doi: 10.1155/2017/5071740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang H., Tong T.K., Kong Z., et al. Exercise training-induced visceral fat loss in obese women: the role of training intensity and modality. Scand J Med Sci Sports. 2021;31(1):30–43. doi: 10.1111/sms.13803. [DOI] [PubMed] [Google Scholar]
- 53.Su L., Fu J., Sun S., et al. Effects of HIIT and MICT on cardiovascular risk factors in adults with overweight and/or obesity: a meta-analysis. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0210644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alexander C.M., Kasza I., Yen C.L., et al. Dermal white adipose tissue: a new component of the thermogenic response. J Lipid Res. 2015;56(11):2061–2069. doi: 10.1194/jlr.R062893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mann S., Beedie C., Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. 2014;44(2):211–221. doi: 10.1007/s40279-013-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silveira L., Pinheiro C., Zoppi C., et al. Regulation of glucose and fatty acid metabolism in skeletal muscle during contraction. Arq Bras Endocrinol Metabol. 2011;55(5):303–313. doi: 10.1590/s0004-27302011000500002. [DOI] [PubMed] [Google Scholar]
- 57.Brun J.F., Myzia J., Varlet-Marie E., Raynaud de Mauverger E., Mercier J. Beyond the calorie paradigm: taking into account in practice the balance of fat and carbohydrate oxidation during exercise? Nutrients. 2022;14(8) doi: 10.3390/nu14081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee D.C., Artero E.G., Sui X., Blair S.N. Mortality trends in the general population: the importance of cardiorespiratory fitness. J Psychopharmacol. 2010;24(4 Suppl):27–35. doi: 10.1177/1359786810382057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Church T.S., LaMonte M.J., Barlow C.E., Blair S.N. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med. 2005;165(18):2114–2120. doi: 10.1001/archinte.165.18.2114. [DOI] [PubMed] [Google Scholar]
- 60.Garber C.E., Blissmer B., Deschenes M.R., et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 61.Batacan R.B., Jr., Duncan M.J., Dalbo V.J., Tucker P.S., Fenning A.S. Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br J Sports Med. 2017;51(6):494–503. doi: 10.1136/bjsports-2015-095841. [DOI] [PubMed] [Google Scholar]
- 62.Sabouri M., Amirshaghaghi F., Hesari M.M. High-intensity interval training improves the vascular endothelial function comparing moderate-intensity interval training in overweight or obese adults: a meta-analysis. Clin Nutr ESPEN. 2023;53:100–106. doi: 10.1016/j.clnesp.2022.11.023. [DOI] [PubMed] [Google Scholar]
- 63.Carroll M.D., Kit B.K., Lacher D.A. Total and high-density lipoprotein cholesterol in adults: national health and nutrition examination survey, 2009-2010. NCHS Data Brief. 2012;(92):1–8. [PubMed] [Google Scholar]
- 64.Cholesterol Treatment Trialists C., Baigent C., Blackwell L., et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Law M.R., Wald N.J., Thompson S.G. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 1994;308(6925):367–372. doi: 10.1136/bmj.308.6925.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim K.B., Kim K., Kim C., et al. Effects of exercise on the body composition and lipid profile of individuals with obesity: a systematic review and meta-analysis. J Obes Metab Syndr. 2019;28(4):278–294. doi: 10.7570/jomes.2019.28.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.