Abstract
Background
Neural tube defects (NTDs) still occur among some women who consume 400 μg of folic acid for prevention. It has been hypothesized that intakes of methyl donors and other micronutrients involved in one-carbon metabolism may further protect against NTDs.
Objectives
To investigate whether intakes of vitamin B6, vitamin B12, choline, betaine, methionine, thiamine, riboflavin, and zinc, individually or in combination, were associated with NTD risk reduction in offspring of women meeting the folic acid recommendations.
Methods
Data were from the National Birth Defects Prevention Study (United States population–based, case-control). We restricted deliveries between 1999 and 2011 with daily periconceptional folic acid supplementation or estimated dietary folate equivalents ≥400 μg. NTD cases were live births, stillbirths, or terminations affected by spina bifida, anencephaly, or encephalocele (n = 1227). Controls were live births without a major birth defect (n = 7095). We categorized intake of each micronutrient as higher or lower based on a combination of diet (estimated from a food frequency questionnaire) and periconceptional vitamin supplementation. We estimated NTD associations for higher compared with lower intake of each micronutrient, individually and in combination, expressed as odds ratios (ORs) and 95% confidence intervals (CIs), adjusted for age, race/ethnicity, education, and study center.
Results
NTD associations with each micronutrient were weak to modest. Greater NTD reductions were observed with concurrent higher-amount intakes of multiple micronutrients. For instance, NTD odds were ∼50% lower among participants with ≥4 micronutrients with higher-amount intakes than among participants with ≤1 micronutrient with higher-amount intake (adjusted OR: 0.53; 95% CI: 0.33, 0.86). The strongest reduction occurred with concurrent higher-amount intakes of vitamin B6, vitamin B12, choline, betaine, and methionine (adjusted OR: 0.26; 95% CI: 0.09, 0.77) compared with ≤1 micronutrient with higher-amount intake.
Conclusions
Our findings support that NTD prevention, in the context of folic acid fortification, could be augmented with intakes of methyl donors and other micronutrients involved in folate metabolism.
Keywords: betaine, choline, folic acid, methionine, methylation, neural tube defects, one-carbon metabolism, vitamin B complex, zinc
Introduction
A wealth of research, including randomized trials, supports that maternal periconceptional folic acid intake reduces the risk of neural tube defects (NTDs) [[1], [2], [3], [4], [5]]. For this reason, starting in 1998, the United States mandated the addition of folic acid to enriched grain products. However, despite folic acid fortification, NTDs still affect ∼3000 pregnancies each year in the United States [6]. It is estimated that 20%–50% of NTDs occur among individuals who consume the recommended 400 μg of folic acid per day for prevention [[1], [2], [3], [4],7], suggesting that some pregnancies are not as responsive to the protective effect of folic acid as others. A hypothesized mechanism by which folic acid prevents NTDs is through its action as a methyl group donor in one-carbon metabolism [8]. One-carbon metabolism is crucial for DNA synthesis in embryogenesis [9,10].
It has been suggested that not only folic acid but the entirety of one-carbon metabolism could be involved in the etiology of NTDs. If so, NTD risk reduction might occur with the intake of other micronutrients that act as methyl donors or cofactors in one-carbon metabolism (herein, referred to as simply “methyl donors”) [11]. In support of this hypothesis, lower NTD risk has been observed with higher intakes of vitamin B12 (cobalamin) [[12], [13], [14]], vitamin B6 (pyridoxine) [12,15], choline [16,17], betaine [16,18,19], methionine [20,21], thiamine (vitamin B1) [12,22], riboflavin (vitamin B2) [12,22], and zinc [12,[22], [23], [24]]. However, null associations have also been reported [12,22,[25], [26], [27]]. A possible explanation is that these micronutrients have tended to be investigated in isolation rather than as a composite exposure. Perhaps, these methyl donors provide better NTD risk reduction when consumed together, following the concept of dietary synergy—that is, the total effect of dietary constituents consumed in combination is greater than the sum of the effects of each constituent when consumed alone [28].
Previously, this hypothesis was explored in the Slone Epidemiology Center Birth Defects Study by evaluating associations between intakes of 5 methyl donors, individually and jointly, and NTD risk reduction among women with estimated folic acid ≥400 μg daily [7]. The findings suggested that concurrent methyl donor intakes may be associated with lower NTD risk. In the current investigation, we sought to replicate those findings in another study population, i.e., the National Birth Defects Prevention Study (NBDPS). Specifically, our objective was to investigate whether intake of methyl donors involved in one-carbon metabolism (i.e., vitamins B6 and B12, choline, betaine, methionine, thiamine, riboflavin, and zinc), individually or in combination, were associated with NTD risk reduction among women whose estimated daily folic acid intake met the recommendations.
Methods
Study design
The NBDPS was a multicenter, population-based, case-control study of >40,000 United States pregnancies whose expected delivery dates (EDDs) were between 1997 and 2011, designed to investigate risk factors for major structural malformations. The study design has been described previously [29,30]. In brief, cases were ascertained from birth defects surveillance systems in Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, and Utah and included live births, stillbirths, and terminations. Each potential case was reviewed by clinical geneticists for inclusion and was then classified according to birth defect group(s) for the study [31]. Our primary case definition was NTDs, including spina bifida, anencephaly, and encephalocele. Cases could be affected by >1 major structural malformation. Cases with syndromes were excluded due to known cause of the defect (e.g., chromosomal abnormality), as were cases that were unable to be categorized due to insufficient information. Controls were live-born infants without a major birth defect, selected from the same populations as the cases through random sampling from hospital records or birth certificates. Participation rates were 76% for spina bifida, 62% for anencephaly, 61% for encephalocele, and 71% for controls. Participants completed a structured telephone interview, including an FFQ, in English or Spanish within 6 wk to 24 mo after their EDD. The length of time between EDD and the interview was similar, although slightly longer, for cases (median [interquartile range]: 9 [5–14] mo) than for controls (median [interquartile range]: 7 [5–11] mo). All participants provided informed consent. The study was approved by the Centers for Disease Control and Prevention institutional review board, along with the institutional review board at each study center.
Folic acid intake
We restricted the study population for this analysis to deliveries between 1999 and 2011 to ensure that all pregnancies coincided with mandatory folic acid fortification. We further restricted participants whose estimated periconceptional folic acid intake, assessed by periconceptional vitamin supplementation or diet, met the current folic acid recommendations [32,33]. We made this restriction in order to focus on the population of interest for this work, i.e., those for whom the recommended amount of folic acid—at least by itself—may not provide sufficient protection against NTDs. Specifically, we included participants with either 1) reported use of a folic acid–containing vitamin supplement—either a folic acid-only supplement or multi-/prenatal vitamin form—every day during the periconceptional period, or 2) estimated daily dietary folate equivalents (DFEs) ≥400 μg based on usual diet reported on the FFQ. DFEs account for the greater bioavailability of synthetic folic acid from fortified foods than naturally occurring folate. We excluded participants without FFQ data or whose estimated total energy was extreme (<500 or >3800 kcal/d).
Micronutrient intake
During the telephone interview, participants reported vitamin supplement use, including the product, frequency, and duration of intake during pregnancy and the 3 mo before pregnancy. For this study, we narrowed the exposure window of interest to the periconceptional period, which we defined as the month before pregnancy through the end of the first month of pregnancy, to align exposure timing with the critical period of development of the central nervous system and NTDs [34]. Reported products were linked to their ingredients using the Slone Drug Dictionary [35]. We identified reports of periconceptional vitamin supplement use for products containing folic acid and the other methyl donors under study [35].
In addition, participants completed a Willett FFQ [36] assessing usual dietary intake during the year prior to the study pregnancy. The Willett FFQ has been validated among women [37]. The participants were presented with the typical serving size for 58 food items, and the participant reported the usual frequency of intake for each. These values were converted to daily dietary estimates for a range of nutrients, including those of interest for our study, using the USDA’s nutrient matrix version 27 [38]. We assumed that the FFQ data represented usual dietary patterns during the periconceptional period since it is likely before any dietary changes related to pregnancy recognition occur [38].
To categorize each micronutrient intake (higher or lower), we used a combination of information from diet and supplements (Table 1). First, we used the residual method to energy-adjust daily dietary estimates of each methyl donor [39]. Adjustment for total energy is thought to lessen the impacts of underreporting and overreporting of food items on nutrient estimates [40]. Then, we regressed the case-control status on each of the energy-adjusted micronutrient values, using restricted cubic splines with ≤5 knots controlling for DFEs as a model covariate. We identified the cut point in the micronutrient distribution where the NTD OR comparing higher compared with lower intake was maximized [41]. We excluded extreme values (>99th percentile) of the estimated intake for each micronutrient, given the instability of spline models at the tail ends of the distribution. Also, in the spline models, we excluded participants who reported any periconceptional use of a vitamin supplement containing only that micronutrient or (for vitamin B6, vitamin B12, thiamine, riboflavin, and zinc) multi-/prenatal vitamins since such products commonly contain those methyl donors. Subsequently, we grouped the supplementers into the respective higher intake category if the typical content of the supplement was expected to be greater than the dietary cutoff [42]. For vitamins B6 and B12, the expected content from supplements was higher than the dietary cut point, so supplementers were categorized in the higher intake categories. For thiamine and riboflavin, the content in supplements was expected to be lower than the dietary cut point, so supplementers were categorized based on their dietary data only. For zinc, intake from a zinc-only supplement would be more than the cut point for higher intake, but intake from a multi-/prenatal vitamin would be less; therefore, we categorized participants who reported zinc-only supplements into the higher intake category and otherwise grouped the multi-/prenatal supplementers based on their dietary data only.
TABLE 1.
Definitions of higher compared with lower intake for each micronutrient
| Micronutrient | Higher intake | Lower intake |
|---|---|---|
| Vitamin B6 | Any periconceptional supplementation with a vitamin B6–containing supplement (i.e., vitamin B6 only, vitamin B complex, multivitamin, or prenatal vitamin) OR Higher dietary intake, defined as estimated daily intake ≥1.5 mg after adjusting for total energy | No periconceptional supplementation with a vitamin B6–containing supplement AND Lower dietary intake, defined as estimated daily intake <1.5 mg after adjusting for total energy |
| Vitamin B12 | Any periconceptional supplementation with a vitamin B12–containing supplement (i.e., vitamin B12 only, vitamin B complex, multivitamin, or prenatal vitamin) OR Higher dietary intake, defined as estimated daily intake ≥3.4 μg after adjusting for total energy | No periconceptional supplementation with a vitamin B12–containing supplement AND Lower dietary intake, defined as estimated daily intake <3.4 μg after adjusting for total energy |
| Betaine | Higher dietary intake, defined as estimated daily intake ≥90 mg after adjusting for total energy | Lower dietary intake, defined as estimated daily intake <90 mg after adjusting for total energy |
| Choline | Higher dietary intake, defined as estimated daily intake ≥200 mg after adjusting for total energy | Lower dietary intake, defined as estimated daily intake <200 mg after adjusting for total energy |
| Methionine | Higher dietary intake, defined as estimated daily intake ≥2.45 g after adjusting for total energy | Lower dietary intake, defined as estimated daily intake <2.45 g after adjusting for total energy |
| Riboflavin | Higher dietary intake, defined as estimated daily intake ≥2.3 mg after adjusting for total energy | Lower dietary intake, defined as estimated daily intake <2.3 mg after adjusting for total energy |
| Thiamine | Higher dietary intake, defined as estimated daily intake ≥2.3 mg after adjusting for total energy | Lower dietary intake, defined as estimated daily intake <2.3 mg after adjusting for total energy |
| Zinc | Any periconceptional supplementation with a zinc-only–containing supplement OR Higher dietary intake, defined as estimated daily intake ≥15 mg after adjusting for total energy | No periconceptional supplementation with a zinc-only–containing supplement AND Lower dietary intake, defined as estimated daily intake <15 mg after adjusting for total energy |
Unless otherwise noted, vitamin supplementation was not considered in the definition since the estimated content is lower than the dietary cutoff.
Covariates
A priori, we considered several potential confounders, i.e., variables that may be predictive of micronutrient intake and NTD risk and are not hypothesized to be on the causal pathway. All variables were ascertained from the maternal interview. These variables included: maternal sociodemographic characteristics (i.e., age, education, income, race/ethnicity, and birthplace), reproductive and pregnancy history (gravidity, pregnancy intention, and fertility treatment), and health-related characteristics and behaviors (prepregnancy BMI [in kg/m2], any cigarette smoking or alcohol use in the month before through 3 mo into the study pregnancy, and study center). Here, we considered the race/ethnicity variable as a proxy for structural racism and other health-related disparities that may influence intake amounts, such as access to more nutritious foods, knowledge of intake recommendations, and cultural differences in dietary patterns. In the original version of the questionnaire (2005 and earlier), participants could select only 1 of the following options: White, non-Hispanic; Black, non-Hispanic; Asian/Pacific Islander; Native American or Alaskan Native; Hispanic; Other (Specify); Refused; Don’t Know. In the later version (2006 and later), the term “non-Hispanic” was removed from the options for White and Black, a new option was added for “Hispanic,” the “Other Specify” option was removed, and participants were allowed to select >1 option (e.g., White and Hispanic). Given the way the data were collected on the original questionnaire, we were unable to define race and ethnicity as 2 separate variables. Participants were categorized as Hispanic if that option was selected; otherwise, participants were grouped based on their race. Missing data were uncommon (≤7% for any covariate) except pregnancy planning (∼20%). Since nonresponses may have their own meaning, we created missing indicator variables to represent unknown or refused to answer in the main analysis.
Statistical analysis
We estimated the Spearman correlations between the continuous estimates of micronutrient dietary intakes among the controls to understand the degree of collinearity.
We used unconditional logistic regression with Firth’s penalized likelihood to estimate crude and adjusted ORs and 95% CIs for associations between the methyl donors and NTD outcomes [43]. First, we examined each micronutrient in isolation, comparing participants with higher intake with those with lower intake. Second, for each participant, we counted the number of methyl donors categorized as higher intake and then compared participants with 2–3 or ≥4 micronutrients in the higher intake category to those with ≤1 micronutrient in the higher category. Third, post hoc, upon noticing that the participants with 2–4 methyl donors in the higher intake range were often consuming the same micronutrients, we created another grouping variable that evaluated specific combinations: higher intakes of vitamins B6 and B12 only; higher intakes of vitamin B6, vitamin B12, and choline only; higher intakes of vitamin B6, vitamin B12, choline, and betaine only; higher intakes of vitamin B6, vitamin B12, choline, betaine, and riboflavin; higher intakes of vitamin B6, vitamin B12, choline, betaine, and zinc; higher intakes of vitamin B6, vitamin B12, choline, betaine, and thiamine; and higher intakes of vitamin B6, vitamin B12, choline, betaine, and methionine. To avoid small cell counts, the latter 4 groupings were nonexclusive. For instance, participants in the higher intakes of vitamin B6, vitamin B12, choline, betaine, and riboflavin category could have also had higher intakes of zinc, thiamine, and/or methionine. These combinations were compared to participants with ≤1 micronutrient in the higher range. All adjusted models controlled for a selection of the aforementioned potential confounders. Upon including each potential confounder in the regression model, none changed the OR point estimates by >10%, so we selected the characteristics whose inclusion in the multivariable-adjusted models changed the ORs the most, i.e., maternal age, race/ethnicity, education, and study center. We did not report an OR if there were fewer than 5 cases or controls in the index or reference exposure category.
Sensitivity and secondary analyses
We conducted 2 sensitivity analyses. First, to assess if our method for handling missing data influenced results, we estimated the NTD associations using participants with no missing FFQ items and no missing covariate values. Second, given the complexities of drawing inferences from combined dietary and supplement data in the main analysis (e.g., by definition, nearly all reference groups contained no supplementers), we restricted to participants who reported no supplementation so that the results were solely based on the dietary data. In this latter sensitivity analysis, we controlled for continuous DFEs as a model covariate to evaluate the degree to which the results may be explained by folic acid intake above the recommendations.
In secondary analyses, to see if associations differed among NTD subtypes, we evaluated spina bifida and anencephaly outcomes separately and also restricted to isolated cases (i.e., those affected by only 1 birth defect).
In stratified analysis, we grouped the spina bifida results by offspring sex to examine if the micronutrients differentially reduce spina bifida odds among females compared with males, which would support a hypothesis to explain known sex ratio differences [44,45].
Results
The analytic sample included 1227 NTD cases (729 spina bifida, 366 anencephalies, 1074 isolated) and 7095 controls (see Supplemental Figure 1 for eligibility flowchart, including numbers and reasons for exclusion). Compared with controls, cases were less likely to have a graduate education or an annual income >$50,000 but were more likely to identify as Hispanic or have a prepregnancy BMI of ≥30.0 kg/m2 (Table 2).
TABLE 2.
Characteristics among participants with estimated folic acid intake ≥400 μg/d, National Birth Defects Prevention Study (1999–2011)
| Characteristic | Cases n = 1227 | Controls n = 7095 |
|---|---|---|
| Maternal age | ||
| <25 y | 32.6 | 32.4 |
| 25–34 y | 54.8 | 55.7 |
| ≥35 y | 12.6 | 11.9 |
| Maternal education | ||
| High school | 17.6 | 15.4 |
| College | 25.8 | 20.8 |
| Graduate | 56.1 | 62.9 |
| Unknown | 0.6 | 0.8 |
| Annual income | ||
| <$10,000 | 18.4 | 16.1 |
| $10,000–$50,000 | 44.9 | 40.5 |
| >$50,000 | 29.4 | 38.0 |
| Unknown | 7.0 | 5.4 |
| Maternal race-ethnicity | ||
| Black, non-Hispanic | 8.6 | 9.5 |
| Hispanic | 31.5 | 24.3 |
| White, non-Hispanic | 53.1 | 58.9 |
| Other/unknown | 6.8 | 7.2 |
| Mother birthplace | ||
| United States | 74.2 | 77.4 |
| Outside the United States | 25.3 | 21.8 |
| Unknown | 0.5 | 0.7 |
| Gravidity1 | 1.7 (1.7) | 1.6 (1.6) |
| Prepregnancy BMI, kg/m2 | ||
| <18.5 | 9.4 | 12.6 |
| 18.5–24.9 | 46.4 | 51.2 |
| 25.0–29.9 | 12.5 | 12.5 |
| ≥30.0 | 25.4 | 19.7 |
| Unknown | 6.3 | 4.2 |
| Periconceptional use of folic acid–containing supplements | ||
| Daily | 40.8 | 40.1 |
| < Daily | 20.4 | 20.7 |
| None | 38.9 | 39.1 |
| Smoking B1-P3 | ||
| Any | 13.5 | 15.3 |
| None | 86.3 | 84.1 |
| Unknown | 0.2 | 0.5 |
| Alcohol B1-P3 | ||
| Any | 31.5 | 36.0 |
| None | 68.1 | 63.2 |
| Unknown | 0.5 | 0.8 |
| Intended pregnancy | ||
| Yes | 48.5 | 51.4 |
| No | 32.2 | 29.3 |
| Unknown | 19.3 | 19.3 |
| Fertility treatment used | ||
| Yes | 6.5 | 6.1 |
| No | 93.5 | 93.9 |
| Study center | ||
| Arkansas | 11.9 | 12.5 |
| California | 19.2 | 10.2 |
| Iowa | 11.4 | 10.8 |
| Massachusetts | 5.1 | 12.0 |
| New Jersey | 2.4 | 4.7 |
| New York | 5.5 | 8.3 |
| Texas | 9.8 | 11.0 |
| CDC/Atlanta | 12.1 | 10.1 |
| North Carolina | 11.3 | 9.4 |
| Utah | 11.3 | 11.1 |
Data are reported as percentages, unless otherwise indicated.
B1-P3, 1 mo before through the third month of pregnancy.
Mean (SD) shown.
Among the controls, correlations between pairs of the energy-adjusted estimates of dietary intake for vitamin B6, vitamin B12, choline, betaine, methionine, riboflavin, thiamine, and zinc were mostly weak to moderate (Spearman r ≤0.4); stronger correlations existed among vitamin B6, vitamin B12, riboflavin, and thiamine, as well as between zinc and vitamin B6, vitamin B12, and methionine (Supplemental Table 1).
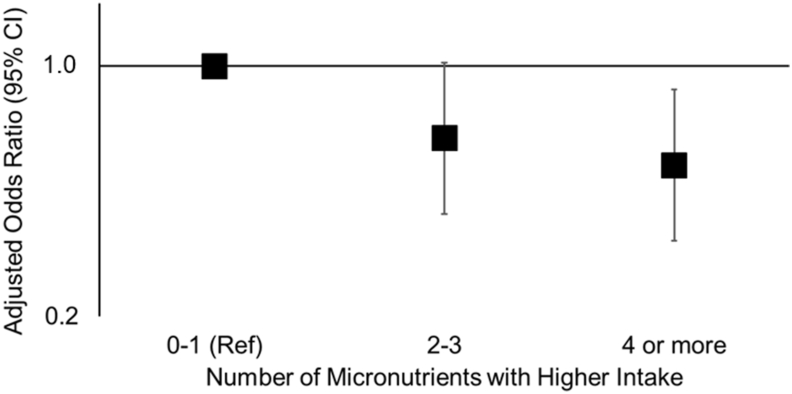
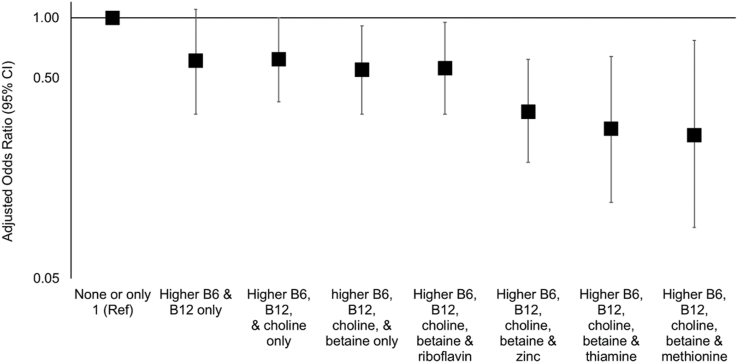
We observed weak to modest inverse NTD associations comparing higher compared with lower intake for each methyl donor in isolation (Table 3). Adjustment for age, race/ethnicity, education, and study center attenuated associations, most noticeably for vitamin B12 and betaine; however, all OR point estimates remained below 1.0. Concurrent intakes of methyl donors in the higher range tended to occur with vitamins B6 and B12, followed by choline, then betaine (Supplemental Table 2). As the number of methyl donors with higher intakes increased, the NTDs odds decreased (Figure 1, Table 3). After covariate adjustment, participants with higher intakes of ≥4 micronutrients had ∼50% lower odds of NTDs compared with participants that had ≤1 micronutrient with intake in the higher range (adjusted OR: 0.53; 95% CI: 0.33, 0.86). Upon evaluating specific combinations, compared with ≤1 micronutrient intake in the higher range, having higher intakes of both vitamins B6 and B12 was associated with an adjusted OR of 0.61 (95% CI: 0.33, 1.1); the addition of higher intakes of choline and betaine lowered the adjusted OR to 0.55 (95% CI: 0.33, 0.91; Figure 2, Table 3). The lowest adjusted OR (0.26) was observed with concurrent higher intakes of vitamin B6, vitamin B12, choline, betaine, and methionine (95% CI: 0.09, 0.77).
TABLE 3.
Associations between methyl donors and other micronutrients involved in one-carbon metabolism and neural tube defects, National Birth Defects Prevention Study (1999–2011)
| Micronutrient | Category1 | Cases (n = 1227) | Controls (n = 7095) | cOR (95% CI) | aOR2 (95% CI) |
|---|---|---|---|---|---|
| Individual | |||||
| Vitamin B6 | Lower | 32 | 148 | 1.0 | 1.0 |
| Higher | 1195 | 6947 | 0.79 (0.54, 1.2) | 0.81 (0.55, 1.2) | |
| Vitamin B12 | Lower | 72 | 336 | 1.0 | 1.0 |
| Higher | 115 | 6759 | 0.79 (0.61, 1.0) | 0.88 (0.67, 1.1) | |
| Betaine | Lower | 672 | 3558 | 1.0 | 1.0 |
| Higher | 555 | 3537 | 0.83 (0.74, 0.94) | 0.91 (0.81, 1.0) | |
| Choline | Lower | 77 | 374 | 1.0 | 1.0 |
| Higher | 1150 | 6721 | 0.83 (0.64, 1.1) | 0.82 (0.63, 1.1) | |
| Methionine | Lower | 1211 | 6974 | 1.0 | 1.0 |
| Higher | 16 | 121 | 0.78 (0.47, 1.3) | 0.82 (0.49, 1.4) | |
| Riboflavin | Lower | 848 | 4675 | 1.0 | 1.0 |
| Higher | 379 | 2420 | 0.86 (0.76, 0.98) | 0.88 (0.77, 1.0) | |
| Thiamine | Lower | 1190 | 6836 | 1.0 | 1.0 |
| Higher | 37 | 259 | 0.83 (0.59, 1.2) | 0.85 (0.60, 1.2) | |
| Zinc | Lower | 1060 | 5974 | 1.0 | 1.0 |
| Higher | 167 | 1121 | 0.84 (0.71, 1.0) | 0.84 (0.70, 1.0) | |
| Number of higher intakes | |||||
| 0–1 | 23 | 73 | 1.0 | 1.0 | |
| 2–3 | 490 | 2469 | 0.62 (0.39, 1.0) | 0.63 (0.39, 1.0) | |
| ≥4 | 714 | 4553 | 0.49 (0.31, 0.79) | 0.53 (0.33, 0.86) | |
| Combinations | |||||
| None or only 1 high | 23 | 73 | 1.0 | 1.0 | |
| High vitamin B6 and high vitamin B12 only | 30 | 160 | 0.59 (0.32, 1.1) | 0.61 (0.33, 1.1) | |
| High vitamin B6, high vitamin B12, and high choline only | 377 | 1876 | 0.63 (0.39, 1.0) | 0.62 (0.38, 1.0) | |
| High vitamin B6, high vitamin B12, high choline, and high betaine only | 272 | 1688 | 0.51 (0.31, 0.82) | 0.55 (0.33, 0.91) | |
| High vitamin B6, high vitamin B12, high choline, high betaine, and high riboflavin3 | 196 | 1293 | 0.48 (0.29, 0.78) | 0.56 (0.33, 0.95) | |
| High vitamin B6, high vitamin B12, high choline, high betaine, and high zinc3 | 90 | 625 | 0.45 (0.27, 0.76) | 0.34 (0.19, 0.62) | |
| High vitamin B6, high vitamin B12, high choline, high betaine, and high thiamine3 | 20 | 155 | 0.41 (0.21, 0.80) | 0.28 (0.12, 0.64) | |
| High vitamin B6, high vitamin B12, high choline, high betaine, and high methionine3 | 10 | 80 | 0.41 (0.18, 0.91) | 0.26 (0.09, 0.77) | |
aOR, adjusted OR; cOR crude OR.
To categorize each micronutrient intake (higher or lower intake), we used a combination of information from diet and supplements. Excluding supplementers, we regressed case-control status on the energy-adjusted dietary estimate of a given methyl donor micronutrient, using restricted cubic splines with ≤5 knots controlling for estimated dietary folate equivalents as a model covariate, and identified a cut point where the OR comparing higher compared with lower intake was maximized. Subsequently, we grouped the supplementers into the respective higher intake category if the typical content of the supplement was expected to be greater than the identified dietary cutoff (refer to Table 1).
Adjusted for age, race, education, and study center.
Nonexclusive. Participants may have high intakes of the other methyl donors under study.
FIGURE 1.
Associations between a number of methyl donors and other micronutrients involved in one-carbon metabolism with higher intake and neural tube defects. Data were from the National Birth Defects Prevention Study (1999–2011). To categorize each micronutrient intake (higher or lower intake), we used a combination of information from diet and supplements. Excluding supplementers, we regressed case-control status on the energy-adjusted dietary estimate of a given methyl donor, using restricted cubic splines with ≤5 knots controlling for estimated dietary folate equivalents as a model covariate, and identified a cut point where the OR comparing higher compared with lower intake was maximized. Subsequently, we grouped the supplementers into the respective higher intake category if the typical content of the supplement was expected to be greater than the identified dietary cutoff (refer to Table 1). ORs and 95% CIs were estimated using unconditional logistic regression with Firth’s penalized likelihood adjusted for age, race/ethnicity, education, and study center. The y-axis is on the log scale. The reference group for all comparisons is intake in the higher range for none or only 1 of the micronutrients of interest. Ref, reference.
FIGURE 2.
Associations between specific groups with concurrent higher intakes of methyl donors and other micronutrients involved in one-carbon metabolism and neural tube defects. Data were from the National Birth Defects Prevention Study (1999–2011). To categorize each micronutrient intake (higher or lower intake), we used a combination of information from diet and supplements. Excluding supplementers, we regressed case-control status on the energy-adjusted dietary estimate of a given methyl donor, using restricted cubic splines with ≤5 knots controlling for estimated dietary folate equivalents as a model covariate, and identified a cut point where the OR comparing higher compared with lower intake was maximized. Subsequently, we grouped the supplementers into the respective higher intake category if the typical content of the supplement was expected to be greater than the identified dietary cutoff (refer to Table 1). ORs and 95% CIs were estimated using unconditional logistic regression with Firth’s penalized likelihood adjusted for age, race/ethnicity, education, and study center. The y-axis is on the log scale. The reference group for all comparisons is intake in the higher range for none or only 1 of the micronutrients of interest. Ref, reference.
In sensitivity analyses restricted to participants with complete data (Supplemental Table 3) and excluding supplementers (Supplemental Table 4), the associations were similar to those in the main analyses.
In secondary analyses of NTD subtypes (Supplemental Table 5), crude associations between isolated cases (i.e., no other accompanying major malformations in other organ systems) and higher compared with lower intake of the methyl donors matched those of the main analysis. Estimates for spina bifida and anencephaly were unadjusted, tended to be imprecise, and should be interpreted with caution. That said, higher riboflavin intake was associated with reduced odds of both spina bifida and anencephaly; higher intake of vitamin B6, vitamin B12, or methionine in isolation was associated with lower odds for spina bifida but not anencephaly; and higher intakes of the other micronutrients in isolation were associated with lower odds for anencephaly but not spina bifida. Crude results of the count analysis were similar to the main analysis for spina bifida; the count ORs were not computed for anencephaly, given the small number of cases in the reference group.
In stratified analyses of spina bifida by offspring sex (Table 4), crude estimates were imprecise, and the CIs were largely overlapping; comparisons should be interpreted with caution. That said, compared with males, females had lower odds of spina bifida with a higher intake of betaine, choline, or thiamine in isolation. Compared with females, males had lower odds of spina bifida with a higher intake of vitamin B6, vitamin B12, or zinc in isolation. Odds of spina bifida for higher compared with lower riboflavin intake were similar for males and females. Methionine could not be compared by sex due to the small number of exposed male cases. As the number of concurrent higher intakes of methyl donors increased, the odds of spina bifida decreased for both males and females, but females demonstrated slightly greater reductions.
TABLE 4.
Crude associations between methyl donors and other micronutrients involved in one-carbon metabolism and spina bifida stratified by infant sex,1 National Birth Defects Prevention Study (1999–2011)
| Females n = 3851 |
Males n = 3948 |
||||||
|---|---|---|---|---|---|---|---|
| Cases n = 331 | Cont N = 3520 | cOR (95% CI) | Cases n = 380 | Cont N = 3568 | cOR (95% CI) | ||
| Individual nutrients2 | |||||||
| Vitamin B6 | <1.45 mg | 7 | 72 | 1.0 | 13 | 76 | 1.0 |
| ≥1.45 mg | 324 | 3448 | 0.91 (0.42, 2.0) | 367 | 3492 | 0.60 (0.33, 1.1) | |
| Vitamin B12 | <3.5 μg | 19 | 165 | 1.0 | 27 | 169 | 1.0 |
| ≥3.5 μg | 312 | 3355 | 0.79 (0.49, 1.3) | 353 | 3399 | 0.64 (0.42, 0.97) | |
| Betaine | <88 mg | 181 | 1724 | 1.0 | 202 | 1828 | 1.0 |
| ≥88 mg | 150 | 1796 | 0.80 (0.64, 1.0) | 178 | 1740 | 0.93 (0.75, 1.1) | |
| Choline | <200 mg | 24 | 184 | 1.0 | 16 | 190 | 1.0 |
| ≥200 mg | 307 | 3336 | 0.69 (0.45, 1.1) | 364 | 3378 | 1.2 (0.74, 2.1) | |
| Methionine | <2.5 g | 324 | 3461 | 1.0 | 378 | 3506 | 1.0 |
| ≥2.5 g | 7 | 59 | 1.3 (0.62, 2.9) | 2 | 62 | NC | |
| Riboflavin | <2.4 mg | 232 | 2326 | 1.0 | 262 | 2344 | 1.0 |
| ≥2.4 mg | 99 | 1194 | 0.83 (0.65, 1.1) | 118 | 1224 | 0.86 (0.69, 1.1) | |
| Thiamine | <2.34 mg | 325 | 3387 | 1.0 | 363 | 3442 | 1.0 |
| ≥2.34 mg | 6 | 133 | 0.51 (0.23, 1.1) | 17 | 126 | 1.3 (0.78, 2.2) | |
| Zinc | ≤15 mg | 280 | 2985 | 1.0 | 328 | 2983 | 1.0 |
| >15 mg | 51 | 535 | 1.0 (0.75, 1.4) | 52 | 585 | 0.81 (0.60, 1.1) | |
| Number of micronutrients with higher intake2 | |||||||
| 0–1 | 8 | 34 | 1.0 | 8 | 39 | 1.0 | |
| 2–3 | 127 | 1222 | 0.42 (0.19, 0.93) | 153 | 1243 | 0.57 (0.27, 1.2) | |
| ≥4 | 196 | 2264 | 0.35 (0.16, 0.76) | 219 | 2286 | 0.45 (0.21, 0.96) | |
Cont, controls; cOR, crude OR; NC, not calculated.
Excludes n = 66 participants where sex was unknown or ambiguous.
To categorize each micronutrient intake (higher or lower intake), we used a combination of information from diet and supplements. Excluding supplementers, we regressed case-control status on the energy-adjusted dietary estimate of a given methyl donor, using restricted cubic splines with ≤5 knots controlling for estimated dietary folate equivalents as a model covariate, and identified a cut point where the OR comparing higher compared with lower intake was maximized. Subsequently, we grouped the supplementers into the respective higher intake category if the typical content of the supplement was expected to be greater than the identified dietary cutoff (refer to Table 1).
Discussion
Higher periconceptional intakes of methyl donors involved in one-carbon metabolism, through diet or supplementation, were associated with lower NTD risk among NBDPS participants who met the clinical recommendations for folic acid. Associations strengthened considerably with concurrent intakes of multiple methyl donors. The strongest association, equating to ∼75% lower NTD risk, occurred with concurrent consumption of higher amounts of vitamin B6, vitamin B12, choline, betaine, and methionine, compared with intake of only 1 or no methyl donors in the higher range. When restricted to women who did not supplement and controlling for DFEs, findings were similar, supporting that the associations were neither due to multivitamin use nor due to underlying folic acid intake. These findings suggest that risk reduction may be possible without supplementation; however, from our estimates, the minority of our sample met the folic acid requirements through diet alone, emphasizing that despite fortification, many women rely on supplements to meet the folic acid guidance.
Overall, our findings were consistent with previous research in the Slone Birth Defects Study (1998–2015), which used a similar design and analytic approach [7]; eligibility criteria barred participants from being in both studies. In that investigation, NTD risk was ∼50% lower with higher intakes of vitamin B6, vitamin B12, choline, and methionine and intake in the middle range for betaine, as compared with having intake for none or only 1 of these nutrients in those ranges (adjusted OR: 0.49; 95% CI: 0.23, 1.08). Unlike our study, that study did not evaluate riboflavin, thiamine, or zinc. Given our findings, it is unclear whether riboflavin, thiamine, and zinc yield much additional benefit in the presence of the aforementioned methyl donors. Another investigation in California (1989–1991) found lower NTD risks when choline and folate, choline and methionine, or choline and betaine were consumed together in higher amounts in the diet; an even stronger association was observed with concurrent higher intakes of choline, betaine, and methionine (OR: 0.17; 95% CI: 0.04, 0.76) [16]. Unlike our study, that study was not restricted to women with recommended folic acid intake.
In our study, we observed some effect heterogeneity between spina bifida and anencephaly, although estimates were unadjusted and imprecise. Another study in the NBDPS (1997–2003) reported inverse associations with both spina bifida and anencephaly for the highest compared with the lowest quartile of individual dietary intakes of choline, thiamine, and riboflavin and with anencephaly only for vitamins B6 and B12 among folic acid supplement users. Estimates were close to (or above) the null for methionine, betaine, and (for spina bifida) vitamins B6 and B12. Differences in findings between the present and prior NBDPS investigations might be due, in part, to the different timeframes, our restriction to participants with estimated folic acid ≥400 μg daily, and our method to dichotomize the nutrient exposures accounting for both dietary and supplement sources compared with their method using quartiles based on diet only.
We observed slightly stronger crude NTD risk reductions with higher intakes of multiple methyl donors among females than males, although the CIs were largely overlapping. NTD sex differences have been hypothesized to relate to epigenetic mechanisms making females more vulnerable to mutations that affect neural tube closure [44]. This may explain why methyl donor intake appears to be more effective for NTD prevention in females. In support of this hypothesis, a comparison of United States studies found a reduction in the preponderance of females with spina bifida after mandatory fortification [31]. A Chinese cohort (1993–1996) found, among pregnancies without periconceptional folic acid supplementation, NTDs were more prevalent in females compared with male offspring (8.8 compared with 4.4 cases/1000 births), but NTD prevalence was similar among pregnancies exposed to supplementation (females: 1.3/1000, males: 1.4/1000) [46].
Strengths and limitations
Strengths of our study include the population-based design, the relatively large number of cases, the rigor of case classification, and the adjustment for sociodemographic factors. Although the length of time from EDD to interview tended to be longer for cases, there is no empirical evidence to suggest that cases would systematically report dietary intakes or supplement use of these micronutrients differently compared with controls. Unlike folic acid, little information was available on the benefit of these micronutrients in relation to NTDs at the time of the study.
Although the incorporation of diet and supplement data could be viewed as a strength, our interpretation has limitations. Dietary intake was assessed through the maternal report, which is vulnerable to measurement errors [47]. Given their semi-quantitative nature, FFQs estimate relative, rather than absolute, intake; our study does not provide insight into clinically relevant thresholds. Nutritional deficiency is uncommon in the study population, so few participants were in the lower intake ranges for some micronutrients, and the reference group for concurrent intakes was small, resulting in imprecise estimates. For products containing vitamin B6 or vitamin B12 and zinc-only supplements, women who reported any periconceptional use (regardless of frequency) were categorized as having higher intake for that nutrient because it was assumed that the supplemental intake in addition to diet would be enough to put them in the higher range. However, this assumption may not hold for all relevant participants. Bioavailability may differ by source and other factors affecting absorption (e.g., food storage and preparation, adiposity, and use of certain medications). Further interrogation of potential differences based on the source of folic acid and other methyl donor intakes is needed in future research. Biomarkers more accurately reflect concentrations in the body, but samples would need to be collected during the periconceptional period, the etiologically relevant time period for NTD occurrence, which would be challenging to implement since that is often before pregnancy is recognized [48]. Given their rarity, we did not account for women at higher risk for NTDs (i.e., those with a prior NTD-affected pregnancy) who may benefit from even higher doses of methyl donor micronutrients, as has been observed for folic acid [49]. With the exception of dietary folic acid, we did not control for the intake of other micronutrients, which may be correlated with those under study. Lastly, due to limitations of the questionnaire, we had to use a combined variable for race and ethnicity, which conflates these 2 constructs.
Conclusions
Our study provides support that the mechanism by which folic acid prevents NTDs is through one-carbon metabolism. Our findings add to growing evidence that higher intakes of methyl donors and other micronutrients involved in one-carbon metabolism may translate to reduced NTD prevalence, including in non-folate sensitive cases. Although definitive evidence may not be possible without a randomized trial, it is unlikely that such a trial would ever be conducted since participants would need to enroll before pregnancy, a massive sample size would be needed to capture a sufficient number of NTD outcomes and the ethical concern of denying access to methyl donors, due to their importance in fetal development in general. Multivitamin supplementation and a healthy diet are already clinically recommended for all women of reproductive age [50]. Given the consistency of observational evidence and the facts that NTDs have persisted despite mandatory folic acid fortification [6] and higher intake of methyl donors is achievable through diet and supplementation, public health messaging should be considered.
Acknowledgments
We thank Steve Kerr for his replication of this analysis, in accordance with the National Birth Defects Prevention Study/Birth Defects Study to Evaluate Pregnancy Exposures data replication policy.
The findings and conclusions in this publication are those of the authors and do not necessarily represent the official position of the California Department of Public Health or the views of the North Carolina Department of Health and Human Services, Division of Public Health.
Author contributions
The authors’ responsibilities were as follows – JMP, GMS, SLC, TAD, EN, AMD, SEP, MDP, MMY, and MMW: designed the research; JMP: analyzed the data, wrote the paper, and takes primary responsibility for its final contents; RSS-W and AMD: provided support in the analysis and writing; GMS, SLC, TAD, EN, SEP, MDP, MMY, and MMW: provided oversight, critical feedback, and expertise in nutrition and birth defects; and all authors: read and approved the final manuscript.
Conflict of interest
The authors report no conflicts of interest.
Funding
This project was supported through CDC cooperative agreements under PA #96043, PA #02081, FOA #DD09-001, FOA #DD13-003, and NOFO #DD18-001 to the Centers for Birth Defects Research and Prevention participating in the National Birth Defects Prevention Study and/or the Birth Defects Study to Evaluate Pregnancy Exposures. This work was also supported by grant number DK56350 from the Nutrition Epidemiology Core of the University of North Carolina Clinical Nutrition Research Center. Coding of vitamin supplement information in the National Birth Defects Prevention Study used the Slone Drug Dictionary under license from the Slone Epidemiology Center of Boston University. The study sponsors had no involvement in study design; collection, analysis, and interpretation of data; or writing of the report; and have no restrictions regarding the submission of the report for publication.
Data availability
The data described in this manuscript, code book, and analytic code may be made available upon request. The process for accessing the data used in this study is described at https://www.cdc.gov/ncbddd/birthdefects/nbdps-public-access-procedures.html.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.05.034.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Berry R.J., Li Z., Erickson J.D., Li S., Moore C.A., Wang H., et al. Prevention of neural-tube defects with folic acid in China. China-U.S. collaborative project for neural tube defect prevention. N. Engl. J. Med. 1999;341(20):1485–1490. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- 2.Kirke P.N., Daly L.E., Elwood J.H. A randomised trial of low dose folic acid to prevent neural tube defects. The Irish vitamin study group. Arch. Dis. Child. 1992;67(12):1442–1446. doi: 10.1136/adc.67.12.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prevention of neural tube defects: results of the medical research council vitamin study. MRC Vitamin Study Research Group. Lancet. 1991;338(8760):131–137. [PubMed] [Google Scholar]
- 4.Werler M.M., Shapiro S., Mitchell A.A. Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA. 1993;269(10):1257–1261. doi: 10.1001/jama.1993.03500100055027. [DOI] [PubMed] [Google Scholar]
- 5.Czeizel A.E., Dudás I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N. Engl. J. Med. 1992;327(26):1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 6.Williams J., Mai C.T., Mulinare J., Isenburg J., Flood T.J., Ethen M., et al. Updated estimates of neural tube defects prevented by mandatory folic acid fortification - United States, 1995-2011. MMWR. Morb. Mortal. Wkly. Rep. 2015;64(1):1–5. ISSN: 1545-861X (Electronic) 0149-2195 (Linking) [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen J.M., Parker S.E., Crider K.S., Tinker S.C., Mitchell A.A., Werler M.M. One-carbon cofactor intake and risk of neural tube defects among women who meet folic acid recommendations: A multicenter case-control study. Am. J. Epidemiol. 2019;188(6):1136–1143. doi: 10.1093/aje/kwz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crider K.S., Yang T.P., Berry R.J., Bailey L.B. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012;3(1):21–38. doi: 10.3945/an.111.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox J.T., Stover P.J. Folate-mediated one-carbon metabolism. Vitam. Horm. 2008;79:1–44. doi: 10.1016/S0083-6729(08)00401-9. [DOI] [PubMed] [Google Scholar]
- 10.James P., Sajjadi S., Tomar A.S., Saffari A., Fall C.H.D., Prentice A.M., et al. Candidate genes linking maternal nutrient exposure to offspring health via DNA methylation: a review of existing evidence in humans with specific focus on one-carbon metabolism. Int. J. Epidemiol. 2018;47(6):1910–1937. doi: 10.1093/ije/dyy153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li K., Wahlqvist M.L., Li D. Nutrition, one-carbon metabolism and neural tube defects: a review. Nutrients. 2016;8(11):741. doi: 10.3390/nu8110741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carmichael S.L., Yang W., Shaw G.M. Periconceptional nutrient intakes and risks of neural tube defects in California. Birth. Defects. Res. A. Clin. Mol. Teratol. 2010;88(8):670–678. doi: 10.1002/bdra.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu Q., Li Y., Cui Z.L., Luo X.P. Homocysteine, folate, vitamin B12 and B6 in mothers of children with neural tube defects in Xinjiang, China. Acta. Paediatr. 2012;101(11):e486–e490. doi: 10.1111/j.1651-2227.2012.02795.x. [DOI] [PubMed] [Google Scholar]
- 14.Ray J.G., Wyatt P.R., Thompson M.D., Vermeulen M.J., Meier C., Wong P.Y., et al. Vitamin B12 and the risk of neural tube defects in a folic-acid-fortified population. Epidemiology. 2007;18(3):362–366. doi: 10.1097/01.ede.0000257063.77411.e9. [DOI] [PubMed] [Google Scholar]
- 15.Candito M., Rivet R., Herbeth B., Boisson C., Rudigoz R.C., Luton D., et al. Nutritional and genetic determinants of vitamin B and homocysteine metabolisms in neural tube defects: a multicenter case-control study. Am. J. Med. Genet. A. 2008;146A(9):1128–1133. doi: 10.1002/ajmg.a.32199. [DOI] [PubMed] [Google Scholar]
- 16.Shaw G.M., Carmichael S.L., Yang W., Selvin S., Schaffer D.M. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am. J. Epidemiol. 2004;160(2):102–109. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- 17.Shaw G.M., Finnell R.H., Blom H.J., Carmichael S.L., Vollset S.E., Yang W., et al. Choline and risk of neural tube defects in a folate-fortified population. Epidemiology. 2009;20(5):714–719. doi: 10.1097/EDE.0b013e3181ac9fe7. [DOI] [PubMed] [Google Scholar]
- 18.Benevenga N.J. Consideration of betaine and one-carbon sources of N5-methyltetrahydrofolate for use in homocystinuria and neural tube defects. Am. J. Clin. Nutr. 2007;85(4):946–949. doi: 10.1093/ajcn/85.4.946. [DOI] [PubMed] [Google Scholar]
- 19.Lavery A.M., Brender J.D., Zhao H., Sweeney A., Felkner M., Suarez L., et al. Dietary intake of choline and neural tube defects in Mexican Americans. Birth. Defects. Res. A. Clin. Mol. Teratol. 2014;100(6):463–471. doi: 10.1002/bdra.23236. [DOI] [PubMed] [Google Scholar]
- 20.Graham A., Brender J.D., Sharkey J.R., Zhu L., Felkner M., Suarez L., et al. Dietary methionine intake and neural tube defects in Mexican-American women, Birth. Defects. Res. A. Clin. Mol. Teratol. 2010;88(6):451–457. doi: 10.1002/bdra.20672. [DOI] [PubMed] [Google Scholar]
- 21.Shaw G.M., Velie E.M., Schaffer D.M. Is dietary intake of methionine associated with a reduction in risk for neural tube defect-affected pregnancies? Teratology. 1997;56(5):295–299. doi: 10.1002/(SICI)1096-9926(199711)56:5<295::AID-TERA1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 22.Chandler A.L., Hobbs C.A., Mosley B.S., Berry R.J., Canfield M.A., Qi Y.P., et al. Neural tube defects and maternal intake of micronutrients related to one-carbon metabolism or antioxidant activity. Birth. Defects. Res. A. Clin. Mol. Teratol. 2012;94(11):864–874. doi: 10.1002/bdra.23068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw G.M., Todoroff K., Schaffer D.M., Selvin S. Periconceptional nutrient intake and risk for neural tube defect-affected pregnancies. Epidemiology. 1999;10(6):711–716. doi: 10.1097/00001648-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Yan L., Wang B., Li Z., Liu Y., Huo W., Wang J., et al. Association of essential trace metals in maternal hair with the risk of neural tube defects in offspring. Birth. Defects. Res. 2017;109(3):234–243. doi: 10.1002/bdra.23594. [DOI] [PubMed] [Google Scholar]
- 25.Mills J.L., Fan R., Brody L.C., Liu A., Ueland P.M., Wang Y., et al. Maternal choline concentrations during pregnancy and choline-related genetic variants as risk factors for neural tube defects. Am. J. Clin. Nutr. 2014;100(4):1069–1074. doi: 10.3945/ajcn.113.079319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molloy A.M., Kirke P., Hillary I., Weir D.G., Scott J.M. Maternal serum folate and vitamin B12 concentrations in pregnancies associated with neural tube defects. Arch. Dis. Child. 1985;60(7):660–665. doi: 10.1136/adc.60.7.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMichael A.J., Dreosti I.E., Ryan P., Robertson E.F. Neural tube defects and maternal serum zinc and copper concentrations in mid-pregnancy: a case-control study. Med. J. Aust. 1994;161(8):478–482. doi: 10.5694/j.1326-5377.1994.tb127560.x. [DOI] [PubMed] [Google Scholar]
- 28.Schoendorfer N.C., Davies P. In: Micronutrients. 1st ed. Betancourt A.I., Gaitan H.F., editors. Nova Science Publishers, Inc; New York: 2012. Micronutrient interrelationships: synergism and antagonism; pp. 159–177. [Google Scholar]
- 29.Yoon P.W., Rasmussen S.A., Lynberg M.C., Moore C.A., Anderka M., Carmichael S.L., et al. The national birth defects prevention study. Public. Health. Rep. 2001;116(Suppl 1):32–40. doi: 10.1093/phr/116.S1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reefhuis J., Gilboa S.M., Anderka M., Browne M.L., Feldkamp M.L., Hobbs C.A., et al. The National birth defects prevention study: a review of the methods, Birth. Defects. Res. A. Clin. Mol. Teratol. 2015;103(8):656–669. doi: 10.1002/bdra.23384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasmussen S.A., Olney R.S., Holmes L.B., Lin A.E., Keppler-Noreuil K.M., Moore C.A., et al. Guidelines for case classification for the national birth defects prevention study, Birth. Defects. Res. A. Clin. Mol. Teratol. 2003;67(3):193–201. doi: 10.1002/bdra.10012. [DOI] [PubMed] [Google Scholar]
- 32.Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR. Recomm. Rep. 1992;41(RR-14):1–7. [PubMed] [Google Scholar]
- 33.Viswanathan M., Treiman K.A., Kish-Doto J., Middleton J.C., Coker-Schwimmer E.J., Nicholson W.K. Folic acid supplementation for the prevention of neural tube defects: an updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317(2):190–203. doi: 10.1001/jama.2016.19193. [DOI] [PubMed] [Google Scholar]
- 34.Botto L.D., Moore C.A., Khoury M.J., Erickson J.D. Neural-tube defects. N. Engl. J. Med. 1999;341(20):1509–1519. doi: 10.1056/NEJM199911113412006. [DOI] [PubMed] [Google Scholar]
- 35.Kelley K.E., Kelley T.P., Kaufman D.W., Mitchell A.A. The Slone drug dictionary: a research driven pharmacoepidemiology tool. Pharmacoepidemiol. Drug. Saf. 2003;12(Suppl 1):S168–S169. [Google Scholar]
- 36.Willett W.C., Reynolds R.D., Cottrell-Hoehner S., Sampson L., Browne M.L. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J. Am. Diet. Assoc. 1987;87(1):43–47. doi: 10.1016/S0002-8223(21)03057-1. [DOI] [PubMed] [Google Scholar]
- 37.Yuan C., Spiegelman D., Rimm E.B., Rosner B.A., Stampfer M.J., Barnett J.B., et al. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am. J. Epidemiol. 2018;187(5):1051–1063. doi: 10.1093/aje/kwx328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.US Department of Agriculture Agricultural Research Service, [Internet] Composition of foods raw, processed, prepared USDA national nutrient database for standard reference. Release 27. August 2014. Available from: https://data.nal.usda.gov/dataset/composition-foods-raw-processed-prepared-usda-national-nutrient-database-standard-reference-release-27. Accessed 28 June 2023.
- 39.Willett W.C., Howe G.R., Kushi L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997;65(4):1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. discussion 1229S. [DOI] [PubMed] [Google Scholar]
- 40.Willett W. Nutritional Epidemiology. Oxford Scholarship Online; 2013. Energy-adjustment and measurement error. [Google Scholar]
- 41.Williams B.A., Mandrekar J.N., Mandrekar S.J., Cha S.S., Furth A.F. 2006. Finding optimal cutpoints for continuous covariates with binary and time-to-event outcomes Technical Report Series. # 79. Mayo Clinic. [Internet]https://www.mayo.edu/research/documents/biostat-79pdf/doc-10027230 Available from: [Google Scholar]
- 42.Willett W.C. 2nd ed. Oxford University Press; New York: 1998. Nutritional Epidemiology; p. 306. [Google Scholar]
- 43.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80(1):27–38. doi: 10.1093/biomet/80.1.27. [DOI] [Google Scholar]
- 44.Juriloff D.M., Harris M.J. Hypothesis: the female excess in cranial neural tube defects reflects an epigenetic drag of the inactivating X chromosome on the molecular mechanisms of neural fold elevation. Birth. Defects. Res. A. Clin. Mol. Teratol. 2012;94(10):849–855. doi: 10.1002/bdra.23036. [DOI] [PubMed] [Google Scholar]
- 45.Shaw G.M., Yang W., Finnell R.H. Male-to-female ratios among NTDs and women’s periconceptional intake of folic acid, Birth. Defects. Res. 2020;112(16):1187–1193. doi: 10.1002/bdr2.1708. [DOI] [PubMed] [Google Scholar]
- 46.Liu J., Li Z., Ye R., Liu J., Ren A. Periconceptional folic acid supplementation and sex difference in prevention of neural tube defects and their subtypes in China: results from a large prospective cohort study. Nutr. J. 2018;17(1):115. doi: 10.1186/s12937-018-0421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subar A.F., Freedman L.S., Tooze J.A., Kirkpatrick S.I., Boushey C., Neuhouser M.L., et al. Addressing current criticism regarding the value of self-report dietary data. J. Nutr. 2015;145(12):2639–2645. doi: 10.3945/jn.115.219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watson K., Angelotta C. The frequency of pregnancy recognition across the gestational spectrum and its consequences in the United States, Perspect. Sex. Reprod. Health. 2022;54(2):32–37. doi: 10.1363/psrh.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petersen J.M., Parker S.E., Benedum C.M., Mitchell A.A., Tinker S.C., Werler M.M. Periconceptional folic acid and risk for neural tube defects among higher risk pregnancies. Birth. Defects. Res. 2019;111(19):1501–1512. doi: 10.1002/bdr2.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Good health before pregnancy: prepregnancy care . 2020. American College of Obstetricians and Gynecologists [Internet]https://www.acog.org/womens-health/faqs/good-health-before-pregnancy-prepregnancy-care [updated 12/2021]. Available from: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data described in this manuscript, code book, and analytic code may be made available upon request. The process for accessing the data used in this study is described at https://www.cdc.gov/ncbddd/birthdefects/nbdps-public-access-procedures.html.