Abstract
Background
Clove volatile oil (CVO) and its major compound, eugenol (EUG), have anxiolytic effects, but their clinical use has been impaired due to their low bioavailability. Thus, their encapsulation in nanosystems can be an alternative to overcome these limitations.
Objectives
This work aims to prepare, characterize and study the anxiolytic potential of CVO loaded-nanoemulsions (CVO-NE) against anxious-like behavior in adult zebrafish (Danio rerio).
Methods
The CVO-NE was prepared using Agaricus blazei Murill polysaccharides as stabilizing agent. The drug-excipient interactions were performed, as well as colloidal characterization of CVO-NE and empty nanoemulsion (B-NE). The acute toxicity and potential anxiolytic activity of CVO, EUG, CVO-NE and B-NE against adult zebrafish models were determined.
Results
CVO, EUG, CVO-NE and B-NE presented low acute toxicity, reduced the locomotor activity and anxious-like behavior of the zebrafish at 4 – 20 mg kg–1. CVO-NE reduced the anxious-like behavior of adult zebrafish without affecting their locomotor activity. In addition, it was demonstrated that anxiolytic activity of CVO, EUG and CVO-NE is linked to the involvement of GABAergic pathway.
Conclusion
Therefore, this study demonstrates the anxiolytic effect of CVO, in addition to providing a new nanoformulation for its administration.
Graphical Abstract
Keywords: Essential oil, Nanoparticle, Biopolymer, Anxiety, Eugenol
Introduction
Anxiety disorders involve a dysfunctional response to potential threats, resulting in excessive worry and fear [1]. Furthermore, anxiety disorders represent the most common type of emotional illnesses, and patients suffering from these conditions have a high degree of functional impairment and a significant decrease in life quality [2]. Despite the predominance of these disorders and their impact on people's mental health, the pharmacotherapy currently used is not effective, showing the importance of researching new drugs to treat these conditions [3].
Volatile oils are colorless and generally aromatic liquids found in practically all parts of plants, such as seeds, bark, flowers and stems [4]. Eugenol (EUG) is a natural phenolic compound and the main constituent of clove volatile oil (CVO), Syzygium aromaticum (L.) Merr. & L.M Perry, which is responsible for most of its pharmacological properties [5, 6]. The anxiolytic activity of the hydroalcoholic extract of S. aromaticum L., investigated using the elevated plus maze and light–dark models, has been reported in previous studies [7]. In parallel, it has been reported that S. aromaticum L. exerts anesthetic and analgesic activities, and that its main constituent, eugenol, is able to activate GABA-A receptors [8].
The traditional use of S. aromaticum L. extends to other conditions of clinical importance; such as skin infections, wound healing, gastric disorders and neurodegenerative diseases. It is important to mention that CVO is also one of the main essences used in aromatherapy for stress reduction and relaxation [9–11]. However, its volatility, chemical instability and hydrophobicity has restricted its clinical use [12]. Therefore, a strategy to overcome these problems may be its encapsulation in nanoemulsions [13].
Nanoemulsions are mechanical dispersions with droplets on nanoscale formed by two immiscible liquids that are stabilized by emulsifiers. These systems have been increasingly used in volatile oil encapsulation in order to improve their application in the pharmaceutical and food industries [14]. Despite the volatile oil of cloves being considered GRAS by the Food and Drug Administration, there are reports in the literature of its irritating effect on the skin and mucosa [15]. Allied to this, the nanoencapsulation of eugenol, the major compound of this volatile oil, was able to reduce its cytotoxicity against human neutrophils and improve its anti-inflammatory activity [16].
Corroborating these properties, the use of nanoemulsions associated with polysaccharides from Agaricus blazei Murill mushroom (PAb) can help to improve the physicochemical features of the formulation containing CVO and to add this formulation in terms of bioactivity. We reported the different biological activities of polysaccharides isolated from Agaricus blazei Murill or Agaricus brasiliensis in a recent review [17]. Thus, the work aims at the development of nanoemulsions loaded with clove volatile oil stabilized by polysaccharides isolated from Agaricus blazei Murill, in order to evaluate its anxiolytic potential through an experimental model using adult zebrafish.
Experimental
Materials
Coconut oil was obtained from Nutiva® (Brazil). Tween 80 and eugenol were purchased from Sigma-Aldrich® (USA). Diazepam (DZP) and flumazenil (FMZ) were obtained from Neo Química® and Sandoz®, respectively. DMSO was purchased from Dinâmica® (Brazil). Agaricus blazei (SisGen: AC29F45) was purchased from Blazei Murril® DEC (São Paulo, Brazil). Clove buds (Syzygium aromaticum L.) were purchased in the Mercado São Sebastião, Fortaleza, Ceará State, Brazil. All other chemicals used were analytical grade. Clove buds were used for extraction of volatile oil by hydrodistillation and characterized by GC–MS [18]. Agaricus blazei Murill polysaccharides were isolated and characterized as reported previously [19].
Preparation of nanoemulsions
Nanoemulsions were prepared according previous work, where a 32 factorial design was employed to choose the vehicle with better physicochemical properties [18]. F8 formulation was chosen for the incorporation of PAb (0.25%, w w–1) in the aqueous phase, composed by Tween 80 (0.05%, w w–1) and ultrapurified water (enough for 10 g of formulation); while the organic phase consisted of coconut oil (0.5%, w w–1) and CVO (0.05%, w w–1). After pouring the aqueous phase into the oil phase, the system was pre-emulsified by magnetic stirring (500 rpm/10 min). Then, the ultrasonication was continued in a Sonifer W-450D device (Branson®) at 30 kHz for 1 min (70% of intensity, 5 s on/10 s off).
Droplet size, polydispersity index (PdI) and zeta potential
Nanoemulsions were diluted in deionized water (1:1000, v v–1) at 25 °C. Droplet size and PdI were measured using a Zetasizer Nano ZS90 particle size analyzer (Malvern®, UK) at 90°. The surface charge of the particles was evaluated by electrophoretic mobility using the same equipment, and the result was expressed as zeta potential in millivolts.
Fourier transform infrared spectroscopy (FTIR)
Infrared spectra were obtained using a spectrometer (70v, Bruker Vertex®, Canada) with an attenuated total reflectance (ATR) accessory. The analysis was performed in the range of 4000 to 600 cm−1.
Differential scanning calorimetry (DSC)
DSC analysis was carried out in a DSC 50 instrument (Shimadzu®, Japan). Where, samples of approximately 5 mg were added in aluminum support. The analyses were carried out on a heating ramp between 25 and 300 °C at 10 °C min–1 under nitrogen atmosphere (50 mL min–1).
Eugenol content and encapsulation efficiency
The determination of eugenol content in nanoemulsions and encapsulation efficiency (EE) was performed by spectrophotometry as reported previously (y = 0.049x + 0.044; R2 = 0.999; LOD = 0.03 µg mL–1 and LOQ = 0.09 µg mL–1) [20]. The assays were conducted in a ThermoScientific® model spectrophotometer (Genesys 6), using 230 nm as reading wavelength. EE was determined using Eq. 1.
| 1 |
Anxiolytic activity in zebrafish
Animals
Adult zebrafish (Danio rerio), males and females, (n = 6/group), aged 60–90 days, sizes of 3.5 ± 0.5 cm and weight 0.4 ± 0.1 g were used for this study. Animals were acclimated for 24 h in 10 L glass aquariums (30 × 15 × 20 cm3), containing dechlorinated water (ProtecPlus® anti-chlorine, 25 °C and pH 7.0), circadian cycle of 12/12 h (light/dark). The fish were fed with commercial food ad libitum 24 h before the experiments. The animals were sacrificed by immersion in cold water (4 °C). All procedures were approved by the Animal Use Ethics Committee of the State University of Ceará (CEUA-UECE), under protocol no. 04983945/2021.
Experimental groups
Zebrafish (n = 6/group) were treated intraperitoneally with 20 µL of the samples: EUG (4, 12 or 20 mg kg–1), CVO (4, 12 or 20 mg kg–1), CVO-NEO (clove volatile oil at 4, 12 or 20 mg kg–1), B-NE (volume equivalent to the CVO-NEO), diazepam (DZP, 40 mg kg–1), flumazenil (FMZ, 4 mg kg–1) or vehicle (control, 3% DMSO, v v–1).
Acute toxicity (96 h)
Toxicity evaluation was performed against adult zebrafish, according to the protocol 203 from OECD to determine the LD50–96 h [21]. During 96 h, the number of dead fish in each experimental group was determined and the LD50 was calculated according to Spearman-Karber method as described previously [22].
Open-field test
After 30 min of intraperitoneal (i.p) treatments, the animals were placed in Petri dishes (10 × 15 cm2), with quadrants, and their locomotor activity was analyzed through the average number of crossed lines during 5 min. The treatments (i.p.) and insulin syringes (0.5 mL; UltraFine® BD) were both implemented with a 30-gauge needle. Thus, through this test it was possible to analyze possible locomotor alterations, such as sedation or muscle relaxation [23].
Light–dark box test
In the light–dark test, fish have a natural preference for the dark side as a defense mechanism due to their aversion to new environments, and anxiolytic drugs induce an increase in the time spent and explored in the light compartment of the aquarium [1, 24]. This experiment consisted in evaluating the animals' permanence in the clear zone of the aquarium (30 × 15 × 20 cm3), which presented a division between clear and dark areas. The animals' anxious behavior was induced by filling the aquarium with water different from the conventional environment. Thirty minutes after the administration of the samples, the animals were placed in the clear zone and the potential anxiolytic-like activity was investigated by measuring the animals' permanence in this region during 5 min of evaluation [25].
GABAergic neuromodulation
The GABAergic system involvement in the mechanism of action of the samples that showed anxiolytic effect was studied through pre-treatment with FMZ (GABAA antagonist) [24]. Initially, the animals were pre-treated with FMZ (4 mg kg–1, 20 μL/i.p.) and after 15 min, the lowest dose with anxiolytic activity was administered, according to the different experimental groups (CVO, EUG, and CVO-NE). Three percent (3%) of DMSO (20 μL/i.p) was used as negative control. DZP (40 mg kg–1, 20 μL/i.p) was the agonist used. After 30 min, the animals were submitted to the light/dark test according to the method aforementioned.
Statistical analysis
Data distribution was analyzed using the Shapiro–Wilk test. One-way ANOVA followed by Tukey's test was used to identify significant differences (confidence interval: 95%, p < 0.05) between experimental groups. Statistical analyses were performed using the software GradPad Prism version 6.0.
Results and discussion
Drug-excipient interactions
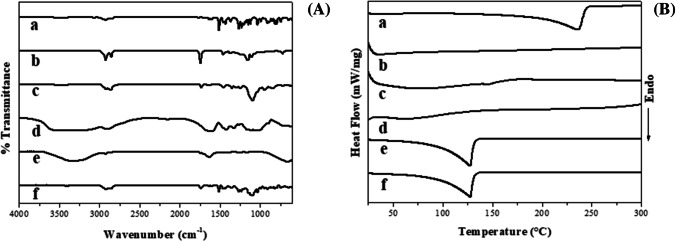
Figure 1A presents the results of the drug-excipient interactions by FTIR. The broad and strong bands presenting around 3200 cm–1 refer to the stretching of the O–H bond of the hydroxyl groups. The low intensity bands located at 2900 cm–1 refer to the stretching of the C-H bond of methylene groups (CH2). The FTIR spectra obtained for coconut oil and PAb showed strong bands between 1750 and 1700 cm–1, which are related to the stretching of the C = O bond of carbonyl groups that are present in the coconut oil’s fatty acids and in the protein fraction of the proteoglycan complex. Around 1100 cm–1 there is a medium intensity band present in the spectra obtained for polysaccharides, coconut oil and Tween 80. This band refers to the stretching of the C-O bond and it is characteristic of polysaccharides, as it indicates the glycosidic bond [21].
Fig. 1.
Drug-excipient compatibility study through FTIR (A) and DSC (B) analysis: (a) Clove volatile oil; (b) coconut oil; (c) Tween 80; (d) Polysaccharides from Agaricus blazei Murill; (e) Clove volatile oil-loaded nanoemulsion and (f) physical mixture
The FTIR spectrum obtained for CVO is very similar to that reported for EUG (major compound of CVO). The characteristic bands of EUG include the O–H (3500 cm–1), C-H (2937 cm–1) and C–C aromatic ring stretches (1636, 1614 and 1604 cm–1). Although these regions are common to the other excipients used in nanoformulation, the bands located at 720 (− CH2 bending) and 890 (–C = CH2 bending) cm–1 confirmed the presence of CVO in the physical mixture. These bands were also verified in eugenol-loaded chitosan nanoparticles in other studies [5, 26]. On the other hand, the FTIR spectrum obtained for CVO-NEO showed bands similar to the constituents of the aqueous phase (mainly PAb), which is suggestive of the almost complete encapsulation of this bioactive in the droplet’s core.
Figure 1B presents the results of the drug-excipient interaction study using DSC. The DSC curve obtained for CVO showed an endothermic peak at 236 °C, which corresponds to its volatilization; in addition, this peak is similar to that reported in the literature for the boiling temperature of EUG [27]. This peak was shifted to 128 °C in the DSC curves obtained for physical mixture and CVO-NE. This reduction may be due to the solubilization of the different hydrophobic constituents of CVO in the emollient used (coconut oil), which then undergoes melting at 35.4 °C. The solubilization of terpenoids from clove volatile oil in coconut oil contributes to the increase of their amorphous character, which requires less energy for physical state changes to occur. Results like this have been reported in the literature for nanoparticles loaded with volatile compounds [28]. The other excipients did not present important thermal events in the temperature range under study, being observed only the event referring to the CVO in the physical mixture and in the nanoformulation.
Nanoemulsion characterization
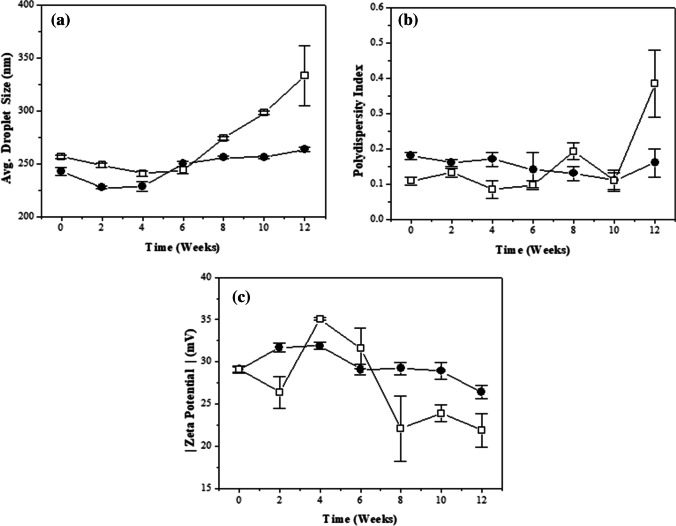
The hydrodynamic diameter of the developed nanoemulsions ranged from 227 to 333 nm (Fig. 2a). The formulation containing the encapsulated CVO showed smaller droplet size variation when compared to the B-NE. PDI values of the CVO-NE remained between 0.1 and 0.3, indicating that the droplet population present in the system is moderately polydisperse (Fig. 2b). After three months, B-NE showed a polydisperse character (PDI > 0.4) [18, 29]. The results obtained for CVO-NE were promising, since drug delivery systems with a hydrodynamic diameter around 200 nm showed efficacy in biodistribution/accumulation in the brain of adult zebrafish [30].
Fig. 2.
Nanoemulsions stability characterization by droplet size (a), polydispersity index (b) and zeta potential (c) during 12 weeks: (□) Blank nanoemulsion (B-NE) and (●) Clove volatile oil-loaded nanoemulsion (CVO-NE)
Both formulations developed in this work showed a negative surface charge, which can be attributed to the ionization of the fatty acids of the emollient employed. The zeta potential of the formulations remained between –32 and –22 mV during the analysis time (Fig. 2c). It is worth mentioning that the CVO-NE showed higher zeta potential modulus values when compared to the B-NE, which indicates that the encapsulation of this bioactive contributes to the increase of the electrostatic stability of the system. This explains why the formulation containing the CVO presented lower hydrodynamic diameter and PDI values during the analysis period.
In addition, the CVO content in the nanoformulation remained above 86% for twelve weeks, while the encapsulation efficiency result was higher than 99%. These facts indicate that practically all the volatile oil is present in the core of the droplets, as confirmed by the characterization of the CVO-NE by FTIR. These results, combined with the stable macroscopic aspect of the prepared systems (absence of signs of creaming, flocculation and sedimentation), indicated that the nanoformulation containing CVO has potential for biological application.
Anxiolytic activity in zebrafish
CVO, EUG, CVO-NE and B-NE have low acute toxicity against adult zebrafish
Before evaluating the efficacy of free bioactives and nanoformulations, their safety was assessed through an acute toxicity test. It is worth mentioning that the use of zebrafish to evaluate the toxicity of new nanomaterials has become widespread in the current scenario, due to its high similarity to the human genome, small size and high reproduction rate [13, 30]. Free bioactives (CVO and EUG), B-NE and CVO-NE showed low toxicity against adult zebrafish in tested doses, since no animals died during the evaluation period (96 h), as shown in Table 1. Based on these results, the evaluation of the potential anxiolytic effect of nanosystems and unencapsulated bioactives was carried out.
Table 1.
Acute toxicity study (96 h) for free bioactives and nanosystems in adult zebrafish model
| Sample | Mortality | 96 h LD50 (mg kg–1, i. p.) |
|||
|---|---|---|---|---|---|
| Control | 4 mg kg–1 | 12 mg kg–1 | 20 mg kg–1 | ||
| CVO | 0 | 0 | 0 | 0 | > 20 |
| EUG | 0 | 0 | 0 | 0 | > 20 |
| CVO-NE | 0 | 0 | 0 | 0 | > 20 |
| B-NE | 0 | 0 | 0 | 0 | > 20 |
CVO clove volatile oil; EUG Eugenol; CVO-NE clove volatile oil-loaded nanoemulsion; B-NE blank nanoemulsion and Control = 3% DMSO. LD50 = lethal dose for 50% of adult zebrafish
Nanoencapsulation prevents locomotor impairment of the CVO in adult zebrafish
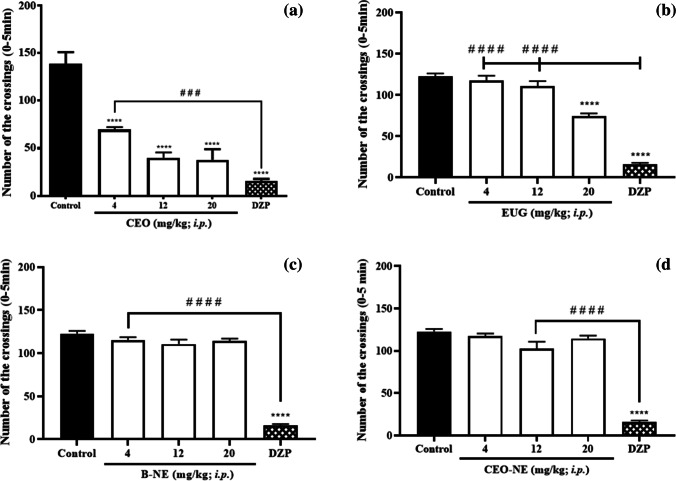
Locomotor activity is an important behavioral parameter for the evaluation of zebrafish exposed to nanoformulations for neuropharmacological application, as it indicates a potential action at central nervous system level [30]. According to the results of the open field test, it was observed that only CVO (4–20 mg kg–1) and EUG (20 mg kg–1) were able to reduce animal locomotion when compared to control group (****p < 0.0001); similarly to DZP (Fig. 3). As the assessment of the locomotor activity of animals is widely used to measure the excitability level of the CNS, this reduction in locomotion is a result of depression of the CNS [31].
Fig. 3.
Effects of CVO (a), EUG (b), B-NE (c) and CVO-NE (d) on locomotor activity of adult zebrafish (Danio rerio) in the Open Field Test (0–5 min). Results are expressed as mean ± SEM. Diazepam (DZP, 40 mg kg–1, 20 µL/i.p.) was used as an anxiolytic standard drug. Where, ****p < 0.0001 vs. Control (3% DMSO, 20 µL/i.p) and # # # p < 0.001 vs. DZP; # # # #p < 0.0001 vs. DZP according to one-way ANOVA followed by Tukey post hoc test
Administration of benzodiazepines, like DZP, reduces anxious-like behavior and locomotor activity in adult zebrafish as a result of reduced neuronal excitation due to increased frequency of chloride channel opening, promoting hyperpolarization [32]. Thus, zebrafish show a significant decrease in parameters associated with locomotion, such as distance traveled, average speed and acceleration, especially compared to the control group [30]. However, from a clinical point of view, motor impairment may favor falls, so benzodiazepines are contraindicated for the elderly population [33]. This result showed that nanoencapsulation was able to prevent the motor impairment caused by the administration of CVO in adult zebrafish.
CVO, EUG and CVO-NE relieve anxiolytic-like behavior in adult zebrafish
In this work, as far as we know, we recorded the first evidence on the anxiolytic effect of nanoemulsions containing clove volatile oil stabilized by polysaccharides isolated from Agaricus blazei Murill mushroom through an experimental model in adult zebrafish (Danio rerio).
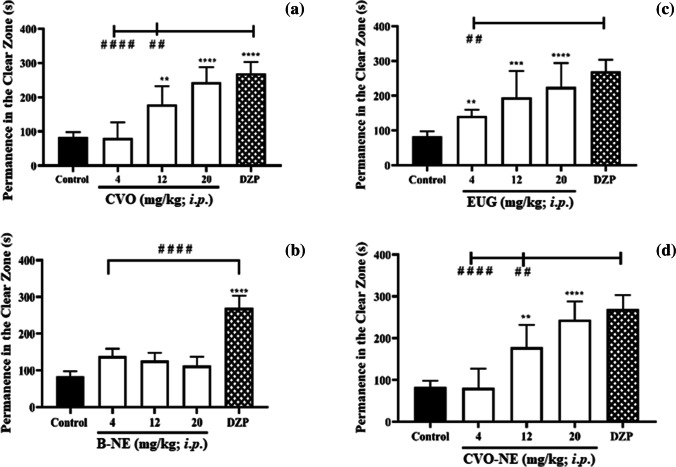
In the light–dark box test, drugs with anxiolytic activity prolong the permanence of animals in the clear region of the aquarium, while drugs that induce anxious behavior reduce this parameter [25]. We observed that CVO, EUG and CVO-NE increased the time of permanence of the animals in the clear area of the aquarium when compared to control group (p < 0.05), similar to the group treated with DZP (Fig. 4a, b and d). In contrast, B-NE did not reverse the anxious-like behavior of the animals (Fig. 4c). This result showed that the nanoencapsulated clove volatile oil had an anxiolytic effect without exerting any side effects at the motor level.
Fig. 4.
Anxiolytic-like effects of CVO (a), EUG (b), B-NE (c) and CVO-NE (d) on light–dark box tests. Results are expressed as mean ± SEM. Diazepam (DZP, 40 mg kg–1, 20 µL/i.p.) was used as an anxiolytic standard drug. Where, **p < 0.01; ***p < 0.001, ****p < 0.0001 vs. Control (3% DMSO, 20 µL/i.p) and # #p < 0.01; # # # #p < 0.0001 according to one-way ANOVA followed by Tukey post hoc test
Several studies have already reported different effects of clove volatile oil and/or EUG on the central nervous system. Zebrafish, at different stages of development, when placed in aquariums containing EUG at 10 and 15 µg mL–1, showed characteristic motor parameters of sedation [34]. Moreover, EUG encapsulation in chitosan-zein coated nanoparticles increased its anesthetic effect against Nile tilapia, where a profound anesthetic effect was verified from 40 mg L–1 in an immersion model [35]. In the same model, CVO (at 100 mg mL–1) did not affect the behavior of Nile tilapia, in terms of reactivity and motivation [36]. Based on these results, we investigated the action mechanism associated with the anxiolytic-like effect of CVO, EUG and CVO-NE.
GABAergic neuromodulation is involved with anxiolytic effect of CVO, EUG and CVO-NE in adult zebrafish
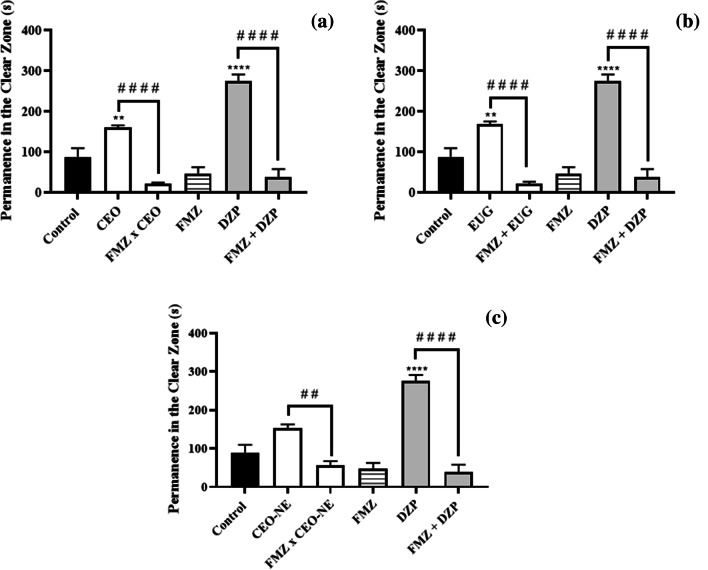
After verifying the effect of CVO, EUG and CVO-NE on locomotor activity and anxious behavior in adult zebrafish, we proceeded with the evaluation of the action mechanism linked to this activity. Thus, the involvement of the GABAergic pathway in the anxiolytic effect of the samples was studied by pretreatment with FMZ (GABAergic antagonist). The anxiolytic effect of CVO, EUG and CVO-NE was significantly reversed (p < 0.05) by pretreatment with FMZ, similarly to DZP (GABAergic agonist). The animals returned to show more permanence in the dark area of the aquarium, which is a characteristic of anxious behavior (Fig. 5a, b and c).
Fig. 5.
Effect of flumazenil (FMZ) on the anxiolytic-like effects of CVO (a), EUG (b) and CVO-NE (c) for evaluation of the GABAergic system involvement. Results are expressed as mean ± SEM. Where, **p < 0.01; ***p < 0.001, ****p < 0.0001 vs. Control (3% DMSO, 20 µL/i.p) and ##p < 0.01; ####p < 0.0001 according to one-way ANOVA followed by Tukey post hoc test
These results corroborate with those described by other authors, who reported the ability of the aqueous extract from clove buds (Syzygium aromaticum L.) and EUG to activate GABAA receptors, contributing to increase the GABAergic neuromodulation [8]. Similarly, Nectandra grandiflora essential oil and its isolated sesquiterpenoids reduce the anxiety-like behavior of zebrafish by acting on GABAergic neurotransmission [37].
Conclusion
Therefore, our findings demonstrate the potential anxiolytic effect of free and nanoencapsulated clove volatile oil against anxious-like behavior in adult zebrafish. The nanoemulsions showed droplets on nanometric scale, low polydispersity, electrostatic stability and high active content for three months. Furthermore, no drug-excipient incompatibility was evidenced through FTIR and DSC analysis. Nanoencapsulation was able to preserve the anxiolytic activity, as well as prevent the occurrence of side effects at locomotor level, which were observed for unencapsulated volatile oil. Nanosystems and unencapsulated bioactives showed low acute toxicity, demonstrating their preclinical safety. The anxiolytic activity observed for clove volatile oil, eugenol and nanoemulsion was related to GABAergic neuromodulation. Thus, this study reports the development and preclinical evaluation of a new nanoformulation with potential for the treatment of anxiety disorders.
Acknowledgements
Authors thank Central Analítica-UFC/CT-INFRA/MCTI-SISNANO/CAPES for the support.
Authors' contributions
MSC, JFCN, ALS and MKAF: data curation, formal analysis, investigation, methodology, writing of the original draft; HSS, NVG, SAS and NMPSR: resources, supervision, writing-review and editing; JESAM and MENPR: conceptualization, funding acquisition, investigation, resources, supervision, writing of original draft and editing.
Funding
This work was funded by the Coordination for the Improvement of Higher Educational Personnel (Finance Code 001, CAPES/PROEX: 23038.000509/2020–82, Nº 1227/2020) and National Council for Scientific and Technological Development (CNPq: N.M.P.S.R., research grant No 309795/2021–4).
Data availability
All the relevant data are reported within the paper. For additional details, data are available on request to the authors.
Declarations
Ethics approval and consent to participate
All procedures were approved by the Animal Use Ethics Committee of the State University of Ceará (CEUA-UECE), under protocol no. 04983945/2021.
Consent for publication
All authors support the submission to this journal.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jane Eire Silva Alencar de Menezes, Email: jane.menezes@uece.br.
Maria Elenir Nobre Pinho Ribeiro, Email: elenir.ribeiro@ufc.br.
References
- 1.Gonçalves NGG, Araújo JIF, Magalhães FEA, Mendes FRS, Lobo MDP, Moreira ACOM, Moreira RA. Protein fraction from Artocarpus altilis pulp exhibits antioxidant properties and reverses anxiety behavior in adult zebrafish via the serotoninergic system. J Funct Foods. 2020;66:103772. doi: 10.1016/j.jff.2019.103772. [DOI] [Google Scholar]
- 2.Moreno-Rius J. The cerebellum in fear and anxiety-related disorders. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;85:23–32. doi: 10.1016/j.pnpbp.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Narasingam M, Vijeepallam K, Mohamed Z, Pandy V. Anxiolytic- and antidepressant-like activities of a methanolic extract of Morinda citrifolia Linn. (noni) fruit in mice: Involvement of benzodiazepine-GABAAergic, serotonergic and adrenergic systems. Biomed Pharmacother. 2017;96:944–952. doi: 10.1016/j.biopha.2017.11.148. [DOI] [PubMed] [Google Scholar]
- 4.Nascimento RV, Alves MS, Pinto TO, Menezes RS, Damasceno-Junior PC, Chaves DSA, Souza MAA. Hydrodistillation extraction kinetics of volatile oils from Varronia curassavica and Laurus nobilis. Rev Bras Farmacogn. 2020;30:503–509. doi: 10.1007/s43450-020-00067-9. [DOI] [Google Scholar]
- 5.Anand T, Anbukkarasi M, Thomas PA, Geraldine P. A comparison between plain eugenol and eugenol-loaded chitosan nanoparticles for prevention of in vitro selenite-induced cataractogenesis. J Drug Deliv Sci Technol. 2021;65:102696. doi: 10.1016/j.jddst.2021.102696. [DOI] [Google Scholar]
- 6.Sisakhtnezhad S, Heidari M, Bidmeshkipour A Eugenol enhances proliferation and migration of mouse bone marrow-derived mesenchymal stem cells in vitro. Environ Toxicol Pharmacol. 2018;57:166–174. doi: 10.1016/j.etap.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Tiwari P, Verma R, Ahirwar D, Chandy A, Dwivedi S. Evaluation of anxiolytic effect of Syzygium aromaticum: a traditional herb of India. Asian Pacific J Trop Dis. 2014;4:S77–S80. doi: 10.1016/S2222-1808(14)60418-7. [DOI] [Google Scholar]
- 8.Sahin S, Eulenburg V, Heinlein A, Villmann C, Pischetsrieder M. Identification of eugenol as the major determinant of GABAA-receptor activation by aqueous Syzygium aromaticum L. (clove buds) extract. J Funct Foods. 2017;37:641–649. doi: 10.1016/j.jff.2017.08.033. [DOI] [Google Scholar]
- 9.Panahzadeh F, Mirnasuri R, Rahmati M. Exercise and Syzygium aromaticum reverse memory deficits, apoptosis and mitochondrial dysfunction of the hippocampus in Alzheimer's disease. J Ethnopharmacol. 2022;286:114871. doi: 10.1016/j.jep.2021.114871. [DOI] [PubMed] [Google Scholar]
- 10.Peng C, Sang S, Shen X, Zhang W, Yan J, Chen P, Jiang C, Yuan Y, Zhu W, Yao M. In vitro anti-Helicobacter pylori activity of Syzygium aromaticum and the preliminary mechanism of action. J Ethnopharmacol. 2022;288:114995. doi: 10.1016/j.jep.2022.114995. [DOI] [PubMed] [Google Scholar]
- 11.Blanco GEO, Souza CWO, Bernardo MP, Zenke M, Mattoso LHC, Moreira FKV. Antimicrobially active gelatin/[Mg-Al-CO3]-LDH composite films based on clove essential oil for skin wound healing. Mater Today Commun. 2021;27:102169. doi: 10.1016/j.mtcomm.2021.102169. [DOI] [Google Scholar]
- 12.Mukurumbira AR, Shellie RA, Keast R, Palombo EA, Jadhav SR. Encapsulation of essential oils and their application in antimicrobial active packaging. Food Control. 2022;136:108883. doi: 10.1016/j.foodcont.2022.108883. [DOI] [Google Scholar]
- 13.Budel RG, Silva DA, Moreira MP, Dalcin AJF, Silva AF, Nazario LR, Majolo JH, Lopes LQS, Santos RCV, Soares FAA, Silva RS, Gomes P, Boeck CR. Toxicological evaluation of naringin-loaded nanocapsules in vitro and in vivo. Colloids Surf B. 2020;188:110754. doi: 10.1016/j.colsurfb.2019.110754. [DOI] [PubMed] [Google Scholar]
- 14.Lucia A, Guzman E. Emulsions containing essential oils, their components or volatile semiochemicals as promising tools for insect pest and pathogen management. Adv Colloid Interface Sci. 2021;287:102330. doi: 10.1016/j.cis.2020.102330. [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Huang G, Ma Y, Liu Y, Huang X, Zheng Q, Yue P, Yang M. Cellulose nanocrystals based clove oil Pickering emulsion for enhanced antibacterial activity. Int J Biol Macromol. 2021;170:24–32. doi: 10.1016/j.ijbiomac.2020.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Lopes AA, Fonseca FN, Rocha TM, Freitas LB, Araújo EVO, Wong DVT, Lima Júnior RCP, Leal LKAM. Eugenol as a promising molecule for the treatment of dermatitis: antioxidant and anti-inflammatory activities and its nanoformulation. Oxid Med Cell Longev. 2018;2018:8194849. doi: 10.1155/2018/8194849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campelo MS, Câmara Neto JF, Lima ABN, Chagas Neto FC, Gonzaga MLC, Soares SA, Leal LKAM, Ribeiro MENP, Ricardo NMPS. Polysaccharides and extracts from Agaricus brasiliensis Murill – A comprehensive review. Int J Biol Macromol. 2021;183:1697–1714. doi: 10.1016/j.ijbiomac.2021.05.112. [DOI] [PubMed] [Google Scholar]
- 18.Campelo MS, Melo EO, Arrais SP, Nascimento FBSA, Gramosa NV, Soares SA, Ribeiro MENP, Silva CR, NobreJúnior HV, Ricardo NMPS. Clove essential oil encapsulated on nanocarrier based on polysaccharide: a strategy for the treatment of vaginal candidiasis. Colloids Surf A: Physicochem Eng. 2021;610:125732. doi: 10.1016/j.colsurfa.2020.125732. [DOI] [Google Scholar]
- 19.Menezes TMF, Campelo MS, Lima ABN, Câmara Neto JF, Saraiva MM, Sousa JAC, Gonzaga MLC, Leal LKAM, Ribeiro MENP, Ricardo NMPS, Soares AS. Effects of polysaccharides isolated from mushrooms (Lentinus edodes Berk or Agaricus blazei Murill) on the gelation of Pluronic® F127. Colloids Surf A Physicochem Eng Asp. 2022;642:128684. doi: 10.1016/j.colsurfa.2022.128684. [DOI] [Google Scholar]
- 20.Mota LB, Campelo MS, Silva GA, Oliveira CLCG, Gramosa NV, Ricardo NMPS, Ribeiro MENP. Spectrophotometric method for quantification of eugenol in volatile oil of clove buds and nanoemulsion. Rev Bras Farmacogn. 2022;32:912–920. doi: 10.1007/s43450-022-00312-3. [DOI] [Google Scholar]
- 21.OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. Organisation for Economic Co-operation and Development. 2013. 10.1787/9789264203709-en.
- 22.Arellano-Aguilar O, Solis-Angeles S, Serrano L, Morales-Sierra E, Mendez-Serrano A, Montero-Montoya R. Use of the zebrafish embryo toxicity test for risk assessment purpose case study. Fish Sci. 2015;9:52–62. [Google Scholar]
- 23.Magalhães FEA, Sousa CAPB, Santos SAAR, Menezes RB, Batista FLA, Abreu AO, Oliveira MV, Moura LFWG, Raposo RS, Campos AR. Adult Zebrafish (Danio rerio): An Alternative Behavioral Model of Formalin-Induced Nociception. Zebrafish. 2017;14:422–429. doi: 10.1089/zeb.2017.1436. [DOI] [PubMed] [Google Scholar]
- 24.Benneh CK, Biney RP, Mante PK, Tandoh A, Adongo DW, Woode E. Maerua angolensis stem bark extract reverses anxiety and related behaviours in zebrafish - Involvement of GABAergic and 5-HT systems. J Ethnopharmacol. 2017;207:129–145. doi: 10.1016/j.jep.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Gebauer DL, Pagnussat N, Piato AL, Schaefer IC, Bonan CD, Lara DR. Effects of anxiolytics in zebrafish: Similarities and differences between benzodiazepines, buspirone and etanol. Pharmacol Biochem Behav. 2011;99:480–486. doi: 10.1016/j.pbb.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Das S, Singh VK, Dwivedy AK, Chaudhari AK, Deepika, Dubey NK. Eugenol loaded chitosan nanoemulsion for food protection and inhibition of Aflatoxin B1 synthesizing genes based on molecular docking. Carbohydr Polym. 2021;255:117339. doi: 10.1016/j.carbpol.2020.117339. [DOI] [PubMed] [Google Scholar]
- 27.Pramod K, Suneesh C, Shanavas S, Hussain S, Ali J. Unveiling the compatibility of eugenol with formulation excipients by systematic drug-excipient compatibility studies. J Anal Sci Technol. 2015;6:1–14. doi: 10.1186/s40543-015-0073-2. [DOI] [Google Scholar]
- 28.Zanetti M, Mazon LR, Meneses AC, Silva LL, Araújo PHH, Fiori MA, Oliveira D. Encapsulation of geranyl cinnamate in polycaprolactone nanoparticles. Mater Sci Eng C. 2019;97:198–207. doi: 10.1016/j.msec.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Coelho EL, Moura CL, Silva RF, Maia DS, Araújo TG, Ricardo NMPS, Ribeiro MENP, Ricardo NMPS. Binary systems of Brij® surfactants with Pluronic® F127 as griseofulvin carrier. Quim Nova. 2017;40:305–309. doi: 10.21577/0100-4042.20170003. [DOI] [Google Scholar]
- 30.Costa KM, Pereira TCB, Valente CA, Pissinate K, Soares JC, Cruz FF, Corte TWF, Machado P, Basso NRS, Bogo MR. Adverse effects of p-TSA-doped polypyrrole particulate exposure during zebrafish (Danio rerio) development. Colloids Surf B Biointerfaces. 2019;177:58–67. doi: 10.1016/j.colsurfb.2019.01.041. [DOI] [PubMed] [Google Scholar]
- 31.Öztürk Y, Aydin S, Beis R, Başer KHC, Berberoǧlu H. Effects of Hypericum perforatum L. and Hypericum calycinum L. extracts on the central nervous system in mice. Phytomedicine. 1996;3:139–46. doi: 10.1016/S0944-7113(96)80027-4. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira MKA, Silva AW, Moura ALS, Sales KVB, Marinho EM, Cardoso JNM, Marinho MM, Bandeira PN, Magalhães FEA, Marinho ES, Menezes JESA, Santos HS. Chalcones reverse the anxiety and convulsive behavior of adult zebrafish. Epilepsy Behav. 2021;117:107881. doi: 10.1016/j.yebeh.2021.107881. [DOI] [PubMed] [Google Scholar]
- 33.Aljawadi MH, Khoja AT, Alhammad AM, Al-Otaibi AD, Al-Shammari SA, Khoja TA. The prevalence of benzodiazepines utilization and its association with falls among Saudi older adults; results from the Saudi national survey for elderly Health (SNSEH) Saudi Pharm J. 2018;26:1112–1119. doi: 10.1016/j.jsps.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirghaed AT, Ghelichpour M, Hoseini SM. Myrcene and linalool as new anesthetic and sedative agents in common carp, Cyprinus carpio - Comparison with eugenol. Aquaculture. 2016;464:165–170. doi: 10.1016/j.aquaculture.2016.06.028. [DOI] [Google Scholar]
- 35.Ferreira AL, Silva IC, Favero GC, Melo NFS, Fraceto LF, Júnior JDC, Luz RK. Chitosan-coated zein nanoparticles containing eugenol potentiates anesthesia in Nile tilápia. Aquaculture. 2020;529:735659. doi: 10.1016/j.aquaculture.2020.735659. [DOI] [Google Scholar]
- 36.Silva DR, Arvigo AL, Giaquinto PC, Delicio HC, Barcellos LJG, Barreto RE. Effects of clove oil on behavioral reactivity and motivation in Nile tilápia. Aquaculture. 2021;532:736045. doi: 10.1016/j.aquaculture.2020.736045. [DOI] [Google Scholar]
- 37.Garlet QI, Rodrigues P, Barbosa LB, Londero AL, Mello CF, Heinzmann BM. Nectandra grandiflora essential oil and its isolated sesquiterpenoids minimize anxiety-related behaviors in mice through GABAergic mechanisms. Toxicol Appl Pharmacol. 2019;375:64–80. doi: 10.1016/j.taap.2019.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the relevant data are reported within the paper. For additional details, data are available on request to the authors.