Abstract
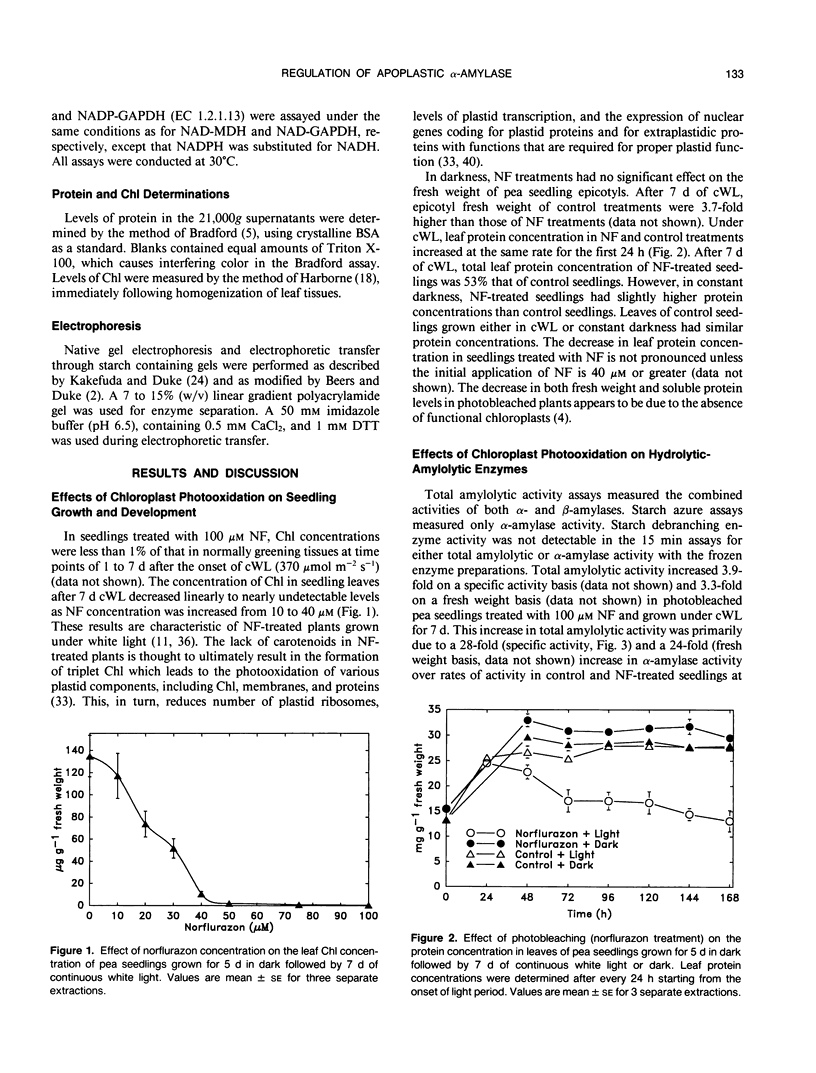
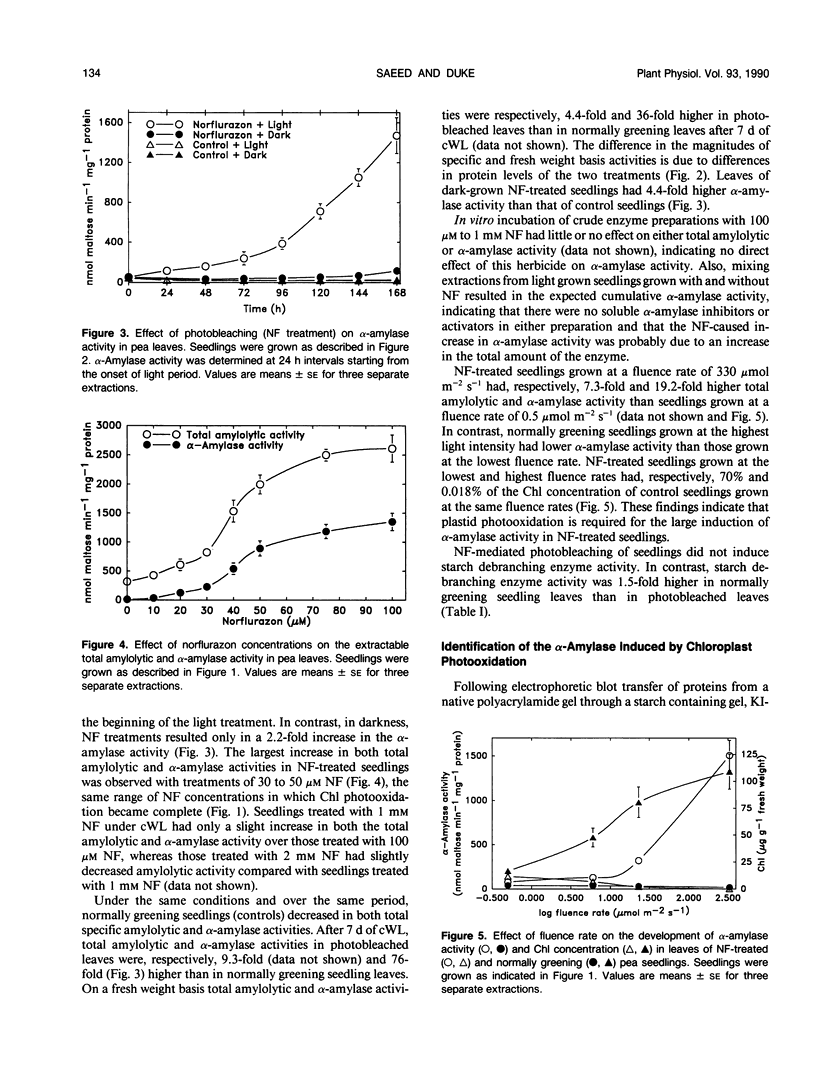
Photobleaching of pea (Pisum sativum L.) seedling leaves by treatment with norflurazon (San 9789) and 7 days of continuous white light caused a 76- to 85-fold increase in the activity of the primary α-amylase, a largely apoplastic enzyme, over normally greening seedlings. Levels of chlorophyll were near zero and levels of plastid marker enzyme activities were very low in norflurazon-treated seedlings, indicating severe photooxidative damage to plastids. As levels of norflurazon or fluence rates were lowered, decreasing photobleaching of tissues, α-amylase activity decreased. Levels of leaf β-amylase and starch debranching enzyme changed very little in norflurazon-treated seedlings. Infiltration extraction of leaves of norflurazon-treated and normally greening seedlings indicated that at least 57 and 62%, respectively, of α-amylase activity was in the apoplast. α-Amylase activity recovered from the apoplast of photobleached leaves of norflurazon-treated seedlings was 18-fold higher than that for green leaves. Inhibitors of photosynthesis (DCMU and atrazine) and an inhibitor of chlorophyll accumulation that does not cause photooxidation of plastid components (tentoxin) had little effect on levels of α-amylase activity, indicating norflurazon-caused loss of chlorophyll and lack of photosynthesis did not cause the large induction in α-amylase activity. An inhibitor of both abscisic acid and gibberellin synthesis (paclobutrazol [PP333]) and an analog of norflurazon which inhibits photosynthesis but not carotenoid synthesis (San 9785) caused only moderate (about five-fold) increases in α-amylase activity. Lincomycin and chloramphenicol increased α-amylase activity in light grown seedings to the same magnitude as norflurazon, indicating that the effect of norflurazon is probably through the destruction of plastid ribosomes. It is proposed that chloroplasts produce a negative signal for the regulation of the apoplastic α-amylase in pea.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batschauer A., Mösinger E., Kreuz K., Dörr I., Apel K. The implication of a plastid-derived factor in the transcriptional control of nuclear genes encoding the light-harvesting chlorophyll a/b protein. Eur J Biochem. 1986 Feb 3;154(3):625–634. doi: 10.1111/j.1432-1033.1986.tb09444.x. [DOI] [PubMed] [Google Scholar]
- Beers E. P., Duke S. H. Characterization of alpha-Amylase from Shoots and Cotyledons of Pea (Pisum sativum L.) Seedlings. Plant Physiol. 1990 Apr;92(4):1154–1163. doi: 10.1104/pp.92.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers E. P., Duke S. H. Localization of alpha-Amylase in the Apoplast of Pea (Pisum sativum L.) Stems. Plant Physiol. 1988 Aug;87(4):799–802. doi: 10.1104/pp.87.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume D. E., McClure J. W. Developmental Effects of Sandoz 6706 on Activities of Enzymes of Phenolic and General Metabolism in Barley Shoots Grown in the Dark or under Low or High Intensity Light. Plant Physiol. 1980 Feb;65(2):238–244. doi: 10.1104/pp.65.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Callis J., Ho T. H. Multiple molecular forms of the gibberellin-induced alpha-amylase from the aleurone layers of barley seeds. Arch Biochem Biophys. 1983 Jul 1;224(1):224–234. doi: 10.1016/0003-9861(83)90206-0. [DOI] [PubMed] [Google Scholar]
- Doehlert D. C., Duke S. H. Specific Determination of alpha-Amylase Activity in Crude Plant Extracts Containing beta-Amylase. Plant Physiol. 1983 Feb;71(2):229–234. doi: 10.1104/pp.71.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierabend J., Schubert B. Comparative Investigation of the Action of Several Chlorosis-inducing Herbicides on the Biogenesis of Chloroplasts and Leaf Microbodies. Plant Physiol. 1978 Jun;61(6):1017–1022. doi: 10.1104/pp.61.6.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloin J. M., Hagedorn D. J. Effects of tentoxin on enzymic activities in cucumber and cabbage cotyledons. Mycopathologia. 1975 Jun 14;55(3):159–162. doi: 10.1007/BF00491501. [DOI] [PubMed] [Google Scholar]
- Jabben M., Deitzer G. F. Effects of the herbicide san 9789 on photomorphogenic responses. Plant Physiol. 1979 Mar;63(3):481–485. doi: 10.1104/pp.63.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J. V., Hanson A. D., Chandler P. C. Water stress enhances expression of an alpha-amylase gene in barley leaves. Plant Physiol. 1986 Feb;80(2):350–359. doi: 10.1104/pp.80.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakefuda G., Duke S. H., Duke S. O. Differential light induction of nitrate reductases in greening and photobleached soybean seedlings. Plant Physiol. 1983 Sep;73(1):56–60. doi: 10.1104/pp.73.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakefuda G., Duke S. H. Electrophoretic transfer as a technique for the detection and identification of plant amylolytic enzymes in polyacrylamide gels. Plant Physiol. 1984 May;75(1):278–280. doi: 10.1104/pp.75.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. P., Spilatro S. R., Preiss J. Subcellular localization and characterization of amylases in Arabidopsis leaf. Plant Physiol. 1988 Jan;86(1):251–259. doi: 10.1104/pp.86.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizotte P. A., Henson C. A., Duke S. H. Purification and Characterization of Pea Epicotyl beta-Amylase. Plant Physiol. 1990 Mar;92(3):615–621. doi: 10.1104/pp.92.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield S. P., Nelson T., Taylor W. C. The Fate of Chloroplast Proteins during Photooxidation in Carotenoid-Deficient Maize Leaves. Plant Physiol. 1986 Nov;82(3):760–764. doi: 10.1104/pp.82.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield S. P., Taylor W. C. Carotenoid-deficient maize seedlings fail to accumulate light-harvesting chlorophyll a/b binding protein (LHCP) mRNA. Eur J Biochem. 1984 Oct 1;144(1):79–84. doi: 10.1111/j.1432-1033.1984.tb08433.x. [DOI] [PubMed] [Google Scholar]
- Sagar A. D., Horwitz B. A., Elliott R. C., Thompson W. F., Briggs W. R. Light effects on several chloroplast components in norflurazon-treated pea seedlings. Plant Physiol. 1988 Oct;88(2):340–347. doi: 10.1104/pp.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain R. R., Dekker E. E. Seed germination studies. II. Pathways for starch degradation in germinating pea seedlings. Biochim Biophys Acta. 1966 Jul 6;122(1):87–100. doi: 10.1016/0926-6593(66)90093-2. [DOI] [PubMed] [Google Scholar]
- Wang S. Y., Sun T., Ji Z. L., Faust M. Effect of paclobutrazol on water stress-induced abscisic Acid in apple seedling leaves. Plant Physiol. 1987 Aug;84(4):1051–1054. doi: 10.1104/pp.84.4.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler P., Beck E. Exoamylase activity in vacuoles isolated from pea and wheat leaf protoplasts. Plant Physiol. 1986 Dec;82(4):1119–1121. doi: 10.1104/pp.82.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]