Abstract
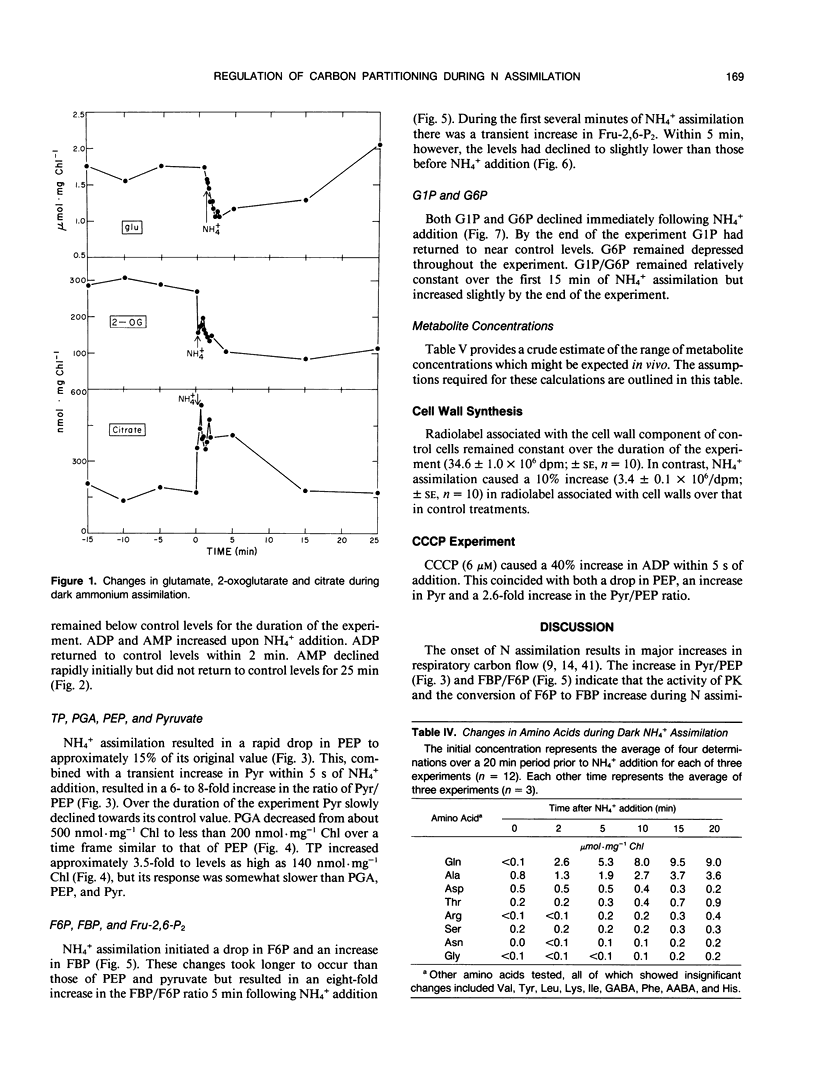
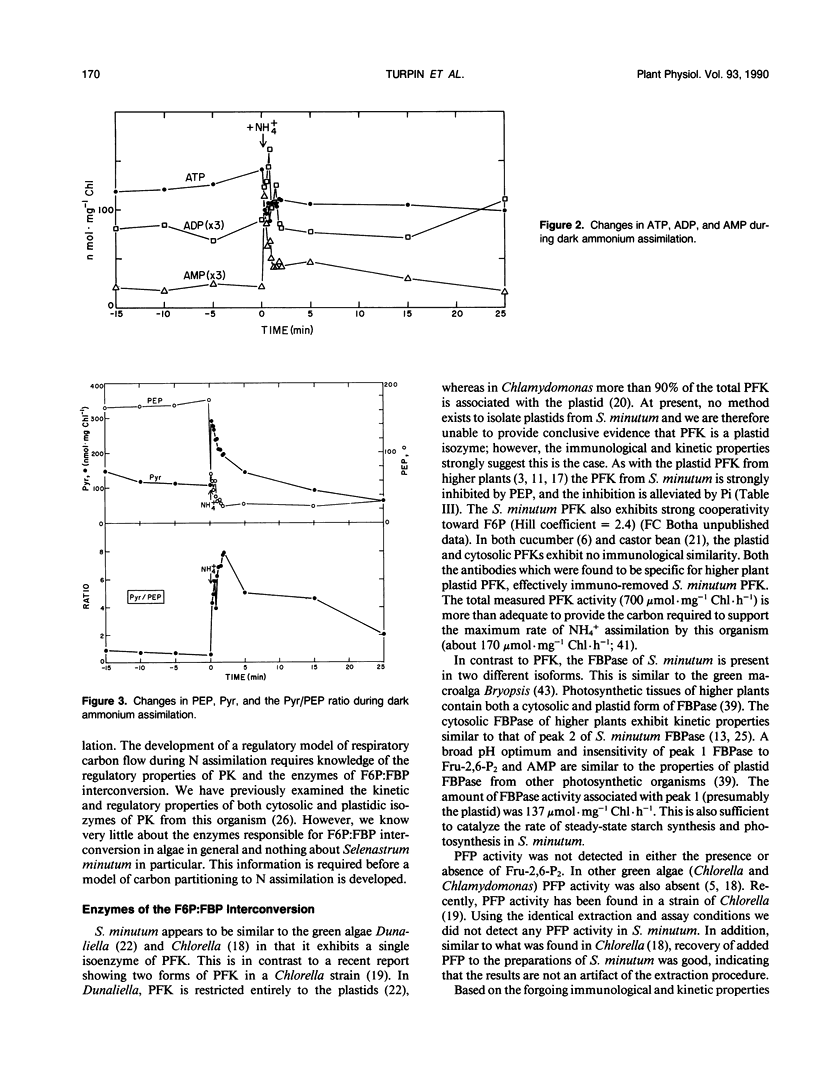
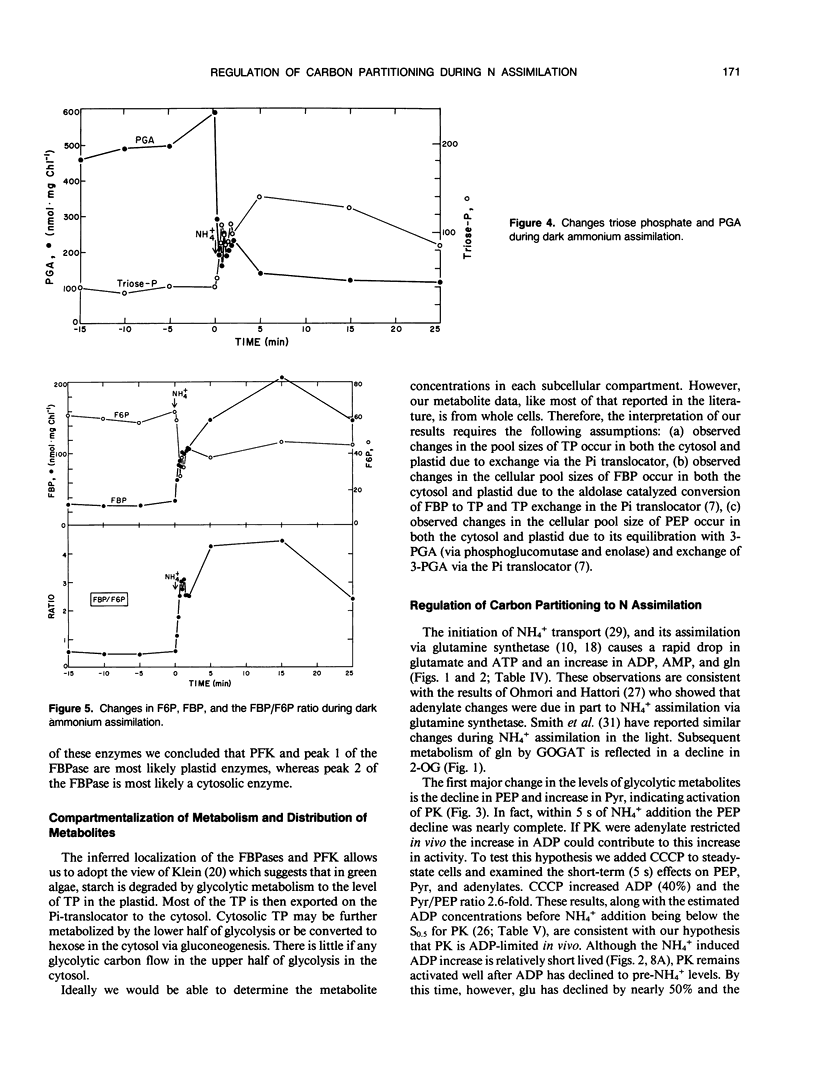
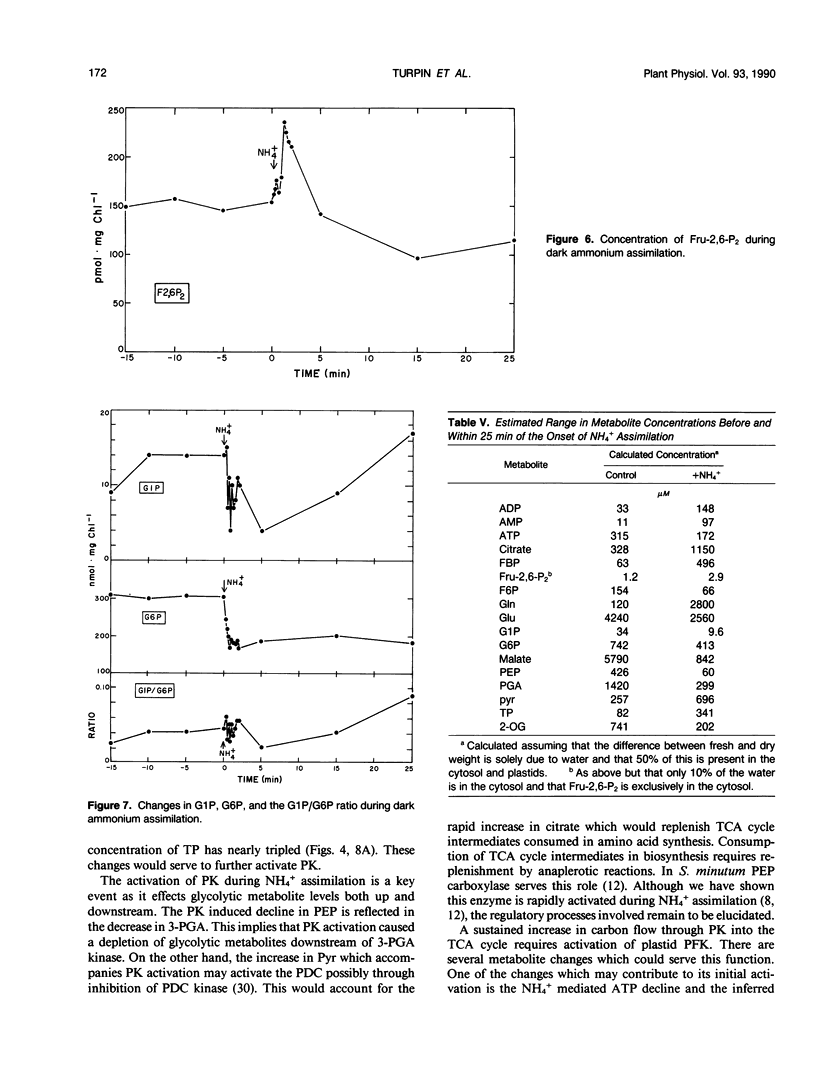
The assimilation of NH4+ causes a rapid increase in respiration to provided carbon skeletons for amino acid synthesis. In this study we propose a model for the regulation of carbon partitioning from starch to respiration and N assimilation in the green alga Selenastrum minutum. We provide evidence for both a cytosolic and plastidic fructose-1,6-bisphosphatase. The cytosolic form is inhibited by AMP and fructose-1,6-bisphosphate and the plastidic form is inhibited by phosphate. There is only one ATP dependent phosphofructokinase which, based on immunological cross reactivity, has been identified as being localized in the plastid. It is inhibited by phosphoenolpyruvate and activated by phosphate. No pyrophosphate dependent phosphofructokinase was found. The initiation of dark ammonium assimilation resulted in a transient increase in ADP which releases pyruvate kinase from adenylate control. This activation of pyruvate kinase causes a rapid 80% drop in phosphoenolpyruvate and a 2.7-fold increase in pyruvate. The pyruvate kinase mediated decrease in phosphoenolpyruvate correlates with the activation of the ATP dependent phosphofructokinase increasing carbon flow through the upper half of glycolysis. This increased the concentration of triosephosphate and provided substrate for pyruvate kinase. It is suggested that this increase in triosephosphate coupled with the glutamine synthetase mediated decline in glutamate, serves to maintain pyruvate kinase activation once ADP levels recover. The initiation of NH4+ assimilation causes a transient 60% increase in fructose-2,6-bisphosphate. Given the sensitivity of the cytosolic fructose-1,6-bisphosphatase to this regulator, its increase would serve to inhibit cytosolic gluconeogenesis and direct the triosephosphate exported from the plastid down glycolysis to amino acid biosynthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carnal N. W., Black C. C. Phosphofructokinase activities in photosynthetic organisms : the occurrence of pyrophosphate-dependent 6-phosphofructokinase in plants and algae. Plant Physiol. 1983 Jan;71(1):150–155. doi: 10.1104/pp.71.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrifi I. R., Turpin D. H. Nitrate and Ammonium Induced Photosynthetic Suppression in N-Limited Selenastrum minutum. Plant Physiol. 1986 May;81(1):273–279. doi: 10.1104/pp.81.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrifi I. R., Turpin D. H. The Path of Carbon Flow during NO(3)-Induced Photosynthetic Suppression in N-Limited Selenastrum minutum. Plant Physiol. 1987 Jan;83(1):97–104. doi: 10.1104/pp.83.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer P., Klein U. Localization of Nitrogen-Assimilating Enzymes in the Chloroplast of Chlamydomonas reinhardtii. Plant Physiol. 1988 Nov;88(3):947–952. doi: 10.1104/pp.88.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland W. J., Dennis D. T. Plastid and cytosolic phosphofructokinases from the developing endosperm of Ricinus communis. II. Comparison of the kinetic and regulatory properties of the isoenzymes. Arch Biochem Biophys. 1980 Oct 1;204(1):310–317. doi: 10.1016/0003-9861(80)90038-7. [DOI] [PubMed] [Google Scholar]
- Guy R. D., Vanlerberghe G. C., Turpin D. H. Significance of Phosphoenolpyruvate Carboxylase during Ammonium Assimilation: Carbon Isotope Discrimination in Photosynthesis and Respiration by the N-Limited Green Alga Selenastrum minutum. Plant Physiol. 1989 Apr;89(4):1150–1157. doi: 10.1104/pp.89.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog B., Stitt M., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : III. Properties of the Cytosolic Fructose 1,6-Bisphosphatase. Plant Physiol. 1984 Jul;75(3):561–565. doi: 10.1104/pp.75.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa T., Kanazawa K., Kirk M. R., Bassham J. A. Regulatory effects of ammonia on carbon metabolism in Chlorella pyrenoidosa during photosynthesis and respiration. Biochim Biophys Acta. 1972 Mar 16;256(3):656–669. doi: 10.1016/0005-2728(72)90201-0. [DOI] [PubMed] [Google Scholar]
- Kanazawa T., Kirk M. R., Bassham J. A. Regulatory effects of ammonia on carbon metabolism in photosynthesizing Chlorella pyrenoidosa. Biochim Biophys Acta. 1970 Jun 30;205(3):401–408. doi: 10.1016/0005-2728(70)90106-4. [DOI] [PubMed] [Google Scholar]
- Kelly G. J., Latzko E. Chloroplast Phosphofructokinase: II. Partial Purification, Kinetic and Regulatory Properties. Plant Physiol. 1977 Aug;60(2):295–299. doi: 10.1104/pp.60.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink E., Kruger N. J., Beevers H. Kinetic properties of pyrophosphate:fructose-6-phosphate phosphotransferase from germinating castor bean endosperm. Plant Physiol. 1984 Feb;74(2):395–401. doi: 10.1104/pp.74.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink E., Wöber G. Chloroplast phosphofructokinase in the green alga, Dunaliella marina: partial purification and kinetic and regulatory properties. Arch Biochem Biophys. 1982 Feb;213(2):602–619. doi: 10.1016/0003-9861(82)90590-2. [DOI] [PubMed] [Google Scholar]
- Kruger N. J., Beevers H. Effect of fructose 2,6-bisphosphate on the kinetic properties of cytoplasmic fructose 1,6-bisphosphatase from germinating castor bean endosperm. Plant Physiol. 1984 Sep;76(1):49–54. doi: 10.1104/pp.76.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger N. J., Hammond J. B., Burrell M. M. Molecular characterization of four forms of phosphofructokinase purified from potato tuber. Arch Biochem Biophys. 1988 Dec;267(2):690–700. doi: 10.1016/0003-9861(88)90078-1. [DOI] [PubMed] [Google Scholar]
- Ohmori M., Hattori A. Transient change in the ATP pool of Anabaena cylindrica associated with ammonia assimilation. Arch Microbiol. 1978 Apr 27;117(1):17–20. doi: 10.1007/BF00689345. [DOI] [PubMed] [Google Scholar]
- Schuller K. A., Randall D. D. Regulation of pea mitochondrial pyruvate dehydrogenase complex : does photorespiratory ammonium influence mitochondrial carbon metabolism? Plant Physiol. 1989 Apr;89(4):1207–1212. doi: 10.1104/pp.89.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weger H. G., Turpin D. H. Mitochondrial Respiration Can Support NO(3) and NO(2) Reduction during Photosynthesis : Interactions between Photosynthesis, Respiration, and N Assimilation in the N-Limited Green Alga Selenastrum minutum. Plant Physiol. 1989 Feb;89(2):409–415. doi: 10.1104/pp.89.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz W., Stitt M., Heldt H. W. Enzymic determination of metabolites in the subcellular compartments of spinach protoplasts. Plant Physiol. 1980 Jul;66(1):187–193. doi: 10.1104/pp.66.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X. H., Kwiatkowska D., Kemp R. G. Inorganic pyrophosphate: fructose-6-phosphate 1-phosphotransferase of the potato tuber is related to the major ATP-dependent phosphofructokinase of E. coli. Biochem Biophys Res Commun. 1988 Jul 15;154(1):113–117. doi: 10.1016/0006-291x(88)90657-2. [DOI] [PubMed] [Google Scholar]


