Abstract
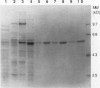
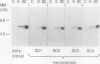
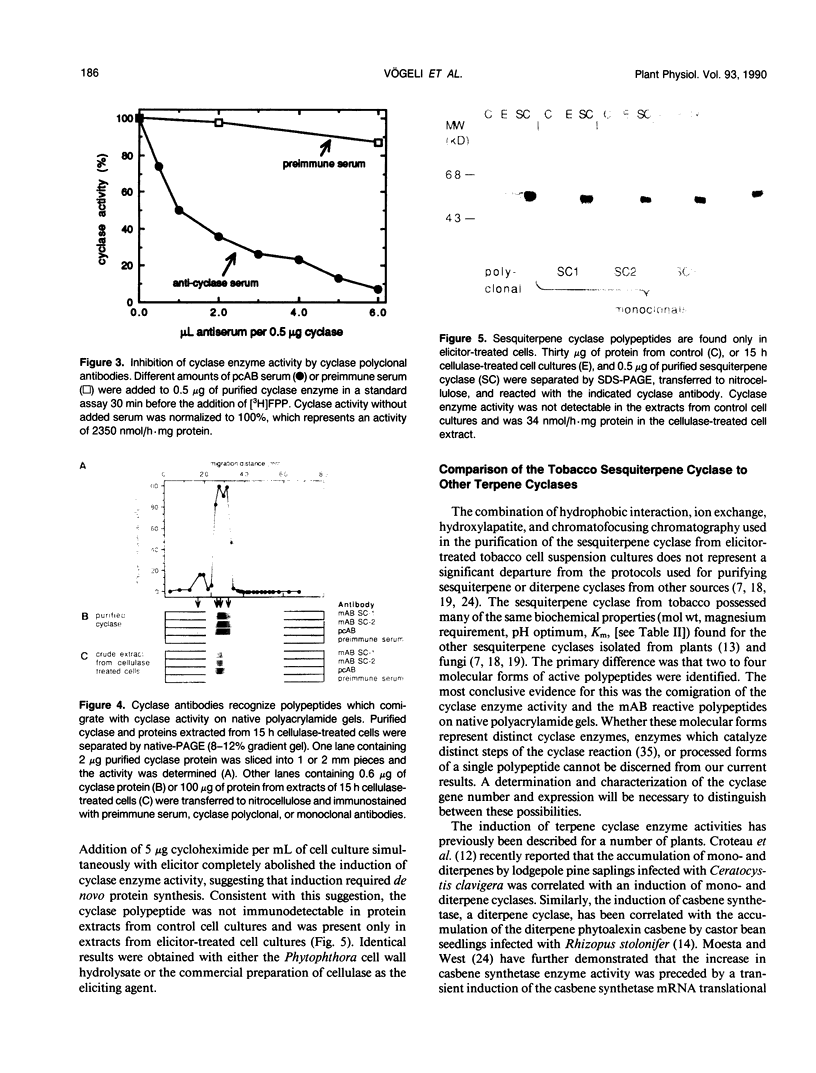
An elicitor-inducible sesquiterpene cyclase, which catalyzes the conversion of farnesyl diphosphate to 5-epi-aristolochene (IM Whitehead, DR Threlfall, DF Ewing [1989] Phytochemistry 28:775-779) and representing a committed step in the phytoalexin biosynthetic pathway in tobacco, was purified by a combination of hydrophobic interaction, anion exchange, hydroxylapatite, and chromatofocusing chromatography. From 2 kilograms of elicited tobacco (Nicotiana tabacum) cells, approximately 500 micrograms of cyclase protein was purified, representing greater than a 130-fold increase in the specific activity of the enzyme and a 4% recovery of the starting activity. The purified enzyme resolved as two major polypeptides of 60 and 62 kilodaltons by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Biochemical characterization of the enzyme activity included an absolute requirement for magnesium, an isoelectric point of 4.5 to 4.9, and a Km for farnesyl diphosphate of 2 to 5 micromolar. The purified cyclase protein was used to generate mouse polyclonal antibodies which efficiently inhibited cyclase activity in an in vitro assay. Electrophoresis of extracts from elicitor-treated cells or purified cyclase enzyme on native polyacrylamide gels separated the cyclase enzyme into four polypeptides as shown by immunoblot analysis using poly- and monoclonal antibodies. Proportionate cyclase enzyme activity comigrated with those polypeptides. No cyclase polypeptides were detectable in extracts of control cells by immunoblot analysis. However, immunoblot analysis of proteins from elicitor-treated cells using four independent monoclonal antibody lines and the polyclonal antibodies detected the same polypeptides, regardless of whether the proteins were separated by native or SDS-PAGE. The results suggest an induction of multiple cyclase polypeptides in elicitor-treated cells resulting from either the expression of multiple genes or multiple post-translational processing events.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers A. R., Ebel J., Valent B., Albersheim P. Host-Pathogen Interactions: X. Fractionation and Biological Activity of an Elicitor Isolated from the Mycelial Walls of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):760–765. doi: 10.1104/pp.57.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cane D. E., Pargellis C. Partial purification and characterization of pentalenene synthase. Arch Biochem Biophys. 1987 May 1;254(2):421–429. doi: 10.1016/0003-9861(87)90120-2. [DOI] [PubMed] [Google Scholar]
- Chappell J., Nable R. Induction of sesquiterpenoid biosynthesis in tobacco cell suspension cultures by fungal elicitor. Plant Physiol. 1987 Oct;85(2):469–473. doi: 10.1104/pp.85.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R., Gundy A. Cyclization of farnesyl pyrophosphate to the sesquiterpene olefins humulene and caryophyllene by an enzyme system from sage (Salvia officinalis). Arch Biochem Biophys. 1984 Sep;233(2):838–841. doi: 10.1016/0003-9861(84)90513-7. [DOI] [PubMed] [Google Scholar]
- Croteau R., Gurkewitz S., Johnson M. A., Fisk H. J. Biochemistry of Oleoresinosis : Monoterpene and Diterpene Biosynthesis in Lodgepole Pine Saplings Infected with Ceratocystis clavigera or Treated with Carbohydrate Elicitors. Plant Physiol. 1987 Dec;85(4):1123–1128. doi: 10.1104/pp.85.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R., Munck S. L., Akoh C. C., Fisk H. J., Satterwhite D. M. Biosynthesis of the sesquiterpene patchoulol from farnesyl pyrophosphate in leaf extracts of Pogostemon cablin (patchouli): mechanistic considerations. Arch Biochem Biophys. 1987 Jul;256(1):56–68. doi: 10.1016/0003-9861(87)90425-5. [DOI] [PubMed] [Google Scholar]
- Dehal S. S., Croteau R. Partial purification and characterization of two sesquiterpene cyclases from sage (Salvia officinalis) which catalyze the respective conversion of farnesyl pyrophosphate to humulene and caryophyllene. Arch Biochem Biophys. 1988 Mar;261(2):346–356. doi: 10.1016/0003-9861(88)90350-5. [DOI] [PubMed] [Google Scholar]
- Dueber M. T., Adolf W., West C. A. Biosynthesis of the Diterpene Phytoalexin Casbene: Partial Purification and Characterization of Casbene Synthetase from Ricinis communis. Plant Physiol. 1978 Oct;62(4):598–603. doi: 10.1104/pp.62.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J. W., Chatterjee A., Ross B. E., Busch H. Epitope distribution and immunochemical characterization of nucleolar phosphoprotein C23 using ten monoclonal antibodies. Mol Cell Biochem. 1985 Sep;68(1):87–96. doi: 10.1007/BF00219392. [DOI] [PubMed] [Google Scholar]
- Hohn T. M., Plattner R. D. Purification and characterization of the sesquiterpene cyclase aristolochene synthase from Penicillium roqueforti. Arch Biochem Biophys. 1989 Jul;272(1):137–143. doi: 10.1016/0003-9861(89)90204-x. [DOI] [PubMed] [Google Scholar]
- Hohn T. M., Vanmiddlesworth F. Purification and characterization of the sesquiterpene cyclase trichodiene synthetase from Fusarium sporotrichioides. Arch Biochem Biophys. 1986 Dec;251(2):756–761. doi: 10.1016/0003-9861(86)90386-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moesta P., West C. A. Casbene synthetase: regulation of phytoalexin biosynthesis in Ricinus communis L. seedlings. Purification of casbene synthetase and regulation of its biosynthesis during elicitation. Arch Biochem Biophys. 1985 Apr;238(1):325–333. doi: 10.1016/0003-9861(85)90171-7. [DOI] [PubMed] [Google Scholar]
- Stermer B. A., Bostock R. M. Involvement of 3-hydroxy-3-methylglutaryl coenzyme a reductase in the regulation of sesquiterpenoid phytoalexin synthesis in potato. Plant Physiol. 1987 Jun;84(2):404–408. doi: 10.1104/pp.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- Vögeli U., Chappell J. Induction of sesquiterpene cyclase and suppression of squalene synthetase activities in plant cell cultures treated with fungal elicitor. Plant Physiol. 1988 Dec;88(4):1291–1296. doi: 10.1104/pp.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]