Abstract
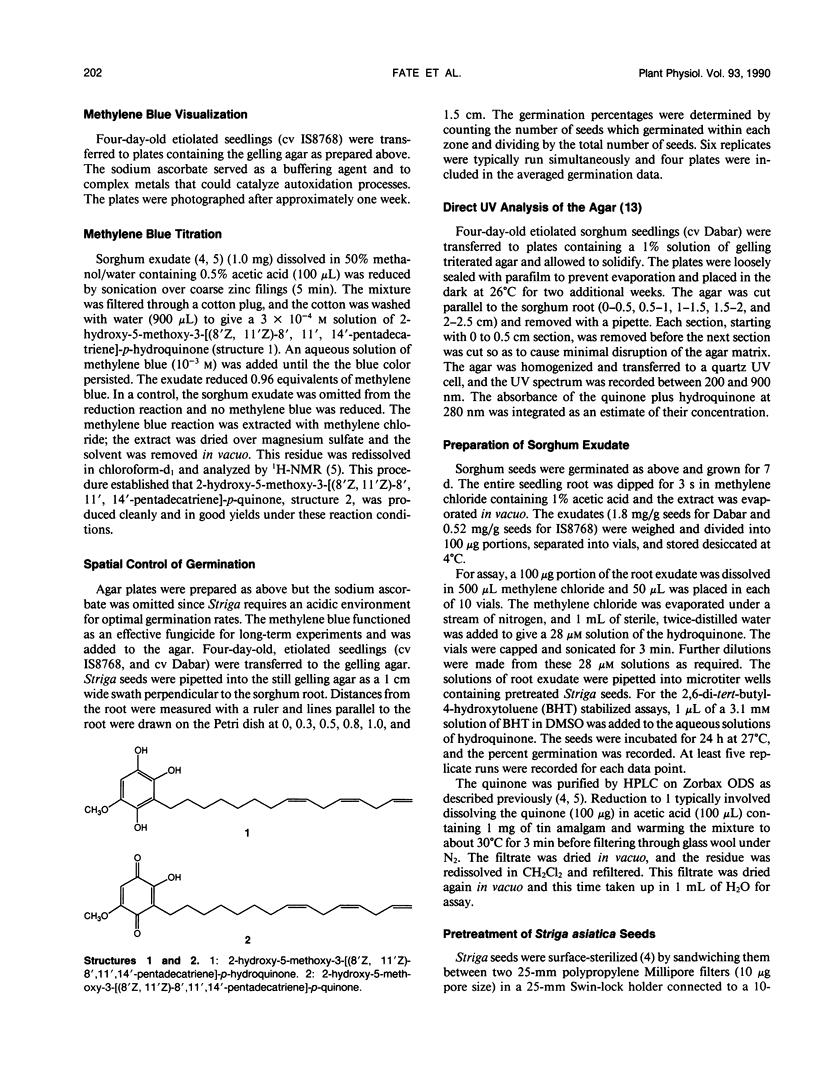
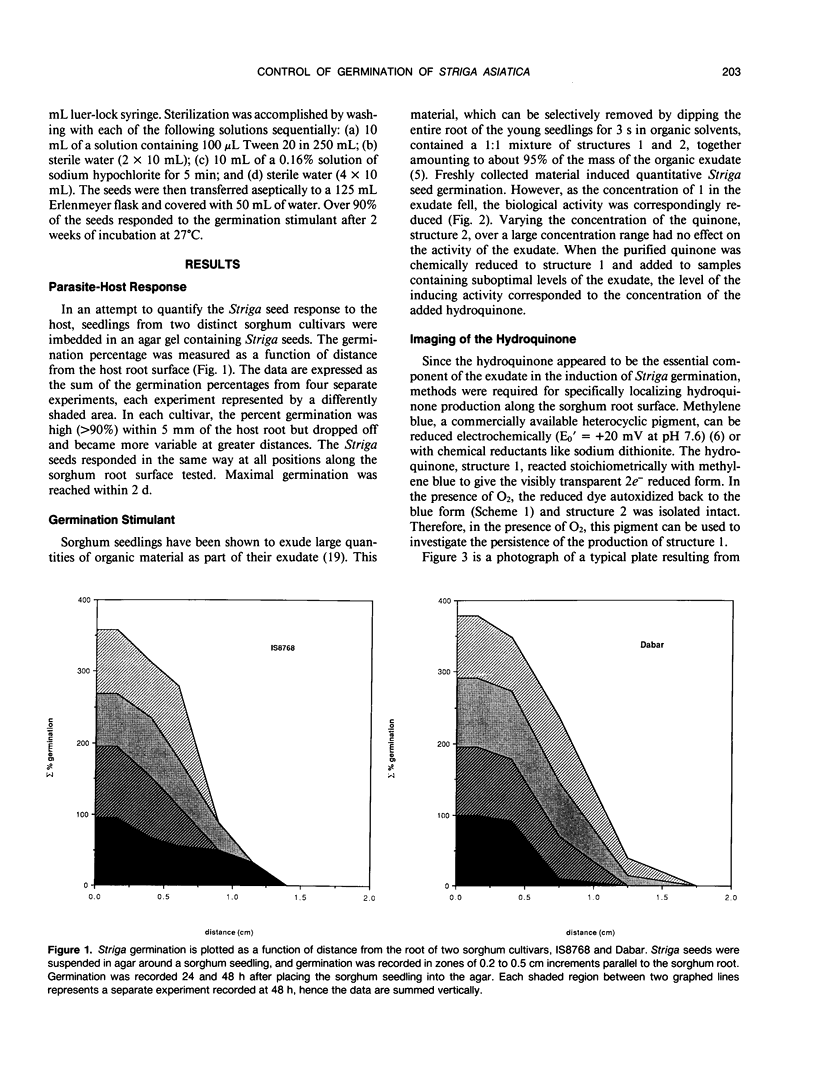
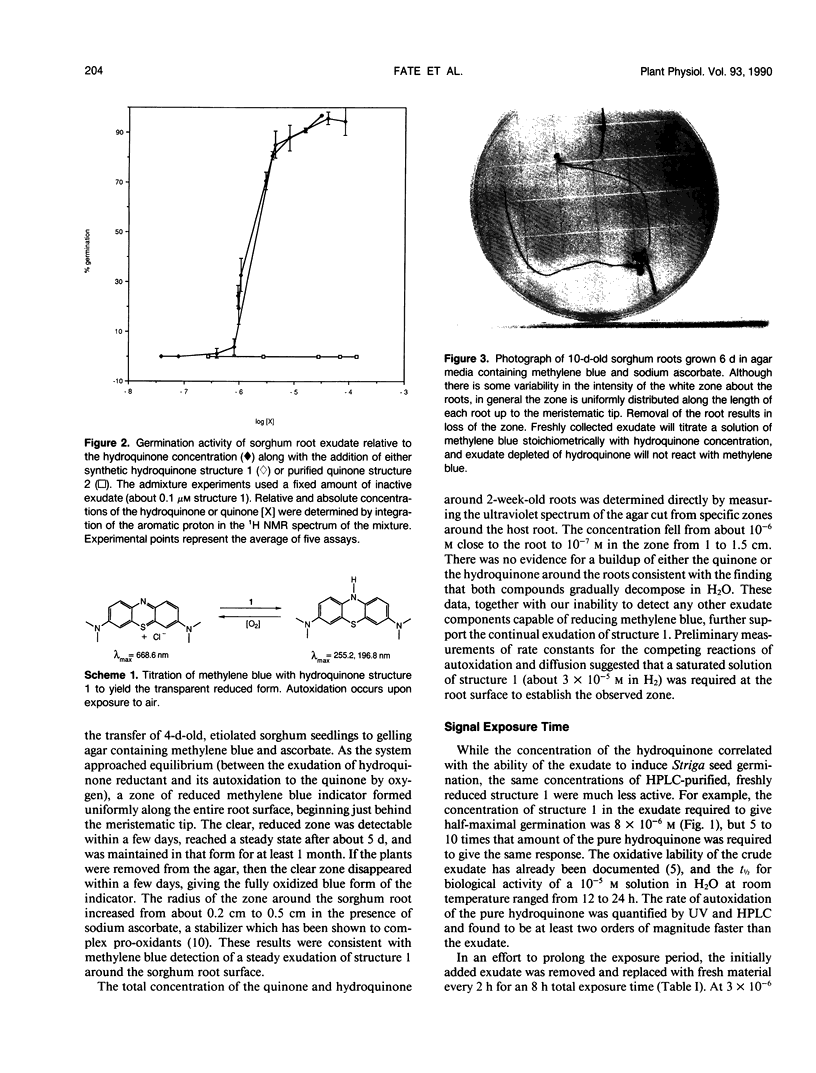
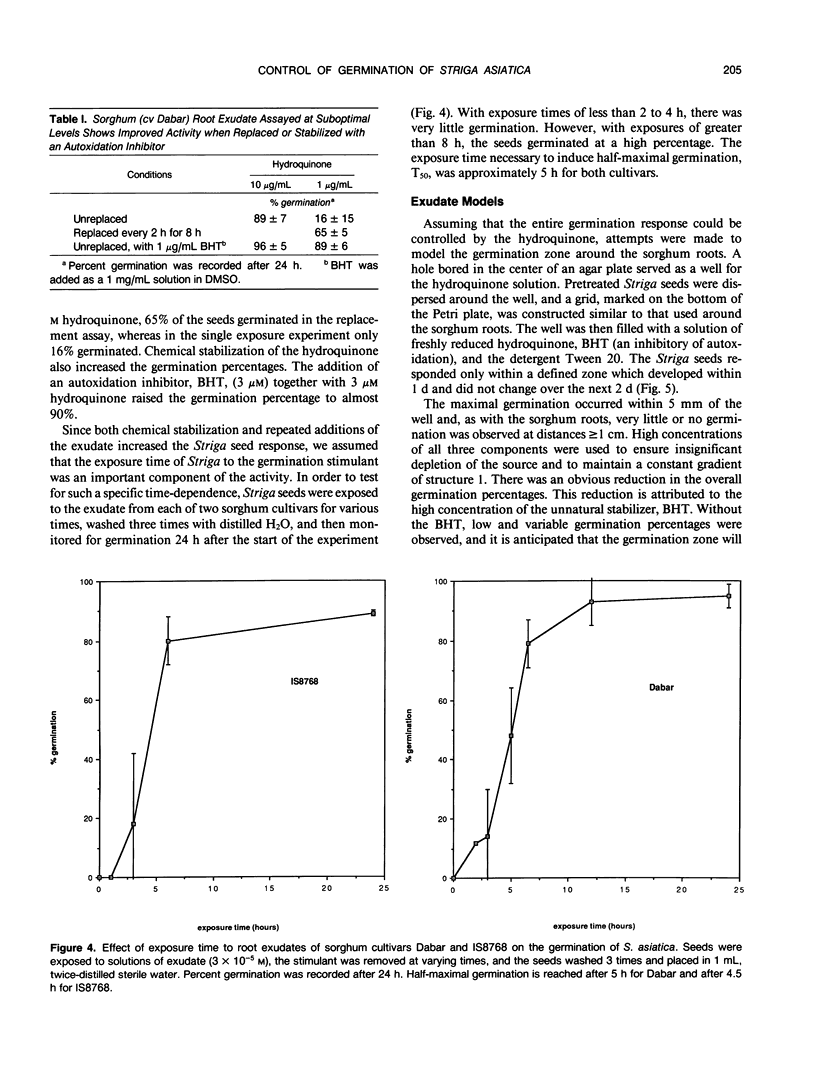
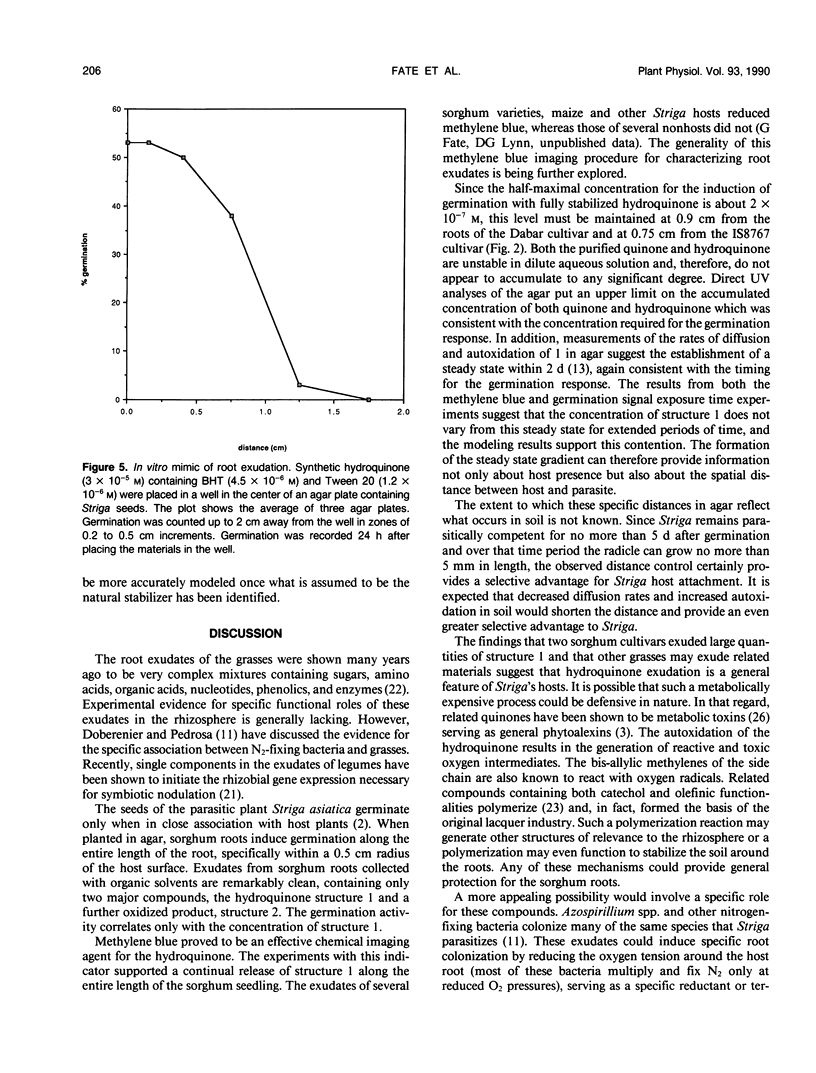
Striga asiatica (Scrophulariaceae), a member of a heterogeneous group known as the parasitic plants, is totally dependent on host root attachment for survival. In agar, Striga seeds germinated in high percentages within 5 millimeters of a sorghum (Sorghum bicolor (L.) Moench) host root surface, and no germination was observed at distances greater than 1 centimeter. This spatially restricted germination may be explained by the chemistry of a single compound, 2-hydroxy-5-methoxy-3-[8′Z, 11′Z)-8′, 11′, 14′ -pentadecatriene]-p-hydroquinone, structure 1, which is exuded by sorghum roots. The presence of the compound was chemically imaged with pigments such as methylene blue. The use of methylene blue suggested that structure 1 was exuded along the entire surface of the root for long periods. This exudation and the inherent instability of structure 1 together establish an apparent steady state concentration gradient of the germination stimulant around the sorghum root. The Striga seed must be exposed to micromolar concentrations of 1 for ≥5 hours before high germination percentages were observed. Such a requirement for a long term exposure to a steady state concentration of an inherently labile, exuded compound would provide an extra degree of resolution to signal detection and host commitment in Striga parasitism.
Full text
PDF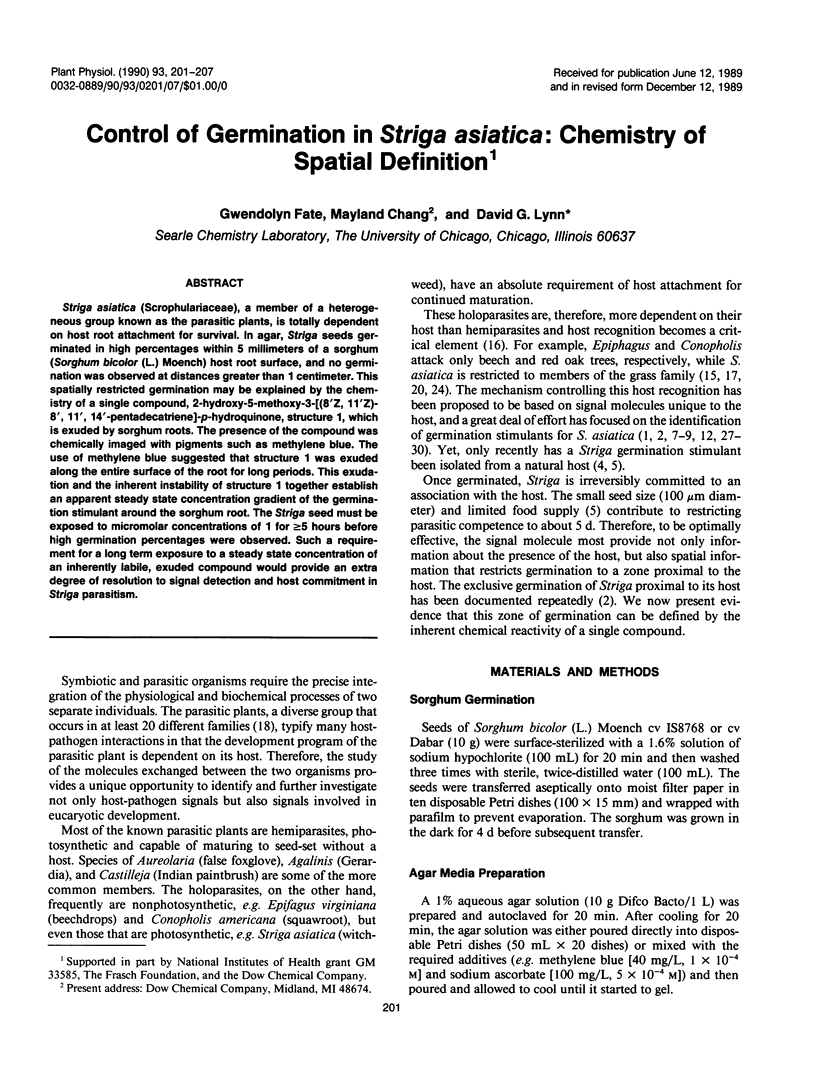

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cook C. E., Whichard L. P., Turner B., Wall M. E., Egley G. H. Germination of Witchweed (Striga lutea Lour.): Isolation and Properties of a Potent Stimulant. Science. 1966 Dec 2;154(3753):1189–1190. doi: 10.1126/science.154.3753.1189. [DOI] [PubMed] [Google Scholar]
- Fossard C., Dale G., Latner A. L. Separation of the proteins of cerebrospinal fluid using gel electrofocusing followed by electrophoresis. J Clin Pathol. 1970 Oct;23(7):586–589. doi: 10.1136/jcp.23.7.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierer A., Meinhardt H. A theory of biological pattern formation. Kybernetik. 1972 Dec;12(1):30–39. doi: 10.1007/BF00289234. [DOI] [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969 Oct;25(1):1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- Wong S. M., Pezzuto J. M., Fong H. H., Farnsworth N. R. Isolation, structural elucidation, and chemical synthesis of 2-hydroxy-3-octadecyl-5-methoxy-1,4-benzoquinone (irisoquin), a cytotoxic constituent of Iris missouriensis. J Pharm Sci. 1985 Oct;74(10):1114–1116. doi: 10.1002/jps.2600741023. [DOI] [PubMed] [Google Scholar]
- Worsham A. D., Moreland D. E., Klingman G. C. Stimulation of Striga asiatica (Witchweed) Seed Germination by 6-Substituted Purines. Science. 1959 Dec 11;130(3389):1654–1656. doi: 10.1126/science.130.3389.1654. [DOI] [PubMed] [Google Scholar]




