Abstract
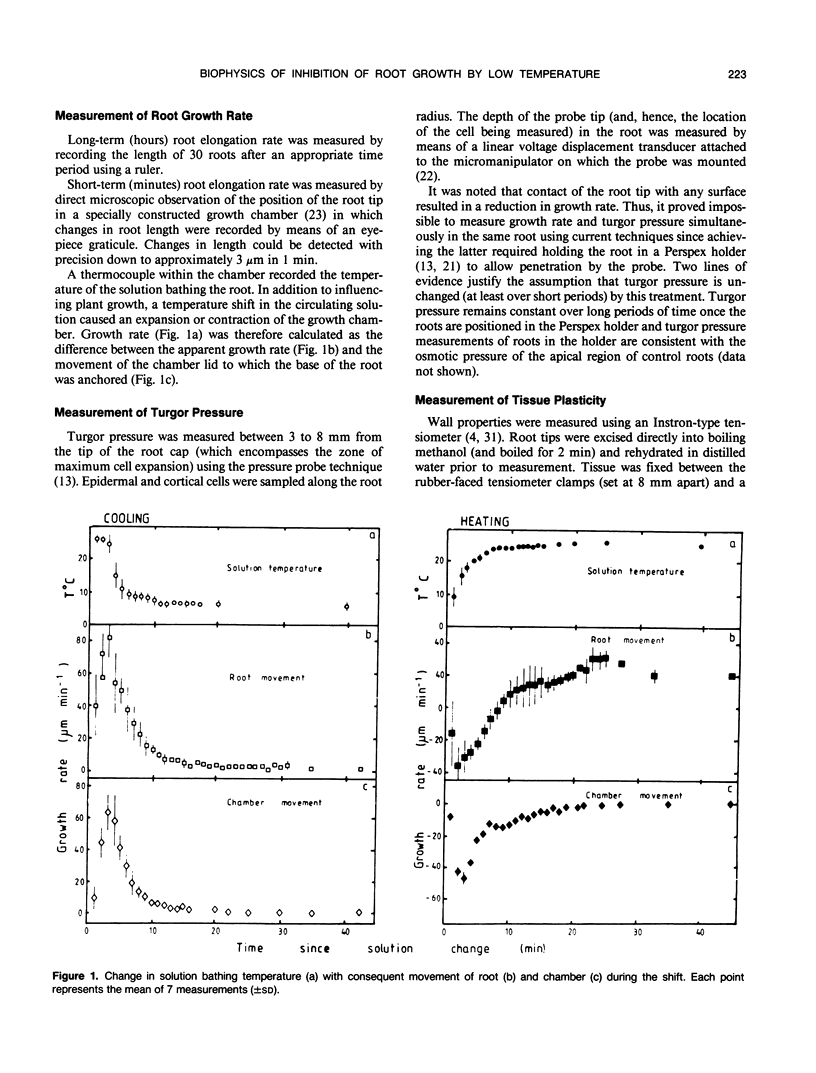
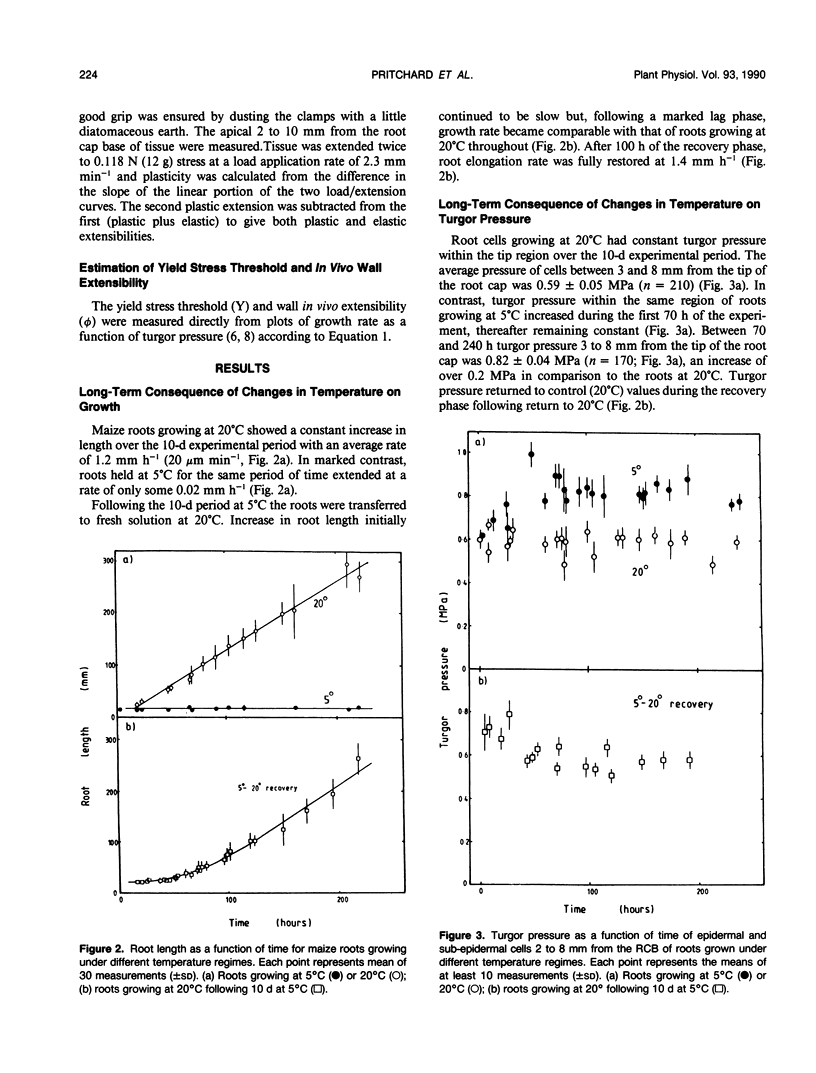
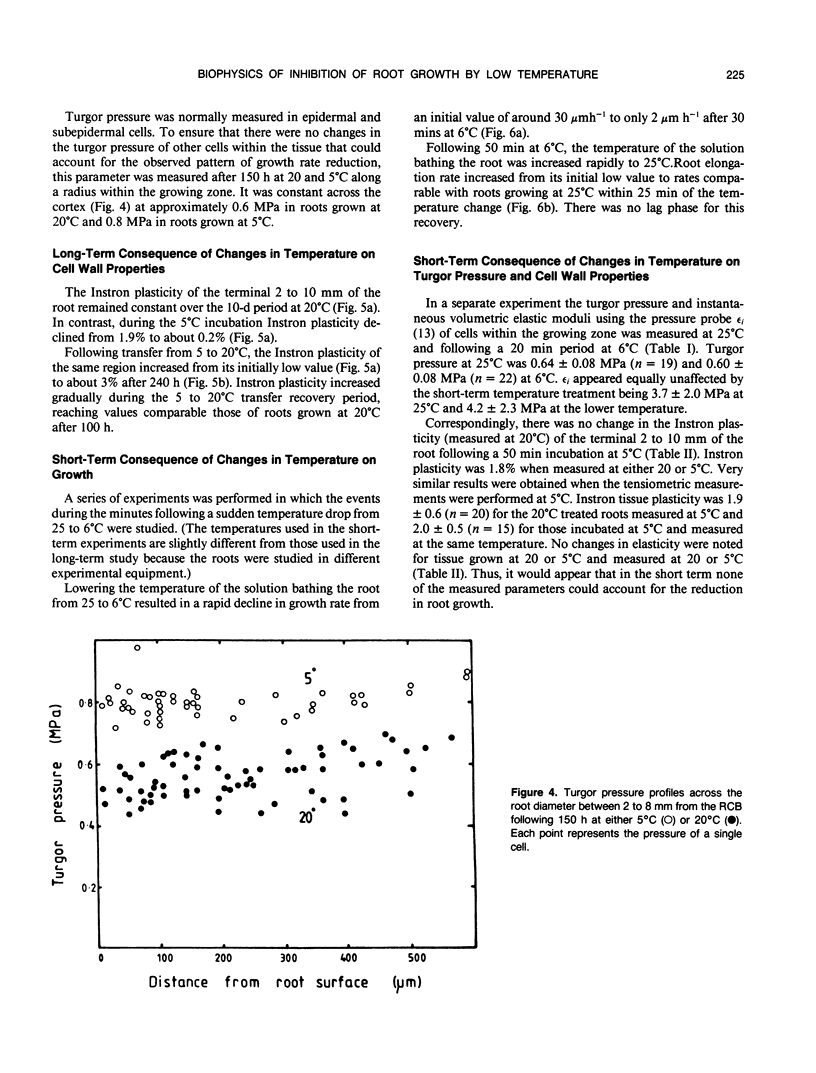
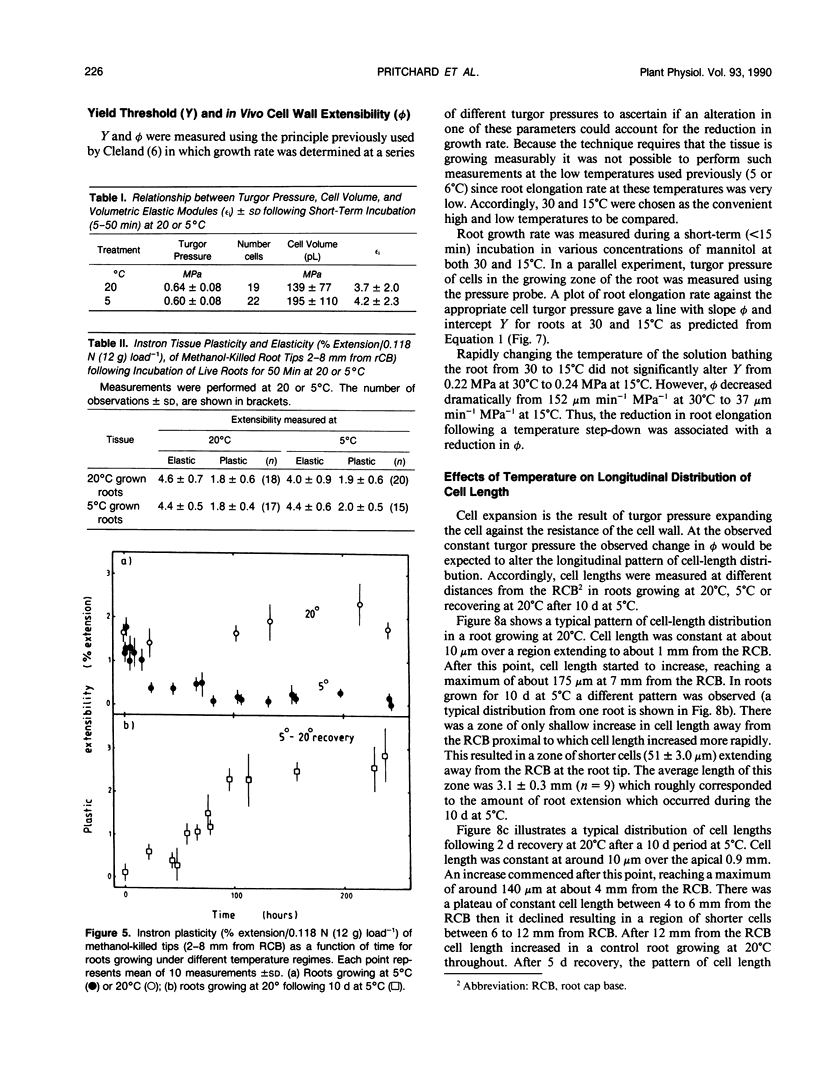
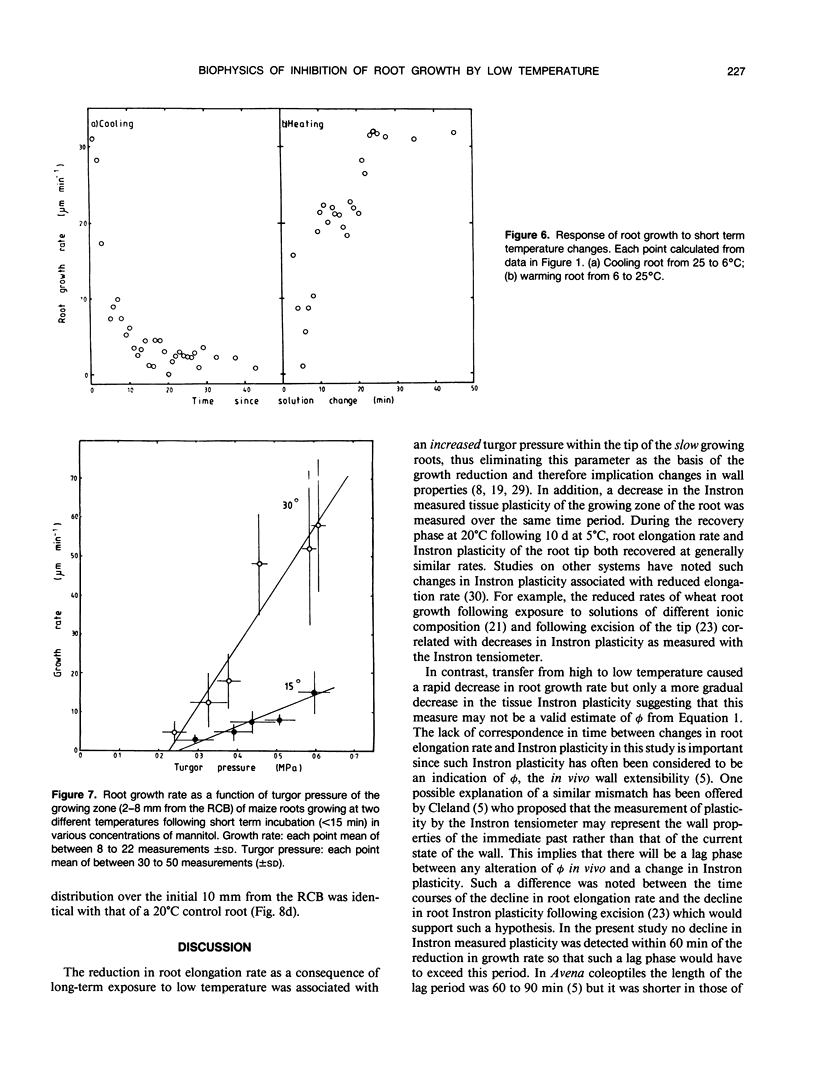
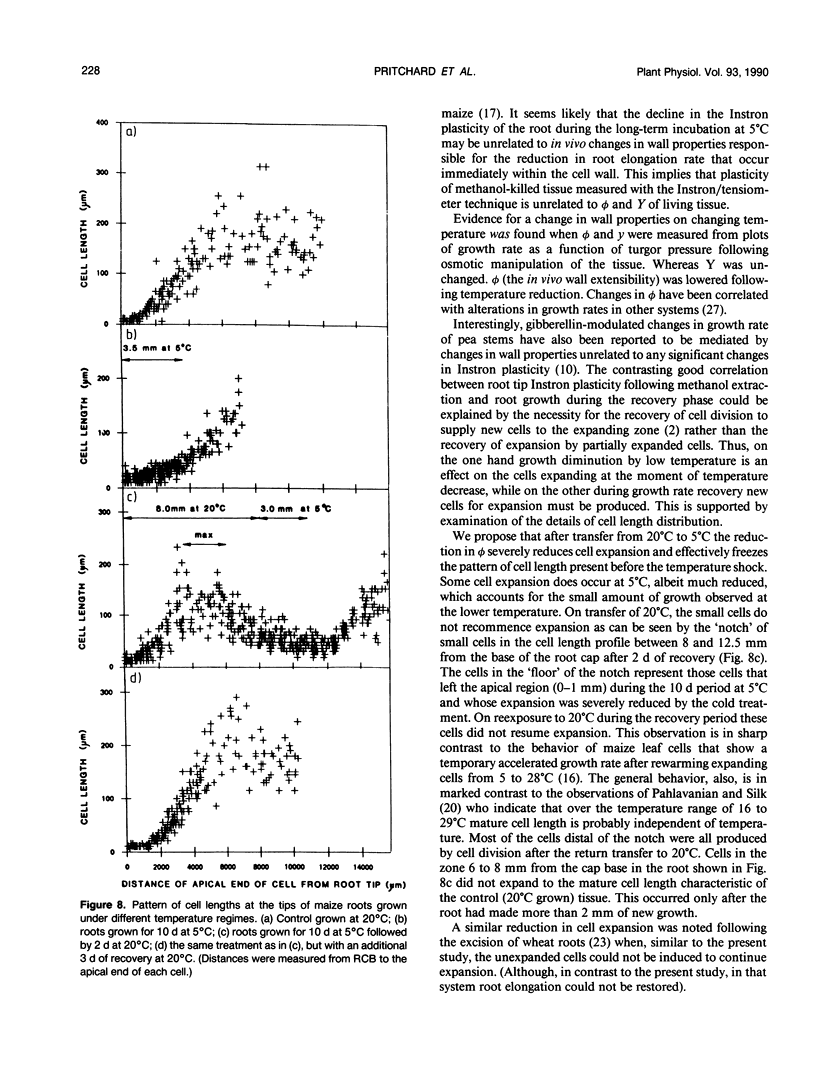
Roots of hydroponically grown maize (Zea mays cv LG11) have a greatly reduced growth rate at 5°C (0.02 millimeters per hour) compared with those at 20°C (1.2 millimeters per hour). Various physical parameters of roots growing at each temperature were compared. Turgor pressure of cells in the elongation zone increased from 0.59 ± 0.05 megapascal at 20°C to 0.82 ± 0.04 megapascal after 70 hours at 5°C; thus, growth rate was not limited by decreased pressure. On cooling, tissue plasticity (measured by Instron/tensiometer) decreased slowly over 70 hours. On rewarming to 20°C from 5°C, growth rate, turgor pressure, and tissue plasticity all returned concertedly to their original values over a period of days. However, immediately following cooling growth rate dropped rapidly from 1.8 to 0.12 millimeters per hour in 30 minutes but turgor pressure and tissue Instron plasticity remained unchanged. A plot of turgor pressure against growth rate indicated that, following cooling from 30 to 15°C, the in vivo wall extensibility of the tissue was reduced by 75%. Yield threshold was unchanged. Cells whose expansion was arrested in the long-term cold treatment do not resume growth. Root growth recovers by the expansion of cells newly produced by the meristem. Cessation of extension growth is an effect on the individual expanding cell. Growth recovery is not a reverse of this effect but requires the generation of fresh cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christiansen M. N., Carns H. R., Slyter D. J. Stimulation of Solute Loss from Radicles of Gossypium hirsutum L. by Chilling, Anaerobiosis, and Low pH. Plant Physiol. 1970 Jul;46(1):53–56. doi: 10.1104/pp.46.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. J., Cleland R. E. Osmotic properties of pea internodes in relation to growth and auxin action. Plant Physiol. 1983 Jun;72(2):332–338. doi: 10.1104/pp.72.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. J., Sovonick-Dunford S. A. Mechanism of gibberellin-dependent stem elongation in peas. Plant Physiol. 1989;89:184–191. doi: 10.1104/pp.89.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. Biophysical control of plant cell growth. Annu Rev Plant Physiol. 1986;37:377–405. doi: 10.1146/annurev.pp.37.060186.002113. [DOI] [PubMed] [Google Scholar]
- Hüsken D., Steudle E., Zimmermann U. Pressure probe technique for measuring water relations of cells in higher plants. Plant Physiol. 1978 Feb;61(2):158–163. doi: 10.1104/pp.61.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart J. A. An analysis of irreversible plant cell elongation. J Theor Biol. 1965 Mar;8(2):264–275. doi: 10.1016/0022-5193(65)90077-9. [DOI] [PubMed] [Google Scholar]
- Pahlavanian A. M., Silk W. K. Effect of temperature on spatial and temporal aspects of growth in the primary maize root. Plant Physiol. 1988 Jun;87(2):529–532. doi: 10.1104/pp.87.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk W. K., Hsiao T. C., Diedenhofen U., Matson C. Spatial distributions of potassium, solutes, and their deposition rates in the growth zone of the primary corn root. Plant Physiol. 1986 Nov;82(3):853–858. doi: 10.1104/pp.82.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk W. K., Wagner K. K. Growth-sustaining Water Potential Distributions in the Primary Corn Root: A NONCOMPARTMENTED CONTINUUM MODEL. Plant Physiol. 1980 Nov;66(5):859–863. doi: 10.1104/pp.66.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse R. E., Zamski E., Tomos A. D. Turgor regulation of sucrose transport in sugar beet taproot tissue. Plant Physiol. 1986 Jun;81(2):478–481. doi: 10.1104/pp.81.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]


